2020 Volume 2 Issue 6 Pages 339-342
2020 Volume 2 Issue 6 Pages 339-342
Background: Chronic inflammation caused by pathogenic immune response is crucial in the pathogenesis of kidney disease. In particular, T-cell-mediated adaptive immune responses evoke pathogenic immunoinflammatory responses and contribute to kidney injury (KI). Cytotoxic T lymphocyte-associated antigen-4 (CTLA-4), a potent negative regulator of T-cell immune responses, protects against immunoinflammatory diseases of the arteries such as atherosclerosis and abdominal aortic aneurysm. However, the role of this molecule in kidney disease remains undetermined.
Methods and Results: To examine the effects of CTLA-4 overexpression on angiotensin II (AngII)-induced KI, we induced KI in CTLA-4 transgenic/apolipoprotein E-deficient (CTLA-4-Tg/Apoe−/−) mice or Apoe−/− mice fed a high-cholesterol diet by continuously infusing AngII. Overexpression of CTLA-4 ameliorated the development of AngII-induced KI and fibrosis. Moreover, CTLA-4-Tg/Apoe−/− mice had decreased expression of pro-inflammatory molecules in the kidney.
Conclusions: CTLA-4 overexpression has a protective effect on AngII-induced KI, and increasing CTLA-4 may be a novel therapeutic strategy to prevent the progression of kidney disease.
Kidney disease is an important cause of mortality in developed and developing countries and has become a significant health problem.1 Kidney disease typically results from diabetes, hypertension, autoimmune disease, or infection. However, interventions to regulate these diseases are not sufficient to slow the progression of kidney dysfunction. Although a large number of experimental and clinical studies have investigated the mechanisms underlying the process of kidney dysfunction, precise mechanisms have not been fully clarified. A thorough understanding of the mechanisms leading to kidney disease could contribute to the development of novel therapeutic strategies to prevent the progression of this disease.
Accumulating evidence suggests that the innate and adaptive immune system plays a critical role in the development and progression of kidney disease.2 In particular, chronic renal inflammation via T-cell-mediated immune responses has been shown to be involved in the pathogenesis of kidney disease. Angiotensin II (AngII) is one of the critical hypertensive stimuli, and its role in various diseases including atherosclerotic disease, hypertension, and kidney disease has been extensively investigated. AngII stimulation activates immune cells including macrophages, dendritic cells, and, in particular, T cells, and mediates several key events of the inflammatory process in the development of cardiovascular and kidney disease. Based on this, it can be speculated that regulation of T-cell immune responses may be a possible strategy to prevent AngII-induced kidney injury (KI).
Naïve T cells are activated by receiving 2 critical signals from antigen-presenting cells. T cells receive the first signal via the T-cell receptor by interacting with antigenic peptide/major histocompatibility complex ligand on the antigen-presenting cells. They also receive the second signal provided by co-stimulatory molecules on antigen-presenting cells to enhance or inhibit their activation depending on the type of co-stimulatory pathways. Co-stimulatory pathways play a crucial role in the regulation of pro-inflammatory effector T cells and anti-inflammatory regulatory T cells, and have a significant influence on atherosclerosis,3 abdominal aortic aneurysm,4 and hypertension.5 Regulatory T cells and activated effector T cells express co-inhibitory molecule cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4), a homolog of CD28 that competitively binds to CD80 and CD86 on antigen-presenting cells, leading to blockade of the co-stimulatory CD80/CD86–CD28 pathway and negative regulation of T-cell function. In addition, effector T-cell responses are negatively regulated by receiving an inhibitory signal through the CD80/CD86–CTLA-4 pathway. We recently generated CTLA-4 transgenic (CTLA-4-Tg) mice on an atherosclerosis-prone background and demonstrated that overexpression of CTLA-4 protected against the development of immunoinflammatory diseases of the arteries such as atherosclerosis6 and abdominal aortic aneurysm.7 However, the effect of CTLA-4 overexpression on kidney disease remains unknown. In the present study, we used the CTLA-4-Tg mice that we have recently established, to investigate the role of CTLA-4 in the development of AngII-induced experimental kidney disease.
We used apolipoprotein E-deficient (Apoe−/−) mice and CTLA-4-Tg/Apoe−/− mice on a C57BL/6 background and fed them a high-cholesterol diet containing 0.2% cholesterol and 21% fat (CLEA, Tokyo, Japan) and water ad libitum. We implanted ALZET mini-osmotic pumps (Model 2004; DURECT, Cupertino, CA, USA) in 12-week-old mice under anesthesia and continuously infused AngII (1,000 ng/kg/min, Sigma, St Louis, MO, USA) for 28 days as described previously.7 The mice were killed at 16 weeks of age under anesthesia to evaluate KI. The mice were housed in specific pathogen-free animal facilities. All animal experiments were approved by the Animal Care Committee of Kobe Pharmaceutical University (Permit Numbers: 2018-003, 2019-008) and conformed to the NIH guidelines.
Kidney HistologyMice were anesthetized and the kidney was perfused with saline. The kidney was embedded in OCT compound (Tissue-Tek; Sakura Finetek, Tokyo, Japan), and 10-μm-thick cross-sections were prepared. Two sections in each mouse (7 mice per group) were stained with hematoxylin-eosin (HE) and the stained sections were digitally captured using an All-in-one Type Fluorescence Microscope (BZ-8000; Keyence, Osaka, Japan). Masson’s trichrome staining was performed to detect the fibrous area of the kidney. The stained sections were digitally captured using an All-in-one Type Fluorescence Microscope, and the percentage of the fibrotic area was calculated using ImageJ (National Institutes of Health, Bethesda, MD, USA). Two randomly chosen fields per section of the kidney were analyzed in each mouse and the average of the 2 sections was used for statistical analysis.
Real-Time Reverse Transcription–Polymerase Chain ReactionTotal RNA was extracted from the kidney after perfusion with RNA (Life Technologies) using TRIzol reagent (Life Technologies). Quantitative reverse transcription–polymerase chain reaction (RT-PCR) was performed using a PrimeScript RT reagent Kit (Takara, Shiga, Japan), a SYBR Premix Ex Taq (Takara), and a StepOnePlus Real-Time PCR System (Thermo Fisher Scientific) according to the manufacturer’s protocol. The following primers were used to amplify interleukin (IL)-1β, IL-6, monocyte chemoattractant protein-1 (MCP-1), CD68 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH): IL-1β, 5’-TCC AGG ATG AGG ACA TGA GCA C-3’ and 5’-GAA CGT CAC ACA CCA GCA GGT TA-3’; IL-6, 5’-CCA CTT CAC AAG TCG GAG GCT TTA-3’ and 5’-GCA AGT GCA TCA TCG TTG TTC ATA C-3’; MCP-1, 5’-GCA TCC ACG TGT TGG CTC A-3’ and 5’-CTC CAG CCT ACT CAT TGG GAT CA-3’; and GAPDH, 5’-TGT GTC CGT CGT GGA TCT GA-3’ and 5’-TTG CTG TTG AAG TCG CAG GAG-3’. Amplification reactions were performed in duplicate and fluorescence curves were analyzed with included software. GAPDH was used as an endogenous control reference.
Statistical AnalysisMann-Whitney U-test was used to detect significant differences between 2 groups. P<0.05 was considered statistically significant. For statistical analysis, GraphPad Prism version 7.0 (GraphPad Software) was used.
Amelioration of AngII-Induced KI CTLA-4 overexpression did not change mouse body weight or plasma lipid profile.7 Although there was a marked increase in systolic blood pressure (SBP) in both Apoe−/− and CTLA-4-Tg/Apoe−/− mice following AngII infusion for 4 weeks, there was no difference in SBP between the 2 groups.7 We next evaluated the effect of AngII infusion on morphological changes in kidney structure in both Apoe−/− and CTLA-4-Tg/Apoe−/− mice by evaluating HE-stained cryosections. On histology of the kidney tissues, AngII-infused Apoe−/− mice had glomerular abnormalities such as decreased Bowman’s space and increased mesangial cells, whereas only minimal changes were observed in the kidney tissues of CTLA-4-Tg/Apoe−/− mice (Figure 1). Collectively, these data indicate that CTLA-4 plays a protective role in AngII-induced KI without lowering plasma lipid profile or blood pressure.

Assessment of glomerular abnormalities in the kidney in 12-week-old apolipoprotein E-deficient (Apoe−/−) mice or cytotoxic T lymphocyte-associated antigen-4 transgenic (CTLA-4-Tg)/Apoe−/− mice fed a high-cholesterol diet and infused with angiotensin II (AngII) for 28 days, and killed at 16 weeks of age. Representative photomicrographs of kidneys stained with hematoxylin-eosin. Asterisks, decreased Bowman’s space. Arrowhead, increased mesangial cells. Scale bar, 100 μm.
The extent of fibrosis in AngII-induced KI was analyzed using Masson’s trichrome staining. Collagen deposition in the kidney of AngII-infused CTLA-4-Tg/Apoe−/− mice was significantly decreased compared with that of AngII-infused Apoe−/− mice (Figure 2).
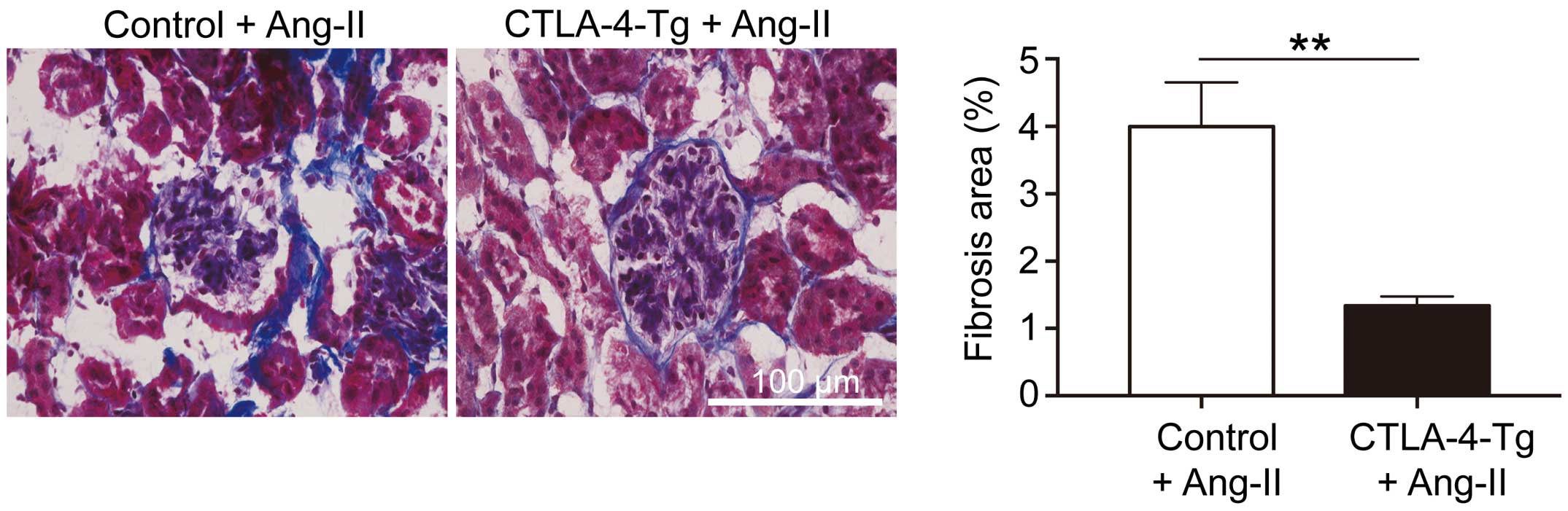
(Left) Representative photomicrographs of renal tissue fibrosis evaluated on Masson’s trichrome staining in 12-week-old apolipoprotein E-deficient (Apoe−/−) mice or cytotoxic T lymphocyte-associated antigen-4 transgenic (CTLA-4-Tg)/Apoe−/− mice fed a high-cholesterol diet and infused with angiotensin II (AngII) for 28 days, and killed at 16 weeks of age. (Right) Percentage of renal fibrosis area (n=7 mice per group). Error bars, s.e.m. **P<0.01 (Mann-Whitney U-test). Scale bar, 100 μm.
To clarify the mechanisms of AngII-induced KI fibrosis, we focused on immunoinflammatory responses in the injured kidney. Consistent with our previous findings of suppressed aortic inflammation in AngII-infused CTLA-4-Tg/Apoe−/− mice,7 on quantitative RT-PCR the mRNA expression of pro-inflammatory cytokines or chemokines (IL-1β, IL-6, MCP-1) and macrophage specific marker CD68 was markedly decreased in the kidney of AngII-infused CTLA-4-Tg/Apoe−/− mice compared with that of AngII-infused Apoe−/− mice (Figure 3). Taken together, these data indicate that CTLA-4 may prevent AngII-induced kidney fibrosis through the downregulation of renal immunoinflammatory responses.

mRNA expression of pro-inflammatory cytokines (interleukin [IL]-1β, IL-6), chemokine monocyte chemoattractant protein (MCP)-1, and macrophage specific marker CD68 on quantitative real-time reverse transcription–polymerase chain reaction and normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH), in 12-week-old apolipoprotein E-deficient (Apoe−/−) mice or cytotoxic T lymphocyte-associated antigen-4 transgenic (CTLA-4-Tg)/Apoe−/− mice fed a high-cholesterol diet and infused with angiotensin II (AngII) for 28 days, and killed at 16 weeks of age (n=5–6 mice per group). Error bars, s.e.m. *P<0.05 (Mann-Whitney U-test).
Kidney disease is one of the leading causes of morbidity and mortality worldwide. However, pharmacological treatment for several risk factors including diabetes or hypertension has little effect on the progression of this disease, and until now there has been no effective medical treatment for this disease. AngII is considered to be one of the main causes of kidney disease. AngII activates T cells and promotes their accumulation in the artery and kidney, leading to vascular and renal dysfunction.8 Therefore, therapeutic interventions to regulate T-cell activation could be effective to prevent AngII-induced kidney damage. Here, we provide evidence that overexpression of the co-inhibitory molecule CTLA-4 inhibits renal inflammation and limits the damage and fibrosis in the kidney in an AngII-induced KI mouse model. The increasing of CTLA-4 is therefore suggested as a possible therapeutic strategy to prevent the progression of kidney disease.
Accumulating experimental and clinical evidence indicates that T-cell-mediated inflammation critically contributes to the pathogenesis of hypertension.8 An experimental study using genetic or antibody-mediated blockade approaches demonstrated that inhibition of the co-stimulatory CD80/CD86–CD28 pathway prevented the development of hypertension by regulating T-cell-mediated vascular inflammation.5 In the present study, however, we found no changes in blood pressure following blockade of the CD80/CD86–CD28 pathway by CTLA-4 overexpression in AngII-infused hypercholesterolemic mice, suggesting that the protective actions of CTLA-4 overexpression in KI may depend on direct anti-inflammatory effects.
With regard to the clinical implications of the present results, CTLA-4-Ig is a soluble fusion protein consisting of the extracellular CTLA-4 portion and mimics the inhibitory effect of CTLA-4 through a cell extrinsic pathway. CTLA-4-Ig has beneficial effects in the treatment of autoimmune diseases such as type 1 diabetes mellitus9 and rheumatoid arthritis,10 which are linked to the progression of kidney disease. Notably, previous experimental studies using CTLA-4-Ig showed that blockade of the CD80/CD86–CD28 pathway prevented renal allograft rejection11 and diabetic nephropathy.12 Furthermore, not only in experimental studies, but also in a case series of focal segmental glomerulosclerosis patients, treatment with CTLA-4-Ig induced partial or complete remission of proteinuria.13 Taken together with the present findings on the protective role of CTLA-4 in AngII-induced KI, it will be of great interest to test the hypothesis that therapeutic interventions to regulate the adaptive immune response by modulating CTLA-4 function would be effective for preventing the development of kidney disease. Although careful observation is needed for application in clinical settings in consideration of the detrimental side-effects such as general immunosuppression by the co-stimulatory blockade, the present data may provide a novel strategy for the treatment and prevention of kidney disease.
We would like to thank Takashi Saito (RIKEN Center for Integrative Medical Sciences) for the generous gift of CTLA-4-Tg mice. This work was supported by JSPS KAKENHI Grant Number 18K08088 (N.S.) and research grants from Takeda Scientific Foundation (N.S.) and Suzuken Memorial Foundation (N.S.).
The authors declare no conflicts of interest.
This study was approved by the Animal Care Committee of Kobe Pharmaceutical University (reference no., 2018-003, 2019-008).