Abstract
Background:
Cardiogenic shock due to acute severe mitral regurgitation is characterized by multiple organ failure and acute pulmonary edema, leading to a high risk of mortality.
Methods and Results:
We report on a patient with acute, severe mitral regurgitation complicated by cardiogenic shock, refractory to both inotrope treatment and intra-aortic balloon pump support. The patient was successfully bridged to surgery with an Impella CP, a percutaneous left ventricular assist device.
Conclusions:
Mechanical support using an Impella CP can stabilize hemodynamics and may be used as a bridge to elective surgery for patients with mitral regurgitation with cardiogenic shock.
Management of patients with cardiogenic shock associated with acute mitral regurgitation is a substantial clinical challenge.1,2
This pathophysiology is often characterized acute pulmonary venous congestion and left ventricular overload, ultimately leading to multiple organ failure and death.1,2
Once hemodynamic instability occurs, prompt surgical intervention is recommended, as this is the only definitive therapy to correct mitral regurgitation; however, the perioperative mortality rate of patients undergoing urgent or emergency mitral valve surgery is 14.8%, which is considered extremely high.2
The Impella (Abiomed, Danvers, MA, USA) is a continuous, non-pulsatile, axial flow pump that provides both left ventricle (LV) volume unloading and active circulatory support by expelling aspirated blood from the LV into the ascending aorta.3
Here, we report a case of acute, severe mitral regurgitation complicated by cardiogenic shock that was refractory to inotrope treatment and intra-aortic balloon pump (IABP) support. The patient was successfully bridged to surgery with the temporary support of the Impella, allowing hemodynamic stabilization and thorough preoperative examination.
Case Report
A 69-year-old man who had been followed-up as an outpatient for moderate mitral regurgitation for 1 year presented with worsening shortness of breath on exertion. Cardiac workup revealed pleural effusion and atrial fibrillation; thus, the patient was referred for surgery.
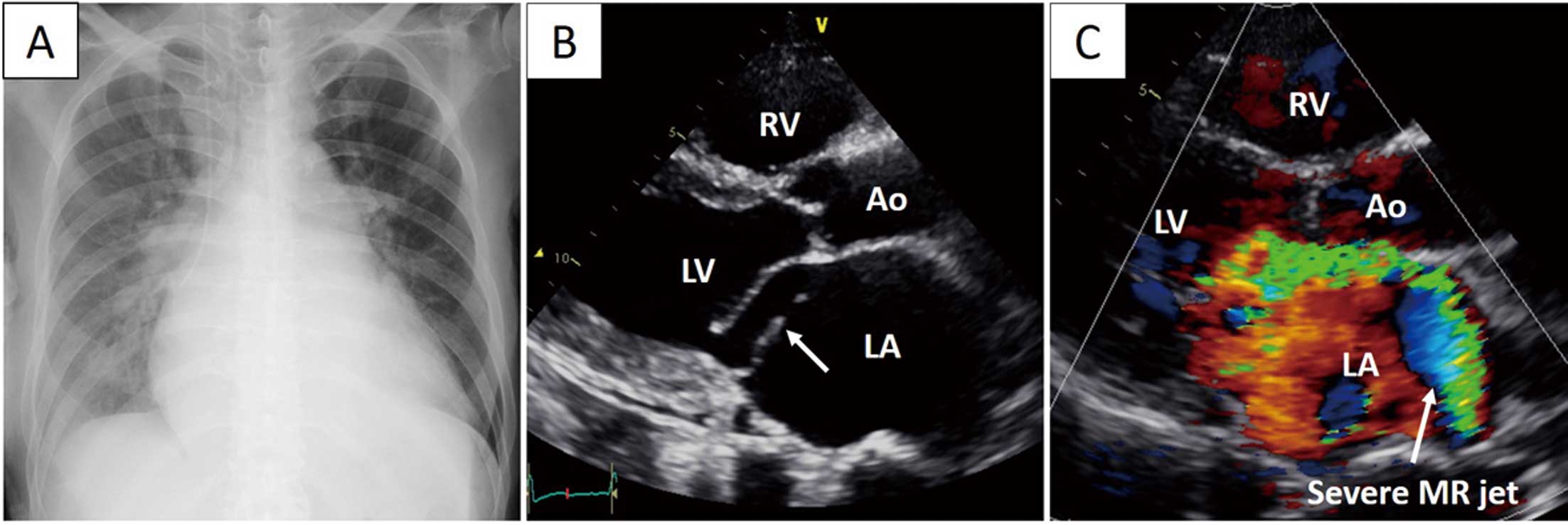
Upon physical examination, the patient was found to have jugular venous distension and peripheral coldness, along with tachycardia and hypotension. An electrocardiogram showed atrial fibrillation, with a heart rate of 128 beats/min, and the patient’s systolic blood pressure was 75 mmHg, consistent with cardiogenic shock. A chest radiograph indicated pulmonary edema and pleural effusion (Figure 1A). Transthoracic echocardiography revealed modest LV systolic dysfunction, with an ejection fraction of 48%, dilated LV end-diastolic and systolic dimensions (63 and 48 mm, respectively), and severely developed mitral regurgitation due to posterior mitral leaflet prolapse (Figure 1B,C).
Laboratory data indicated substantial liver dysfunction, as indicated by a prolonged international normalized ratio of 1.9, an aspartate transferase level of 429 IU/L, an alanine transferase level of 544 IU/L, and a total bilirubin level of 2.4 mg/dL.
The patient was diagnosed with cardiogenic shock due to severe mitral regurgitation, which may have been triggered by the onset of atrial fibrillation. Intravenous inotrope therapy was given; however, a significant improvement in hemodynamics and an increase in urinary output was not seen.
Right heart catheterization revealed high pulmonary artery pressures of 42/23/32 mmHg, a pulmonary wedge pressure of 27 mmHg, a cardiac index of 1.6 L·min−1·m−2, and mixed venous oxygen saturation (SvO2) of 57%; therefore, an IABP was introduced. Contrary to expectations, the patient’s hemodynamic status did not stabilize over a couple of hours after introduction of the IABP, as evidenced by a persistently low cardiac index (1.6 L·min−1·m−2) and SvO2
(59%). Emergency mitral valve surgery was considered to extremely high risk based on a European System for Cardiac Operative Evaluation (EuroSCORE) II of 25% and a 13% mortality risk based on the Society of Thoracic Surgeons (STS) score.
Therefore, we decided to exchange the IABP for the Impella CP for further hemodynamic support and LV unloading. The Impella CP was introduced through the left femoral artery and its pump was placed in the LV. Simultaneously, coronary angiography was performed, revealing vessel disease of both the left anterior descending artery and obtuse marginal branch. The patient’s cardiac index quickly improved to 2.3 L·min−1·m−2, and mean pulmonary artery pressure decreased from 32 to 24 mmHg. In addition, sinus rhythm was promptly restored, along with a decrease in the heart rate to 70 beats/min. Urine volume gradually, but steadily, increased over time. Consequently, the chest radiograph performed the following day showed that pulmonary congestion was relieved (Figure 2A). Furthermore, transthoracic echocardiography showed that the grade of mitral regurgitation had decreased to mild (Figure 2B,C). The course over the first 2 days of hospitalization is summarized in
Figure 3.


During Impella CP support, mild hemolysis and non-sustained ventricular tachycardia occurred; these were managed by adjusting pump position and reducing the degree of machine support (P-level) from P8 to P3. After 5 days of Impella CP support, elective mitral valve repair was performed through resection and suture of the P2, along with mitral annuloplasty and 2-vessel coronary artery bypass grafting. The Impella CP could then be removed. At that time, the EuroSCORE II was 8.6% and the STS risk of mortality was 5.6%. The patient soon recovered and was discharged 3 weeks after the operation. The postoperative echocardiogram showed the absence of mitral regurgitation, and LV function improved from 46% before surgery to 52% at 2 weeks, 57% at 2 months, and 62% at 12 months after surgery.
Discussion
Preoperative stabilization of cardiogenic shock with acute severe mitral regurgitation is complex due to multiple organ failure and acute pulmonary edema with hemodynamic collapse, making mitral valve surgery a high-risk procedure. This report shows that the Impella CP effectively relieved cardiogenic shock complicated by liver dysfunction that was refractory to inotrope treatment and IABP support, thus allowing us to conduct the elective surgery for severe mitral regurgitation; without hemodynamic stabilization, the surgery would have been high risk. In addition, coronary angiography may not have been possible without LV unloading and hemodynamic improvement, and could have resulted in untreated diseased coronary lesions and perioperative ischemic insult. We therefore believe that the Impella CP played a vital role through hemodynamic stabilization, as well as enabling thorough preoperative examination.
A novel aspect of this case is that we used the Impella CP for a patient with cardiogenic shock and mild LV systolic dysfunction. The patient had mild hemolysis and infrequent, non-sustained ventricular arrhythmia, which are known limitations of mechanical circulatory support.4
We found that the degree of hemolysis and ventricular arrhythmia rapidly improved after adjusting the pump position and the level of Impella CP support. This approach is consistent with computational flow models showing that hemolysis is caused by the high rotatory shear forces of the impeller and the small cleaning space between the impeller and its housing.5
This case report highlights the need to understand the complications during Impella CP support for patients with mildly impaired or normal LV function, as well as potential solutions.
In conclusion, we report a case of acutely developed severe mitral regurgitation complicated by cardiogenic shock refractory to inotrope treatment and IABP support, in which the patient was successfully bridged to surgery via temporary support from the Impella CP. By presenting this case, we hope that clinicians encountering high-risk cases with similar conditions in the future will be better equipped to establish a diagnosis and respond appropriately. Further investigations are mandatory to elucidate the potential benefits of mechanical support with the Impella CP in patients with acute mitral regurgitation and cardiogenic shock.
Disclosures
Y. Sawa is a member of
Circulation Reports’ Editorial Board.
IRB Information
This study was approved by the Osaka University Graduate School of Medicine (Reference no. 16105).
References
- 1.
Akodad M, Schurtz G, Adda J, Leclercq F, Roubille F. Management of valvulopathies with acute severe heart failure and cardiogenic shock. Arch Cardiovasc Dis 2019; 112: 773–780.
- 2.
Lorusso R, Gelsomino S, De Cicco G, Beghi C, Russo C, De Bonis M, et al. Mitral valve surgery in emergency for severe acute regurgitation: Analysis of postoperative results from a multicentre study. Eur J Cardiothorac Surg 2008; 33: 573–582.
- 3.
Chung JS, Emerson D, Ramzy D, Akhmerov A, Megna D, Esmailian F, et al. A new paradigm in mechanical circulatory support: 100-Patient experience. Ann Thorac Surg 2020; 109: 1370–1377.
- 4.
Nalluri N, Patel N, Saouma S, Anugu VR, Anugula D, Asti D, et al. Utilization of the Impella for hemodynamic support during percutaneous intervention and cardiogenic shock: An insight. Expert Rev Med Devices 2017; 14: 789–804.
- 5.
Konstantinou K, Keeble TR, Kelly PA, Alsanjari O, Napp LC, Karamasis GV, et al. Protected percutaneous coronary intervention with Impella CP in a patient with left main disease, severe left ventricular systolic dysfunction and established hemolysis. Cardiovasc Diagn Ther 2019; 9: 194–199.