Abstract
Background: Guidelines for the prevention and management of cardiovascular disease (CVD) highly recommend cardiac rehabilitation (CR) on the basis of abundant evidence of its effectiveness. However, the current understanding and dissemination of CR in Japan are far from sufficient.
Methods and Results: The Japanese Association of Cardiac Rehabilitation Registry (J-CARRY) is an academic society-led prospective multicenter observational registry conducted by the Registration and Facility Accreditation System Committee of the Japanese Association of Cardiac Rehabilitation. Data are collected prospectively using an electronic data capture system. Items related to patients’ clinical background and CR, as well as mortality and major adverse cardiac and cerebrovascular events, will be collected in all cases. This Registry started in May 2014, and the number of participating medical institutions is expected to increase to >30; the targeted number of cases exceeded 3,000 per year as of April 30, 2022. Focusing on late Phase II data collection is a novel and significantly different approach compared with previous studies. The results of this study are currently under investigation.
Conclusions: J-CARRY will provide real-world data regarding the current status and prognosis of CVD in patients who undergo Phase II CR in Japan.
Recent advances in the treatment and prevention of cardiovascular diseases (CVD) have significantly improved survival rates and reduced morbidity.1 However, CVD remains a leading cause of death and mortality on a global scale. Cardiac rehabilitation (CR) is a highly recommended medically supervised program in the guidelines developed for CVD due to robust evidence of its effectiveness.2–5 The Japanese Association of Cardiac Rehabilitation (JACR) was established in 1995, having been preceded by the Cardiac Rehabilitation Research Conference in Japan. The JACR has numerous members from a wide range of disciplines, including physicians, nurses, physical therapists, occupational therapists, clinical laboratory technicians, dietitians, clinical psychologists, health fitness programmers, pharmacists, and researchers. The JACR aims to promote the widespread implementation of CR and improve its quality as an advanced cardiovascular therapy with preventive intervention approaches, enhancing the quality of life and long-term prognosis of patients with CVD and contributing to the health and welfare of Japanese citizens.6 To achieve these goals, the JACR is committed to make every effort possible so as to promote and develop scientific research on CR, and thus work towards human resources development through education, training, and the academic exchange of ideas and information. The JACR also intends to promote mutual cooperation and collaboration with related organizations both in Japan and overseas, and disseminate related information, raise awareness, and provide guidance and recommendations on CR to health professionals and the general public.7
A report from the European Cardiac Rehabilitation Registry and Database with patient data from 12 countries has shown that clinical characteristics, indications, and programs for patients undergoing CR vary among countries.8 In addition, underutilization of CR, especially in female patients and those with chronic heart failure (HF), is a prevalent issue in all European countries, as well as in patients with acute coronary syndrome in some countries. A systematic review of CR registries comprising 11 articles from 7 national registries and 1 international registry, which collectively included a total of 265,608 patients, has recently highlighted the importance of benchmarking in terms of improving the quality of CR.9
Recently, nationwide evidence of CR has been reported in Japan.10–12 However, the current understanding and dissemination of CR in Japan are insufficient, thus hindering the wide implementation of this medically supervised program. The Japanese Association of Cardiac Rehabilitation Registry (J-CARRY) is an academic society-led multicenter observational registry conducted by the Registration and Facility Accreditation System Committee of the JACR. The objectives of the Registry are to accurately understand the actual status of CR in Japan and build evidence for the effectiveness of CR based on patients who are either undergoing or have already undergone Phase II CR by investigating its effects on prognosis to achieve the mission of the JACR.
Methods
Study Population
J-CARRY is an academic society-led, real-world, prospective observational database for patients with CVD, including patients after cardiac surgery, those with acute myocardial infarction (AMI), angina pectoris (AP), HF, aortic disease, peripheral artery disease, and post-structural heart disease interventions after transcatheter aortic valve implantation. All patients in the Registry participated in Phase II CR (Figure 1). The CR period following hospital admission for CVD was defined as the acute (Phase I), early recovery (early Phase II), late recovery (late Phase II), or maintenance (Phase III) phase, in accordance with the respective guidelines.2,3 In principle, early Phase II CR is defined as a comprehensive program performed in the general cardiovascular ward, whose primary objective is to prepare patients for a return to society following discharge from the coronary care unit or intensive care unit (ICU). Late Phase II CR is defined as a comprehensive program performed in an outpatient rehabilitation center that prepares patients for a return to society and helps them establish new and healthy lifestyle habits.2,3

Participating institutions in J-CARRY are training facilities accredited by the JACR and facilities that provide CR according to the standard program for CR recommended by the JACR.
Data Acquisition and Analysis
This study is an academic society-led registry organized by the JACR, with data collected prospectively using the electronic data capture system (DATATRAK Enterprise Cloud). All related sites provide their data through an electronic case report form, which is then assessed by qualified members of the Registry and by the Analysis Committee of the JACR to ensure optimal data quality.
The data collected will include patient characteristics, coronary risk factors, medical history, blood test, urinalysis, echocardiography, exercise capacity, 6-min walking test, and medications at the beginning and end of Phase II CR. The status of exercise implementation, such as the number of times that patients participate in supervised exercise therapy, the duration of home-based exercise therapy, the total duration of supervised and home-based exercise therapy, and the number of steps completed, will be surveyed at the end of Phase II CR. In addition, patients’ work status, New York Heart Association classification, the duration of exercise therapy, number of steps, prognosis including total mortality, HF hospitalization, unstable AP/AMI, revascularization (percutaneous coronary intervention/coronary artery bypass graft), ischemic heart accidents, or other cardiovascular events requiring hospitalization, fractures, pneumonia, and medical disability (other than cardiac events requiring discontinuation of exercise therapy) will be surveyed at the end of the study (Figure 1).
Continuous variables will be presented as the mean±SD when normally distributed or otherwise as the median and interquartile range; categorical variables will be presented as frequencies and percentages. Data at the beginning and end of CR recovery (late Phase II) will be compared for each patient using paired t-tests to evaluate the singular effects of CR. Odds ratios will be estimated using a logistic regression model, and will be presented with 95% confidence intervals and P values. Furthermore, in a multivariate logistic regression model, we will include statistically significant covariates in the univariate logistic regression analysis of factors associated with major adverse cardiovascular events (all-cause death or rehospitalization for AMI/unstable angina/worsening HF). The same variable selection method will be used in the Cox regression model for cardiovascular death during the follow-up period. All tests will be 2-tailed, and a P value of <0.05 will be considered statistically significant. The analyses will be performed using the JMP Version 13 software for Windows (SAS Institute, Cary, NC, USA).
Study Schedule
Trial registration started in facilities belonging to the Registry and the Analysis Committee of the JACR from May 1, 2014, with full registration starting on November 11, 2016. The mandatory registration of Phase I cases ended on October 31, 2017. After that, the total number of patients who underwent only Phase I CR was reported once a year. The electronic data capture system was updated on December 8, 2021, and will continue to recruit participants in the JACR Registry facilities.
Ethics
This study has been registered with the University Hospital Medical Information Network (UMIN) Clinical Trial Registry (ID: UMIN000021647) and conducted in accordance with the ethical principles of the Declaration of Helsinki. The study protocol was approved by the ethics committees of the participating institutions. All participants will provide written informed consent, and subjects may withdraw their consent at any time.
Results
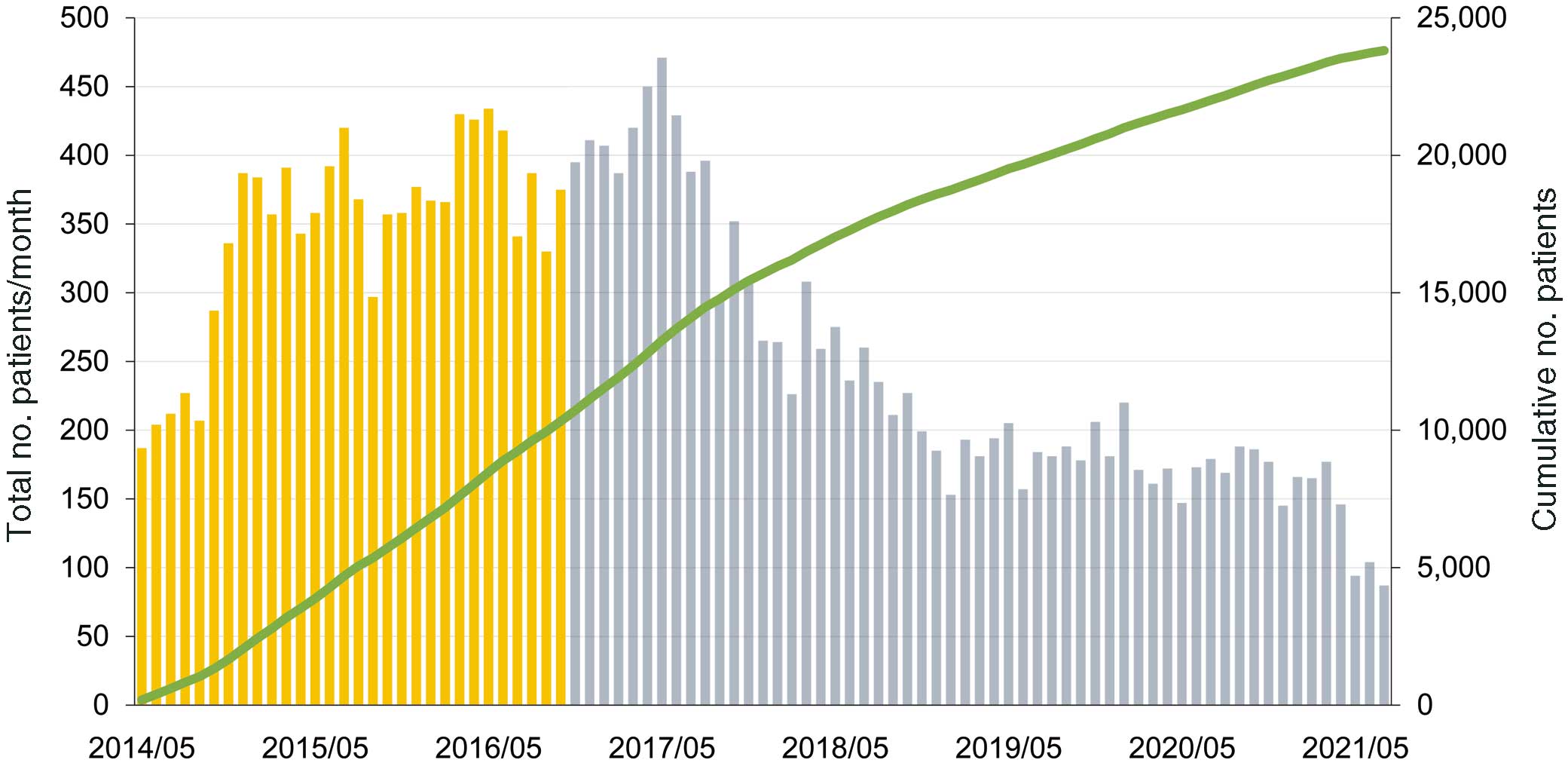
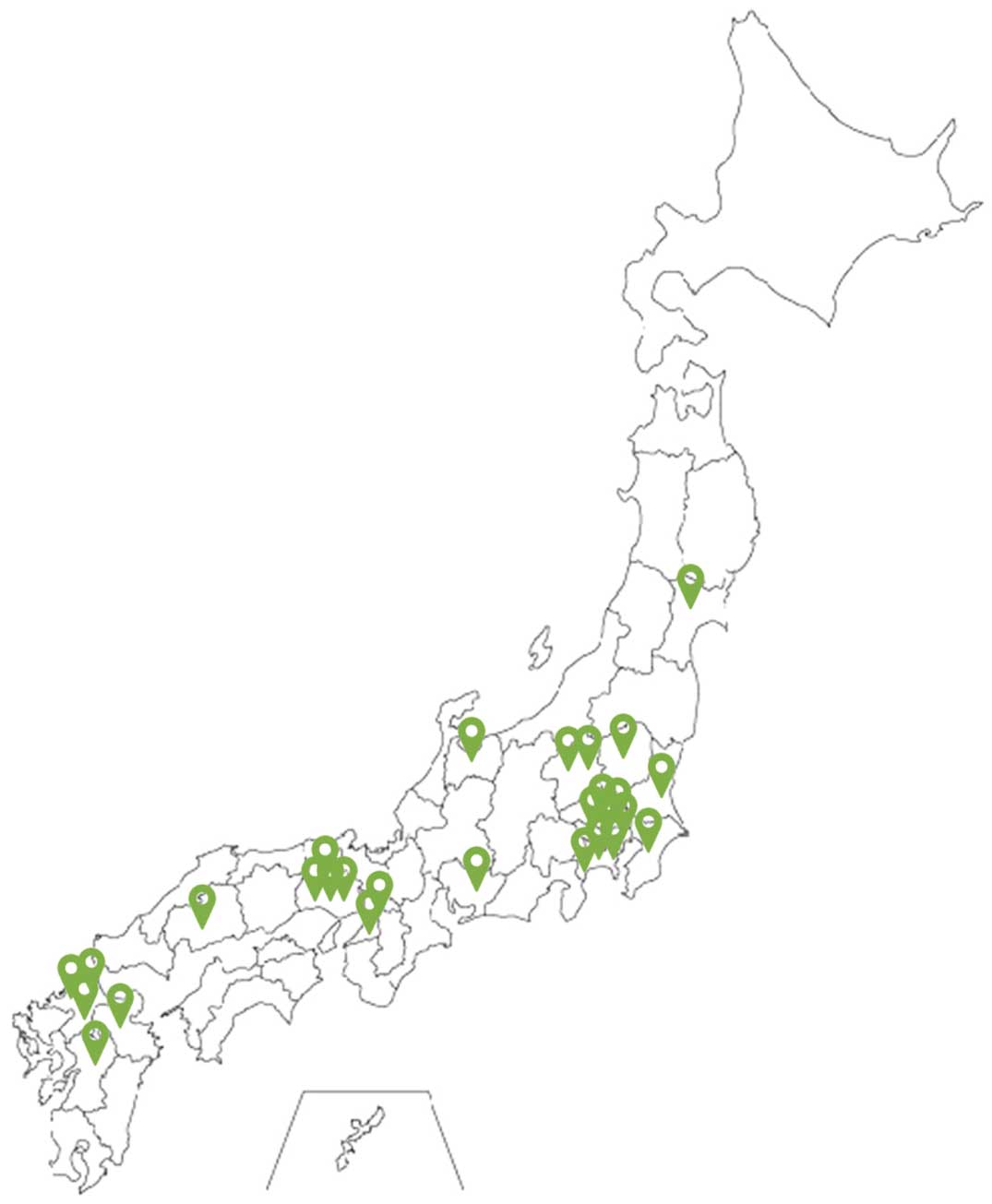
Figure 2 demonstrates the number of monthly accumulated participating centers and the registered CR cases within the first 7 years since the beginning of this registry. J-CARRY launched trial registration in May 2014, with full registration starting in November 2016. The number of participating medical institutions is expected to increase to >30, and, as of April 30, 2022, the targeted number of cases exceeds 3,000 per year. Figure 3 shows the geographic distribution of the participating institutions as of December 2021. Detailed analysis of this study is currently underway and will be published in the near future.
Discussion
J-CARRY is the largest JACR-led multicenter registry of CR in Japan, and is an important initiative for understanding the current status of CR and building evidence to improve guideline adherence and treatment quality. We believe that it is our responsibility to gather more data and provide more evidence pertaining to the clinical benefits of CR.
Evidence of CR in Japan
Epidemiological studies on CR have already been reported in Japan using the Diagnosis Procedure Combination database (a Japanese administrative database).13–15 In Japan, the number of CR-certified hospitals expanded rapidly between 2010 and 2017, leading to increased inpatient CR participation among CVD patients. In contrast, outpatient CR has remained extremely underutilized. These results are similar to the findings of the AMED-CHF study.9 Immediate action is urgently required to increase the use of outpatient CR.16 In addition, potential benefit of acute phase initiation of CR for short-term clinical outcomes in hospitalized patients with acute HF has been reported.14 A retrospective multicenter study in Japan also reported that patients participating in CR have a significantly lower risk of all-cause mortality and HF rehospitalization.17 J-CARRY is the largest JACR-led prospective multicenter registry of CR with an indefinite duration. In addition, facilities with superior programs accredited by the JACR have been participating in the Registry, and therefore high-quality data will be expected, with a particular focus on late Phase II data collection, which is novel and different from previous studies.13–15,18
Limitations and Future Challenges
In addition to J-CARRY, other database research studies on CR in Japan include JROAD (The Japanese Registry Of All cardiac and vascular Diseases) and JROAD-DPC (JROAD-Diagnosis Procedure Combination).18,19 J-CARRY is characterized by the use of individual patient data and detailed information on exercise therapy, and therefore may have considerable potential to contribute to evidence regarding all CR-adapted CVD. Conversely, it will be difficult to investigate the penetration rate and regional characteristics of CR in Japan because the Registry consists of data for patients in whom CR has already been implemented. In the future, it will be important to expand the number of participating facilities, including CR training facilities, and increase the number of registrations.
Conclusions
J-CARRY will provide real-world data regarding the current status of CR and prognosis of CVD in patients who undergo Phase II CR in Japan.
Acknowledgments
The authors thank Mayuko Ichikawa, Takeshi Nagasono, Yuji Nishizaki, Muneko Nojiri, Kazutoshi Fujibayashi, Koshi Kataoka, Sakiko Kitamura, Jiro Kosaka, Satomi Uchiyama, Koichi Tamita, Kazuya Miyamoto, Nobushige Akagi, Shinya Minatoguchi, and Kazuhiko Nishigaki for their assistance with this work. The authors acknowledge the contributions made by participants of J-CARRY in the 30 Japanese Association of Cardiac Rehabilitation (JACR) Registry facilities to manage the registry. Participants of J-CARRY in the 30 JACR Registry facilities are listed in the Supplementary Appendix.
Sources of Funding
This registry system and work were supported by the Japanese Association of Cardiac Rehabilitation.
Disclosures
H.D., Y.S., H.I., Y.O., and S. Miura are members of Circulation Reports’ Editorial Team. The remaining authors have no conflicts of interest to declare.
IRB Information
The study protocol was approved by Juntendo Hospital Ethics Committee (No. H13-58) and the ethics committees of participating institutions.
Data Availability
The datasets will not be publicly available because patient consent in each institute does not allow for such publication. The corresponding authors will respond to inquiries regarding data analyses.
Supplementary Files
Please find supplementary file(s);
http://dx.doi.org/10.1253/circrep.CR-22-0071
References
- 1.
Tsao CW, Aday AW, Almarzooq ZI, Alonso A, Beaton AZ, Bittencourt MS, et al. Heart disease and stroke statistics – 2022 update: A report from the American Heart Association. Circulation 2022; 145: e153–e639.
- 2.
JCS Joint Working Group. Guidelines for rehabilitation in patients with cardiovascular disease (JCS 2012). Circ J 2014; 78: 2022–2093.
- 3.
Makita S, Yasu T, Akashi Y, Adachi H, Izawa H, Ishihara S, et al; on behalf of the Japanese Circulation Society/the Japanese Association of Cardiac Rehabilitation Joint Working Group. JCS/JACR 2021 guideline on rehabilitation in patients with cardiovascular disease. Circ J 2022
(in press).
- 4.
JCS/JHFS Joint Working Group. JCS 2017/JHFS 2017 guideline on diagnosis and treatment of acute and chronic heart failure digest version. Circ J 2019; 83: 2084–2184.
- 5.
Tsutsui H, Ide T, Ito H, Kihara Y, Kinugawa K, Kinugawa S, et al. JCS/JHFS 2021 guideline focused update on diagnosis and treatment of acute and chronic heart failure. Circ J 2021; 85: 2252–2291.
- 6.
Goto Y. Current state of cardiac rehabilitation in Japan. Prog Cardiovasc Dis 2014; 56: 557–562.
- 7.
JACR statement mission of the Japanese Association of Cardiac Rehabilitation. 2013. https://www.jacr.jp/en/jacr-statement/ (accessed June 23, 2022).
- 8.
Benzer W, Rauch B, Schmid JP, Zwisler AD, Dendale P, Davos CH, et al. Exercise-based cardiac rehabilitation in twelve European countries results of the European cardiac rehabilitation registry. Int J Cardiol 2017; 228: 58–67.
- 9.
Poffley A, Thomas E, Grace SL, Neubeck L, Gallagher R, Niebauer J, et al. A systematic review of cardiac rehabilitation registries. Eur J Prev Cardiol 2017; 24: 1596–1609.
- 10.
Kamiya K, Yamamoto T, Tsuchihashi-Makaya M, Ikegame T, Takahashi T, Sato Y, et al. Nationwide survey of multidisciplinary care and cardiac rehabilitation for patients with heart failure in Japan: An analysis of the AMED-CHF study. Circ J 2019; 83: 1546–1552.
- 11.
Kanaoka K, Soeda T, Terasaki S, Nishioka Y, Myojin T, Kubo S, et al. current status and effect of outpatient cardiac rehabilitation after percutaneous coronary intervention in Japan. Circ Rep 2021; 3: 122–130.
- 12.
Ohtera S, Kato G, Ueshima H, Mori Y, Nakatani Y, Ozasa N, et al. A nationwide survey on participation in cardiac rehabilitation among patients with coronary heart disease using health claims data in Japan. Sci Rep 2021; 11: 20096.
- 13.
Kanazawa N, Yamada S, Fushimi K. Trends in the use of cardiac rehabilitation in Japan between 2010 and 2017: An epidemiological survey. Circ Rep 2021; 3: 569–577.
- 14.
Kaneko H, Itoh H, Kamiya K, Morita K, Sugimoto T, Konishi M, et al. Acute-phase initiation of cardiac rehabilitation and clinical outcomes in hospitalized patients for acute heart failure. Int J Cardiol 2021; 340: 36–41.
- 15.
Kanaoka K, Iwanaga Y, Fukuma N, Nakai M, Sumita Y, Nishioka Y, et al. Trends and factors associated with cardiac rehabilitation participation: Data from Japanese nationwide databases. Circ J, doi:10.1253/circj.CJ-22-0095.
- 16.
Tamura Y, Yasu T. There are not enough facilities for outpatient cardiac rehabilitation: What is the solution? Circ J, doi:10.1253/circj.CJ-22-0375.
- 17.
Kamiya K, Sato Y, Takahashi T, Tsuchihashi-Makaya M, Kotooka N, Ikegame T, et al. Multidisciplinary cardiac rehabilitation and long-term prognosis in patients with heart failure. Circ Heart Fail 2020; 13: e006798.
- 18.
Kanaoka K, Okayama S, Yoneyama K, Nakai M, Nishimura K, Kawata H, et al. Number of board-certified cardiologists and acute myocardial infarction-related mortality in Japan: JROAD and JROAD-DPC registry analysis. Circ J 2018; 82: 2845–2851.
- 19.
Gohbara M, Nishimura K, Nakai M, Sumita Y, Endo T, Matsuzawa Y, et al. Low activities of daily living associated with increased cardiovascular disease mortality in Japan: Analysis of health records from a nationwide claim-based database, JROAD-DPC. Circ Rep 2018; 1: 20–28.