2022 Volume 4 Issue 12 Pages 563-570
2022 Volume 4 Issue 12 Pages 563-570
Background: Perioperative management of body fluid levels after cardiovascular surgery with cardiopulmonary bypass is essential. Fluid management using tolvaptan with conventional diuretics is effective in maintaining urine output without worsening renal function. This study aimed to improve the in-out balance in the early perioperative phase using low-dose tolvaptan (3.75 mg/day).
Methods and Results: This prospective, single-center, randomized, open-label study included 199 patients who underwent cardiovascular surgery with cardiopulmonary bypass in Kobe City Medical Center General Hospital between September 2018 and December 2020. Treatment with tolvaptan and loop diuretics (tolvaptan group; 99 patients) was compared with treatment with loop diuretics alone (control group; 100 patients) to evaluate achievement of preoperative body weight as the primary outcome. Secondary outcomes were urine volume, the incidence of worsening renal function (WRF), and postoperative paroxysmal atrial fibrillation (POAF). There was no significant difference between groups in the return to preoperative body weight on postoperative Day 6. The tolvaptan group had significantly increased urine volume (2,530 vs. 2,150 mL/day) and decreased total furosemide dose (24 vs. 32 mg) compared with the control group. No significant differences were observed in the development of WRF and POAF between the 2 groups.
Conclusions: Although low-dose tolvaptan administration did not shorten the time to achieving preoperative body weight, it did significantly increase urine volume without WRF and POAF.
In general, cardiovascular surgery (CVS) with cardiopulmonary bypass (CPB) is more invasive than other operations without CPB and leads to excess fluid build up in the body. Controlling excess fluid is necessary for patients receiving CVS with CPB to allow early discharge postoperatively.1,2 In addition to treating fluid retention, preventing postoperative acute kidney injury (AKI), which increases mortality and morbidity postoperatively, is important because it leads to improved body fluid balance in the early postoperative period and prevents a prolonged hospital stay.1,3
Although loop diuretics are often used in postoperative fluid management, they cause worsening renal function (WRF) and postoperative paroxysmal atrial fibrillation (POAF), which leads to dehydration, electrolyte abnormalities, and sympathetic nervous system stimulation.2 Moreover, loop diuretic-related hyponatremia reduces their efficacy, with increased dosages required to maintain diuresis.4 These complications are associated with clinical exacerbations, leading to prolonged hospital stays and intensive care unit (ICU) utilization.5
Recently, combination therapy using loop diuretics and tolvaptan, a new diuretic agent, has been suggested to decrease volume and stabilize hemodynamics with reduced side effects.6 Tolvaptan promotes electrolyte-free diuresis and does not activate the renin-angiotensin system or stimulate the sympathetic nervous system. Tolvaptan has been approved for the management of heart failure.7–9 Recent studies have reported that tolvaptan administration (7.5 mg/day) in the early postoperative period may reduce the incidence of WRF and POAF.1,10 However, urine output in the tolvaptan group was reported to be approximately 300 mL higher than in the control group.1 In addition, Nakamura et al reported that both the tolvaptan and control groups used >100 mg furosemide, with a POAF rate of >40% in the control group.10
We hypothesized that even low-dose (3.75 mg/day) tolvaptan, added to low-dose loop diuretics, may maintain adequate urine volume and may be useful in reducing volume without intravascular hypovolemia and postoperative complications. Thus, the present study investigated whether low-dose tolvaptan improves the in-out balance in the early perioperative phase in addition to sparing loop diuretics.
Altogether, 240 consecutive patients aged >20 years who received CVS with CPB at Kobe City Medical Center General Hospital between September 2018 and December 2020 were enrolled in the study. Patients with chronic kidney disease, hemodialysis, an estimated glomerular filtration rate (eGFR) <30 mL/min/1.73 m2, low cardiac output with an ejection fraction <40%, an admission related to heart failure or infection within 4 weeks before operation, and those undergoing urgent or emergency operation, thoracoabdominal replacement or off-pump coronary artery bypass procedures, and mechanical ventilation or reintubation over the last 24 h were excluded from the study.
Study ProceduresThis was a single-center prospective randomized open-label study. Enrolled patients were randomized in a 1 : 1 ratio to treatment with either tolvaptan and loop diuretics (tolvaptan group) or loop diuretics alone (control group). Randomization was performed using random allocation. In all cases, information was collected for each at discharge, and data were entered manually from patient records.
All patients were administered azosemide orally at a dose of 30 mg/day on postoperative Day (POD) 2. Furosemide was administered intravenously at a dose of 10–40 mg/day from POD1 at the discretion of physicians based on urine output <0.5 mL/kg/h. Patients in the tolvaptan group were administered 3.75 mg/day tolvaptan orally from POD2 in addition to 30 mg/day azosemide orally. Tolvaptan was discontinued if the serum sodium concentration was ≥149 mEq/L. These diuretics were maintained until return to preoperative body weights or until POD6; no other diuretics, such as human atrial natriuretic peptide, or oral diuretics (except azosemide) were used (Figure 1A).
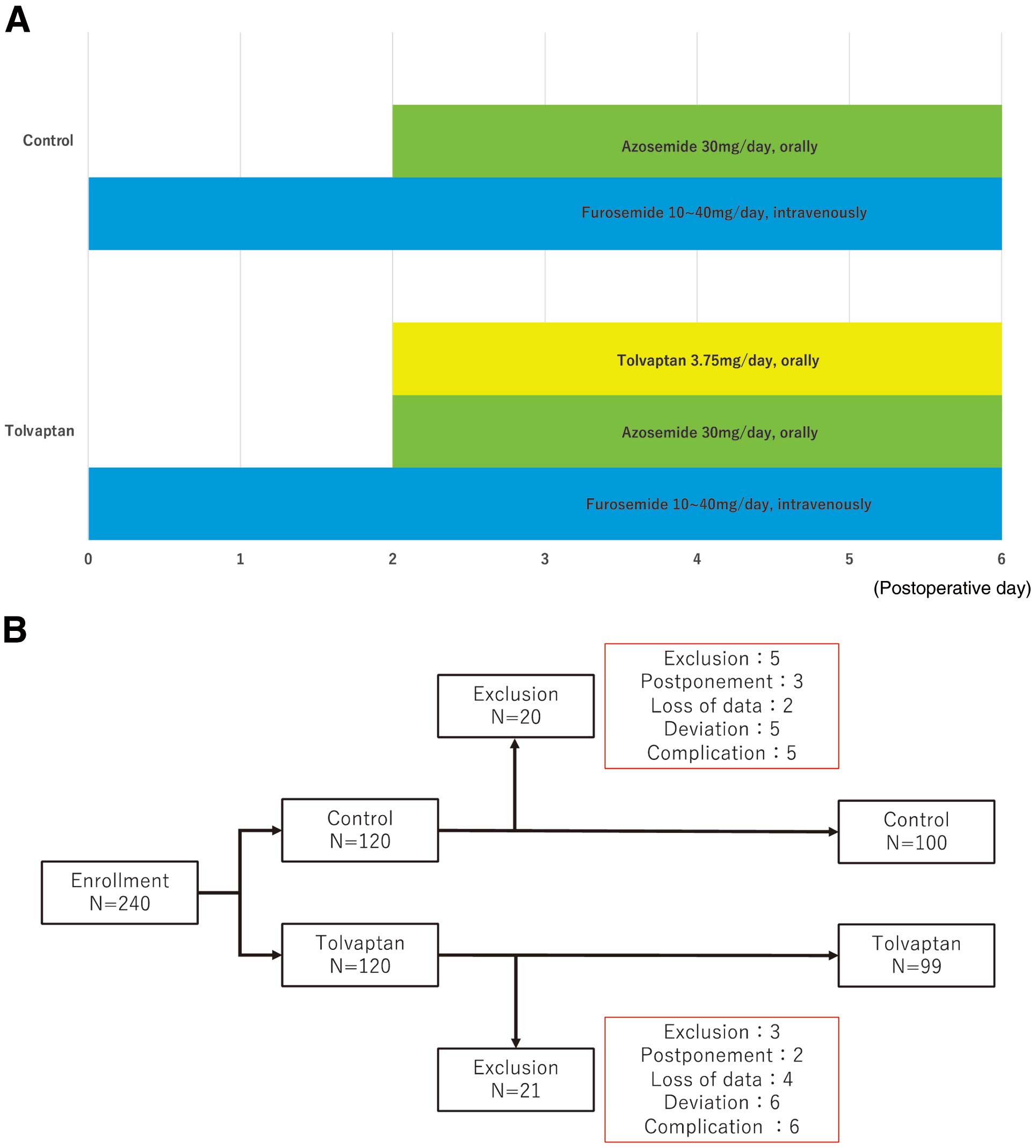
(A) Study procedures. All patients were administered 30 mg/day, p.o., azosemide from Postoperative Day (POD) 2. In addition, furosemide was administered at doses of 10–40 mg/day, i.v., from POD1 at the discretion of physicians based on urine output <0.5 mL/kg/h. In the tolvaptan group, tolvaptan was administered at a dose of 3.75 mg/day, p.o., from POD2 in addition to 30 mg/day, p.o., azosemide. These diuretics were continued until patients had returned to their preoperative body weight up to POD6. (B) Patient enrolment. In all, 240 consecutive patients were enrolled between September 2018 and December 2020 and randomly allocated to the tolvaptan or control group. After randomization, 8 patients who met the exclusion criteria, 5 patients whose surgery was postponed, 6 patients with insufficient data, 11 patients who deviated from the treatment protocol, and 11 patients with complications were excluded. Hence, 199 patients were randomized into 2 groups (tolvaptan, n=99; control, n=100).
The primary outcome was achieving preoperative body weight by POD6; secondary outcomes were renal function and treatment-related complications. In particular, we examined urine volume, the incidence of WRF, the cumulative amount of furosemide, maximum serum creatinine concentrations, and electrolyte imbalance (hypernatremia and hyperkalemia), as well as factors related to WRF. In the present study, WRF was defined based on the Kidney Disease: Improving Global Outcomes clinical practice guidelines for AKI. Stage 1 AKI was defined as an increase in serum creatinine concentrations to ≥0.3 mg/dL or a ≥1.5- to 2-fold increase from the baseline value. Stage 2 AKI was defined as a >2- to 3-fold increase from the baseline value. Stage 3 AKI was defined as a >3-fold increase from the baseline value or an absolute serum creatinine concentration ≥4.0 mg/dL, with a rapid increase of at least 0.5 mg/dL on renal replacement therapy.11
This study was conducted in accordance with the principles of the Declaration of Helsinki. Written informed consent was obtained from all patients.
Operative TechniqueSurgical procedures were performed via a right lateral incision for minimally invasive cardiac surgery (MICS) or mid-sternotomy without MICS. CPB was performed for all patients under normothermic conditions. Myocardial protection was maintained with intermittent antegrade and retrograde blood cardioplegia. For aortic surgery, selective cerebral perfusion was performed using the open distal technique at a rectal temperature of 28–30℃.
Postoperative ManagementPostoperatively, all patients were admitted to the ICU and administered inotropic agents when necessary. Patients were extubated within 48 h postoperatively. Urine volume was measured and blood samples were checked until POD7. Body weight was measured daily from POD1 to discharge. Electrocardiographic monitoring was continued until discharge.
Statistical AnalysisAll data were analyzed using SPSS Statistics ver. 25 (IBM SPSS, Chicago, IL, USA). The sample size was determined to have an α level of 0.05, and a power of 0.8. Fisher’s exact test was used to compare frequencies, and unpaired t-tests were used to compare continuous variables between groups. Two-way analysis of variance with Scheffé’s test for repeated measures was used comparisons of urine volume and results of blood tests. In all analyses, 2-sided P<0.05 was considered significant.
Altogether, 240 consecutive patients undergoing CVS with CPB between September 2018 and December 2020 were randomly allocated to the tolvaptan and control groups. After randomization, 8 patients who met the exclusion criteria, 5 patients whose surgery was postponed, 6 patients who had insufficient data, 11 patients who deviated from the protocol, and 11 patients with complications were excluded. Hence, 199 patients were randomized to the tolvaptan (n=99) and control (n=100) groups (Figure 1B). There were no significant differences in baseline characteristics (age, sex, preoperative complications, echocardiographic data, renal function, oral diuretic therapy, and etiology) or surgical data (surgical procedures and operation data) between the 2 groups (Tables 1,2). In both groups, the 30-day mortality rate was 0%. There was no difference in ICU and hospital stays between the 2 groups; in addition, there were no differences in the time to mechanical ventilation and time to discontinuation of oxygen administration between the 2 groups (Table 3).
| Control group | Tolvaptan group | P value | |
|---|---|---|---|
| Patient background | |||
| Age (years) | 69±12 | 68±12 | 0.413 |
| Sex (male/female) | 57/43 | 54/45 | 0.776 |
| Height (cm) | 162±10 | 162±11 | 0.785 |
| Weight (kg) | 59±12 | 59±14 | 0.750 |
| BSA (m2) | 1.62±0.19 | 1.61±0.23 | 0.72 |
| Hypertension | 58 (58) | 66 (67) | 0.243 |
| Diabetes | 10 (10) | 12 (12) | 0.571 |
| Dyslipidemia | 34 (34) | 44 (44) | 0.148 |
| Atrial fibrillation | 32 (32) | 26 (26) | 0.436 |
| Cerebral infraction | 10 (10) | 11 (11) | 0.822 |
| Diuretics | 28 (28) | 22 (22) | 0.414 |
| Preoperative renal function | |||
| BUN (mg/dL) | 17±6 | 17±7 | 0.828 |
| Cr (mg/dL) | 0.81±0.22 | 0.83±0.24 | 0.669 |
| eGFR (mL/min/1.73 m2) | 68±16 | 67±17 | 0.695 |
| Preoperative echocardiographic data | |||
| LVDd (mm) | 50±8 | 50±8 | 0.519 |
| LVDs (mm) | 32±8 | 33±8 | 0.459 |
| LAD (mm) | 40±9 | 40±9 | 0.789 |
| EF (%) | 63±6 | 62±6 | 0.783 |
| Asynergy | 13 (13) | 14 (14) | 0.839 |
Unless indicated otherwise, data are presented as the mean±SD or n (%). BSA, body surface area; BUN, blood urea nitrogen; Cr, creatinine; EF, ejection fraction; eGFR, estimated glomerular filtration rate; LVDd, left ventricular diastolic diameter; LVDs, left ventricular systolic diameter.
| Control group | Tolvaptan group | P value | |
|---|---|---|---|
| Surgical procedure | |||
| AVP/AVR (n) | 3/35 | 0/26 | 0.095 |
| MVP/MVR (n) | 32/9 | 30/6 | 0.561 |
| TAP/TVR (n) | 23/0 | 15/1 | 0.284 |
| HAR | 15 (15) | 12 (12) | 0.680 |
| TAR | 14 (14) | 21 (21) | 0.197 |
| ARR | 6 (6) | 13 (13) | 0.097 |
| CABG | 9 (9) | 11 (11) | 0.645 |
| MAZE | 13 (13) | 10 (10) | 0.658 |
| MICS | 14 (14) | 10 (10) | 0.515 |
| Reoperation | 16 (16) | 21 (21) | 0.368 |
| Concomitant operation | 21 (21) | 11 (11) | 0.081 |
| Surgical data | |||
| Surgical time (min) | 345±86 | 365±102 | 0.125 |
| CPB time (min) | 178±56 | 188±60 | 0.221 |
| In-out balance (mL) | 4,190±1,940 | 4,360±2,300 | 0.563 |
| Bleeding volume (mL) | 270±230 | 300±230 | 0.388 |
| Transfusion (mL) | 2,050±1,180 | 2,320±1,230 | 0.070 |
Unless indicated otherwise, data are presented as the mean±SD or n (%). ARR, aortic valve replacement; AVP, aortic valve plasty; AVR, aortic valve replacement; CABG, coronary artery bypass grafting; CPB, cardiopulmonary bypass; HAR, hemiarch replacement; MICS, minimally invasive cardiac surgery; MVP, mitral valve plasty; MVR, mitral valve replacement; TAP, tricuspid annuloplasty; TAR, total arch replacement.
| Control group | Tolvaptan group | P value | |
|---|---|---|---|
| 30-Day mortality | 0 | 0 | 1.000 |
| Intubation (h) | 7.3±4.2 | 7.5±3.9 | 0.643 |
| ICU stay (days) | 3.0±1.4 | 2.9±1.2 | 0.439 |
| Hospital stay (days) | 15±5 | 15±6 | 0.362 |
| Time to preoperative weight (days) | 4.1±1.1 | 4.0±1.2 | 0.529 |
| Oxygen therapy (days) | 3.2±2.2 | 3.2±2.3 | 0.931 |
| Transfer to rehabilitation hospital | 11 (11) | 9 (9) | 0.814 |
| Medications | |||
| Furosemide (mg) | 32±35 | 24±29 | 0.086 |
| Dobutamine (mL) | 33±48 | 36±105 | 0.815 |
| Norepinephrine (mL) | 19±68 | 14±37 | 0.549 |
| Complications | |||
| POAF | 13 (19) | 16 (22) | 0.835 |
| Stroke | 1 (1) | 1 (1) | 1.000 |
| Urinary infection | 4 (4) | 1 (1) | 0.369 |
| Pneumonia | 2 (2) | 0 | 0.497 |
| Hypernatremia | 0 | 0 | 1.000 |
Unless indicated otherwise, data are presented as the mean±SD or n (%). ICU, intensive care unit; POAF, postoperative atrial fibrillation.
There was no significant difference in the primary endpoint (i.e., achieving preoperative body weight by POD6) between the control and tolvaptan groups (78.0% vs. 77.8%, respectively; P=0.553). The mean (±SD) time to achieving preoperative body weight was 4.1±1.1 and 4.0±1.2 days in the control and tolvaptan groups, respectively (P=0.529; Table 3). In terms of secondary endpoint, urine volume from POD3 to POD4 was significantly increased in the tolvaptan compared with control group (Figure 2). There was no significant difference in changes in postoperative body weight relative to preoperative body weight up to POD5 between the 2 groups (Figure 3). The total dose of furosemide tended to be lower in the tolvaptan than control group (mean [±SD] 24±29 vs. 32±35 mg, respectively; P=0.086; Table 3). There was no correlation between the amount of furosemide administered and the effect of tolvaptan (P=0.086).
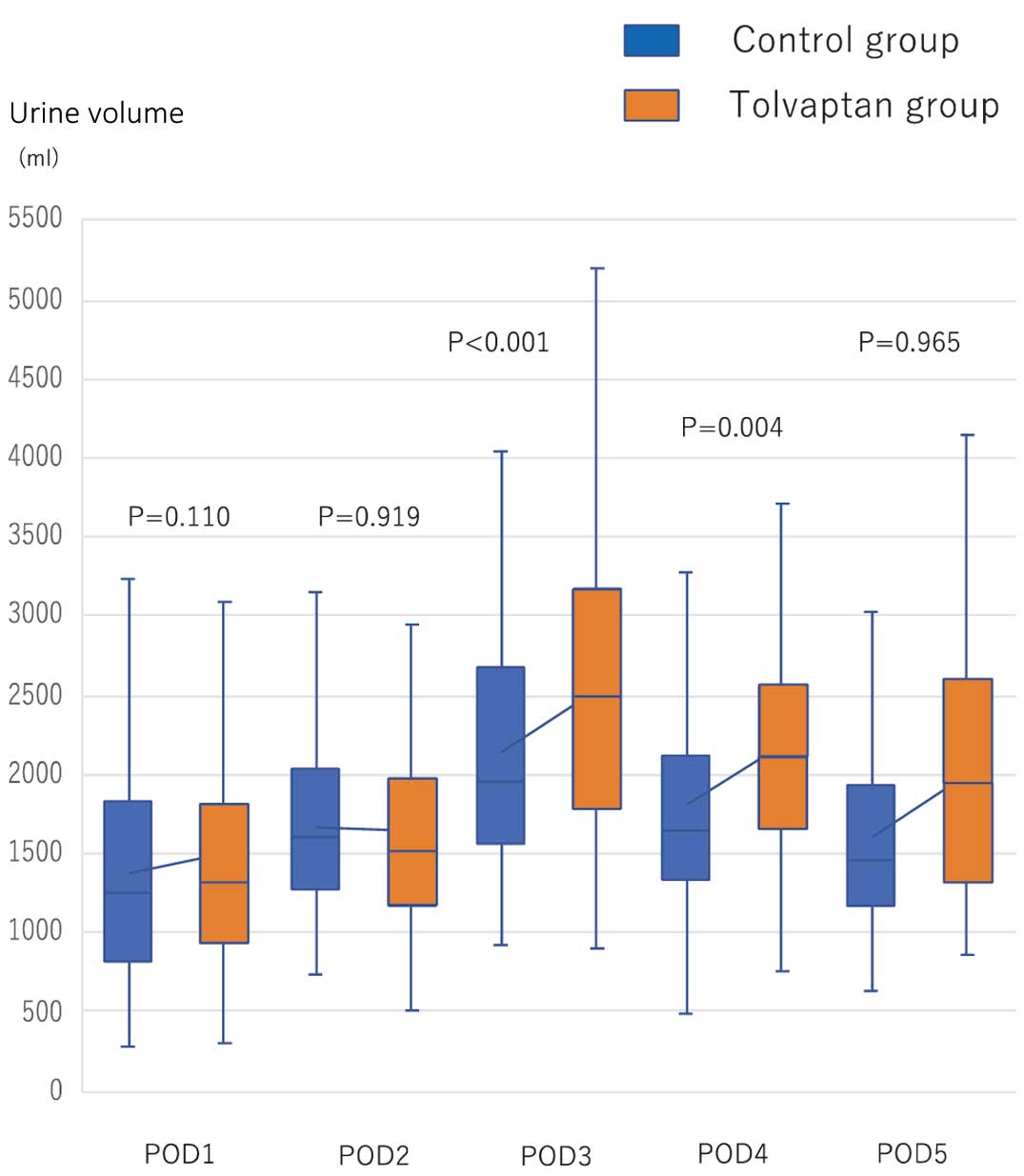
Urine volume from Postoperative Day (POD) 1 to POD5 in the tolvaptan and control groups. Urine volume was significantly increased in the tolvaptan compared with control group on POD3 (mean [±SD] 2,530±890 vs. 2,150±810 mL, respectively; P<0.001) and POD4 (mean [±SD] 2,180±750 vs. 1,810±760 mL, respectively; P=0.004). The boxes show the range of standard deviation, with the median value indicated by the horizontal line; whiskers show the range.
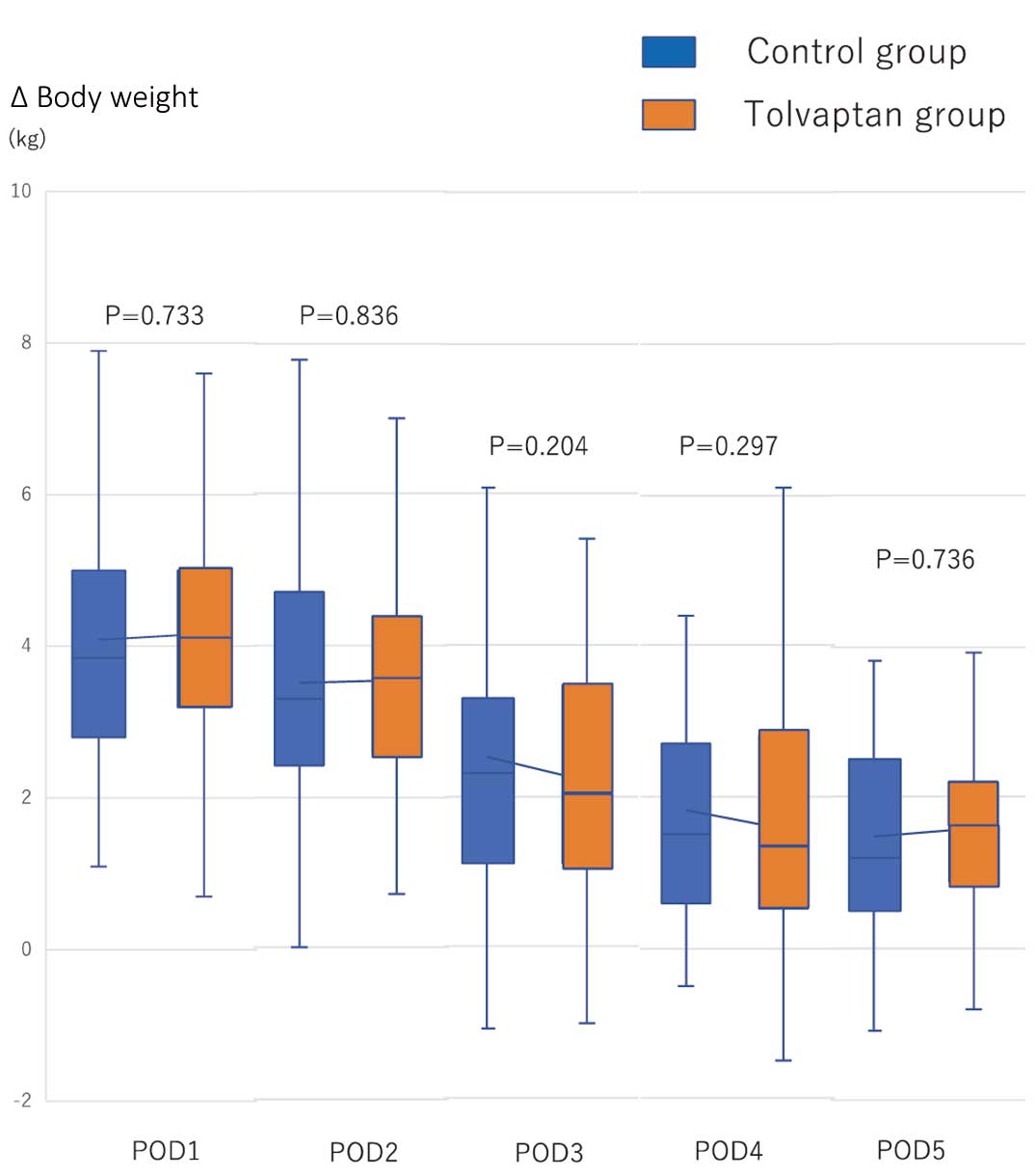
Serial changes in differences between pre- and postoperative body weight from Postoperative Day (POD) 1 to POD5 in the tolvaptan and control groups. There were no significant differences between the 2 groups (P>0.05). The boxes show the range of standard deviation, with the median value indicated by the horizontal line; whiskers show the range.
There was no difference in creatinine levels or eGFR between the 2 groups (Figure 4). In addition, there were no significant differences in the development of WRF between the tolvaptan and control groups (Table 4). Only Stage 2 AKI was observed (n=7 [7%] in each group). The maximum creatinine level did not differ significantly between the control and tolvaptan groups (mean [±SD] 0.89±0.26 vs. 0.93±0.31 mg/dL, respectively; P=0.326).
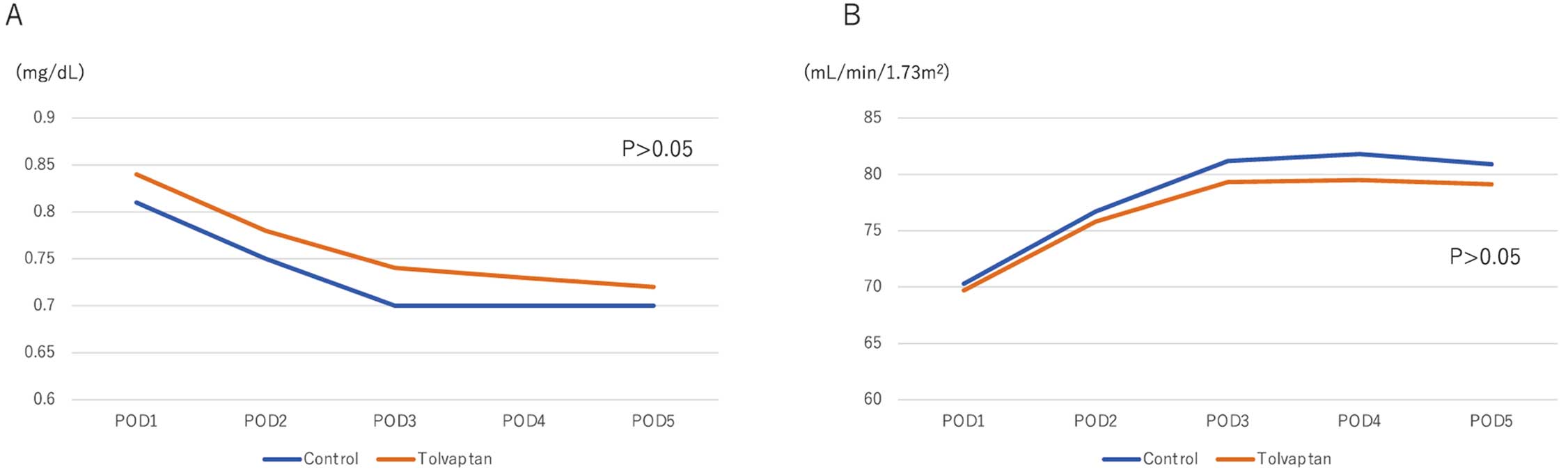
Serial changes in (A) creatinine concentrations and (B) estimated glomerular filtration rate (eGFR) in the control and tolvaptan groups. There were no significant differences between the 2 groups in either creatinine concentrations or eGFR (P>0.05). Data are the mean values for creatinine and eGFR at each time point.
| Control group | Tolvaptan group | P value | |
|---|---|---|---|
| AKI | 7 (7) | 7 (7) | 1.000 |
| Stage 1 | 7 (7) | 7 (7) | 1.000 |
| Stage 2/3 | 0 | 0 | 1.000 |
| Maximum Cr (mg/dL) | 0.89±0.26 | 0.93±0.31 | 0.326 |
Unless indicated otherwise, data are presented as the mean±SD or n (%). AKI, acute kidney injury; Cr, creatinine.
With regard to POAF, 141 patients (68 control, 73 tolvaptan) were evaluated. Of these, 58 with fibrillation were excluded. Among the remaining patients, POAFs were reported in 13 (19%) and 16 (22%) patients in the control and tolvaptan groups, respectively (P=0.835).
Although serum sodium concentrations from POD3 to POD5 were significantly higher in the tolvaptan than control group (P<0.001), there were no cases in which sodium concentrations exceeded 149 mEq/L. In addition, there were no significant differences in serum potassium concentrations between the 2 groups and there were no cases of hyperkalemia in either group.
Tolvaptan was approved for heart failure with volume overload in 2010, and its treatment with tolvaptan for heart failure was standardized. Recently, it has become common in Japan to use tolvaptan in the early postoperative stage as primary therapy because the postsurgical fluid management is the most important factor in managing fluid retention after CVS with CPB.6 In addition to acting as a diuretic, tolvaptan can contribute to the maintenance of renal blood flow without activating the renin-angiotensin-aldosterone and sympathetic nervous systems.1,12–14 Hence, using tolvaptan could decrease the total amount of furosemide required, reducing the untoward side effects of furosemide use, such as intravascular dehydration, electrolyte abnormalities, and sympathetic nervous system stimulation. Therefore, tolvaptan could preserve renal function and help patients achieve an early return to their preoperative body weight without postoperative complications such as WRF and POAF.10 Several studies have revealed that postoperative management with tolvaptan is effective in terms of renal protection and management of excess body fluid after CVS with CPB.15–17 In addition, tolvaptan administration reduces the amount of furosemide required while maintaining urine output.15–17
In the present study we used a lower dose of tolvaptan (3.75 mg/day, p.o.) than that used in previous studies (7.5 mg/day, p.o.), with no significant difference in the primary endpoint (achieving preoperative body weight by POD6) between the tolvaptan and control groups (P=0.553). The mean (±SD) time required to return to preoperative body weight was 4.1±1.1 and 4.0±1.2 days in the control and tolvaptan groups, respectively (P=0.529). With regard to secondary outcomes, urine volume from POD3 to POD4 was significantly greater in the tolvaptan than control group (Figure 2). Moreover, there was a tendency for a decrease in the total dose of furosemide administered to patients in the tolvaptan compared with control group (mean [±SD] 24±29 vs. and 32±35 mg, respectively; P=0.086; Table 3). Compared with total doses of >100 mg furosemide reported in previous studies, the amount of loop diuretics used in the present study was insignificant yet effective in increasing urine volume.
POAF is generally the most frequent complication after CVS with CPB, and the incicence of POAF remains unchanged at 10–60% for decades.18,19 POAF promotes the development of other complications (heart failure, WRF, and embolic cerebral infarction) and can lead to prolonged hospital stays.20 To prevent POAF, the American Heart Association guidelines recommend the use of β-blockers (Class 1 recommendation).21 However, using β-blockers routinely in patients with unstable hemodynamics can be difficult in the early postoperative period. Recent studies have suggested that volume overload, WRF, abnormal electrolyte levels, and sympathetic nervous system activation are risk factors for POAF.5 Hypovolemia is also a risk factor for POAF.22 Therefore, in addition to using β-blockers, managing excessive fluid levels postoperatively is important to prevent POAF. In general, for fluid management after CVS with CPB, patients are administered loop diuretics, which reduce renal blood flow and activate the renin-angiotensin-aldosterone and sympathetic nervous systems.23,24 Thus, tolvaptan tends to be used in the early postoperative period because high doses of loop diuretics may increase the risk of POAF. Several studies have reported no significant differences in the incidence of atrial fibrillation after CVS.1,6,15,25–29 In contrast, Nakamura et al reported that tolvaptan effectively reduced the amount of furosemide and the incidence of POAF due to improvements in volume overload with low dosages of loop diuretics in the early postoperative period.10 However, in that study, POAF occurred in 35 (42.2%) patients in the control group,10 which is higher than the incidence of POAF in the present study. In the present study, there were no significant differences in the incidence of POAF between the control and tolvaptan groups (n=13 [19%] and n=16 [22%], respectively; P=0.835; Table 3), and the occurrence of POAF was much lower than in previous studies. The standard dose of tolvaptan and high-dose loop diuretics bear the risk of dehydration, and low doses of tolvaptan and loop diuretics may prevent POAF, especially in high-risk patients.
WRF is one of the most significant adverse effects of diuretics for body fluid management because it prolongs the length of the hospital stay, and previous studies have reported that tolvaptan prevents WRF compared with conventional therapy.30–32 Kishimoto et al reported that the rate of increase in postoperative creatinine concentrations was significantly lower in the tolvaptan than control group.1 Yamada et al reported that tolvaptan provided adequate urine volume without leading to a deterioration in renal function, and the renal protective effect may become stronger as the stage of chronic kidney disease increases.16 In the present study, 7 (7%) patients in the control group had WRF, and there was no significant difference in the development of WRF between the 2 groups. However, the rate of WRF in the control group was >10% despite preoperative creatinine concentrations being almost the same as in previous reports.1,10
Therefore, the difference in the time to achieving preoperative body weight was <1 day between the present study and previous trials. In addition, the differences in urine output between the control and tolvaptan groups were almost the same in the present compared with previous studies, and there was a higher rate of POAF and WRF in the control group in the present study than in previous studies in which >100 mg furosemide was administered.1,10 These results suggest that although the differences in total urine volume, POAF, and WRF may be the result of tolvaptan, they are more likely related to the total amount of loop diuretics, which activate the renin-angiotensin system or stimulate the sympathetic nervous system. However, it is possible that, in addition to urine volume, significant differences may be seen in WRF and POAF when severe cases, such as emergency, severe renal failure, and low output patients, are included; such patients were not included in the present study. We believe that low-dose tolvaptan is effective because even a small amount of tolvaptan can increase urine output, and it may be shown to be effective in preventing WRF and POAF compared with previous studies, provided that severe cases are included in the analysis.1,10,16 To this end, further studies are needed to demonstrate the efficacy of tolvaptan (3.75 vs. 7.5 mg).
Serum sodium concentrations were significantly higher in the tolvaptan than control group (P<0.01), but none of the patients was found to have significant hypernatremia (serum sodium >149 mEq/L). Moreover, there were no significant differences in serum potassium concentrations between the 2 groups (P>0.05). There were no discontinuations of tolvaptan due to electrolyte imbalance, so its use appeared to be safe.
Study LimitationsThis study has several limitations. First, this was a prospective randomized study from a single institution; thus, selection bias is inherent. Second, the dose of intravenous furosemide was determined by physicians who were not blinded. Third, it remains unclear when diuretics should be started; assessing the optimal diuretic period for each patient may be challenging. Fourth, we excluded patients with severe backgrounds (urgent or emergency operation, low cardiac output with an ejection fraction <40%, and eGFR <30 mL/min/1.73 m2). Finally, the efficacy of tolvaptan treatment has not been examined; for that, responders to tolvaptan should be investigated, because no evidence of the optimal amount of tolvaptan in this setting exists as yet.
Low-dose tolvaptan administration with low-dose loop diuretics could be safe and effective for postoperative fluid management in patients after CVS with CPB and may decrease the total dose of loop diuretic.
The authors thank Editage (www.editage.com) for help with English language editing.
This study received no funding or grants from any agency or institution.
The authors have no conflicts of interest to disclose.
The trial protocol (n180801) was approved by the Ethics Committee of Kobe City Medical Center General Hospital on August 25, 2018.
The deidentified participant data will not be shared.