Abstract
Background: One-month dual antiplatelet therapy (DAPT) in high bleeding risk (HBR) patients undergoing percutaneous coronary intervention (PCI) with the Resolute OnyxTM
zotarolimus-eluting stents (ZES) is safe and effective. Asian patients have a unique ischemia/bleeding risk profile. Here, we compare the outcomes between Asian and non-Asian patients after PCI and 1-month DAPT.
Methods and Results: Onyx ONE Clear was a prospective, multicenter study enrolling HBR patients undergoing PCI with the Resolute Onyx ZES (ClinicalTrials.gov identifier NCT03647475). Event-free patients after 1-month DAPT transitioned to single antiplatelet therapy. Clinical outcomes between 1 month and 2 years were compared between patients from Asian and non-Asian countries after 1 : 1 propensity score matching accounting for baseline differences. Patients from Asian countries represented 18% (n=273) of the study group (n=1,507). Non-Asian patients had greater clinical complexity; however, these differences were minimal after matching. There were no significant differences in ischemic outcomes between matched cohorts from 1 month to 2 years, including the primary composite endpoint of cardiac death or myocardial infarction (12% vs. 12%; P>0.99). However, there were significantly fewer Bleeding Academic Research Consortium types 3–5 bleeding events in the Asian vs. non-Asian cohort (4% vs. 9%; P=0.007), despite similar bleeding risk profiles after matching.
Conclusions: After propensity score matching, HBR patients from Asian countries undergoing PCI treated with 1-month DAPT had similar ischemic outcomes but fewer bleeding events between 1 month and 2 years compared with patients from non-Asian countries.
A persistent challenge after percutaneous coronary intervention (PCI) using drug-eluting stents (DES) in high bleeding risk (HBR) patients is to carefully balance ischemic and bleeding risks with the appropriate type and duration of antiplatelet therapy. Multiple recent trials assessing 1-month dual antiplatelet therapy (DAPT) following PCI have found that an abbreviated DAPT strategy maintains a low incidence of ischemic events in HBR patients while reducing the incidence of bleeding.1–3 Onyx ONE Clear, a follow-up, pre-specified analysis of the Onyx ONE US/Japan study and the Onyx ONE randomized control trial, specifically examined outcomes beginning 1 month after PCI in HBR patients on abbreviated DAPT.4 After implantation of the Resolute OnyxTM
zotarolimus-eluting stent (ZES), patients who were ‘clear’ of ischemic events in the first 30 days transitioned from DAPT to single antiplatelet therapy (SAPT) with or without an oral anticoagulant (OAC). The Onyx ONE Clear study met the prespecified performance goal, underscoring the safety and efficacy of an abbreviated DAPT strategy following ZES implantation during PCI.
The safety and efficacy of 1-month DAPT after ZES implantation, in particular HBR subgroups in Onyx ONE Clear, such as patients with diabetes, small coronary vessels, or patients with complex coronary lesions, has recently been demonstrated.5–7 Asian patients have distinct ischemic and bleeding profiles compared with non-Asian patients, characterized by a reduced ischemic risk but an increased bleeding risk after PCI.8–11 Bleeding events following stent implantation can be fatal, may occur post-discharge, and have an increased association with mortality beyond 30 days after the procedure.12,13 Thus, to appropriately balance ischemic and bleeding risks in Asian patients, a reduced DAPT strategy may be warranted. In this post-hoc subgroup analysis, we assess safety outcomes from 1 month to 2 years in Asian vs. non-Asian patients in the Onyx ONE Clear study.
Methods
Design
The Onyx ONE Clear was a prospective, multicenter, single arm, international study including HBR patients enrolled in either the Onyx ONE randomized control trial or the Onyx ONE US/Japan study and who received the Resolute Onyx ZES (ClinicalTrials.gov identifier NCT03647475).4 To be eligible, patients were required to adhere to 1-month DAPT and be ‘clear’ of adverse events through the first 30 days after PCI that would otherwise prohibit the discontinuation of DAPT at 1 month. Patients were enrolled if they met a least one of the HBR criteria (Supplementary Table 1). The Onyx ONE Clear study was conducted in accordance with the Declaration of Helsinki and was approved by the ethics committee or institutional review board at each enrollment center.
Procedures
As previously described, patients underwent PCI with implantation of the Resolute Onyx ZES.4 Following PCI, patients were mandated to adhere to 1-month DAPT, 75–100 mg aspirin and the standard daily dose of P2Y12
inhibitor (P2Y12i), followed by SAPT with or without OAC for the duration of the study. Patients already at the time of the procedure were permitted to continue OAC use along with SAPT for the duration of the study. The study only included those who were free of adverse events in the first month that would prevent discontinuation of DAPT, including myocardial infarction (MI; excluding periprocedural MI), repeat PCI or coronary artery bypass graft (CABG), stroke, definite/probable stent thrombosis (ST), or death. Patients receiving a stent other than the Onyx ZES were excluded from the study analysis. Clinical outcomes were assessed from 1-month post-procedure to 2 years.
Endpoints
The primary endpoint of the Onyx ONE Clear study was the composite incidence of cardiac death (CD) and MI from 1 month post-procedure to 1 year. Secondary endpoints were all-cause death, CD, MI, clinically driven target lesion revascularization (TLR), target lesion failure (TLF; composite of CD, target vessel MI, TLR), target vessel failure (TVF; CD, target vessel MI, or clinically driven repeat target vessel revascularization [TVR]), definite/probable ST, stroke, and bleeding. MI was defined according to the Third Universal Definition.14 Definite/probable ST was defined according to the Academic Research Consortium guidelines.15 Bleeding events were defined according to the Bleeding Academic Research Consortium (BARC) guidelines.16 Lesion success was defined as attainment of <30% residual stenosis and Thrombolysis in Myocardial Infarction (TIMI) 3 flow using any percutaneous method.17 Device success was based on lesion success with the assigned study device. Procedural success was based on lesion success and the absence of in-hospital major adverse cardiac events.
Statistical Analysis
Categorial data are reported as percentages (counts) and were compared between groups using Fisher’s exact test. Continuous data are reported as means±standard deviations and were compared between groups using the two sample t-test. The Kaplan-Meier method was used to generate time-to-first event curves. Propensity score 1 : 1 matching between groups was performed using a multivariable logistic regression model. The covariates included were the 13 HBR criteria (OAC use at discharge, age ≥75 years, hemoglobin <11 g/dL or transfusion within 4 weeks before procedure, prior intracerebral bleed, stroke in the past 12 months, hospital admission for major bleeding in the past 12 months, non-skin cancer diagnosed or treated within 3 years, nonsteroidal anti-inflammatory drug [NSAID; other than aspirin] or steroids ≥30 days after PCI, planned surgery in the next 12 months requiring interruption of DAPT, creatine clearance >40 mL/min, thrombocytopenia [platelet <105/mm3], severe chronic liver disease, and expected non-compliance to prolonged DAPT), as well as sex, body mass index (BMI), previous PCI or CABG, peripheral vascular disease, maximum lesion length, multivessel coronary artery disease, potent P2Y12i use at discharge, and intravascular imaging by intravascular ultrasound (IVUS) or optical coherence tomography (OCT). Patients were matched based on the closest possible value of propensity score (nearest neighbor matching). A logistic regression was performed to compare procedural success based on intracoronary imaging of Asian and non-Asian patients, with a P value <0.05 indicating a statistically significant interaction for procedural success between intracoronary imaging vs. angiography alone and region (Asian vs. non-Asian). Statistical analyses were performed using SAS (version 9.4; SAS Institute, Cary, NC, USA).
Results
Comparison of Asian and Non-Asian Patient Demographics and Procedural Characteristics
Among 1,507 patients in Onyx ONE Clear to 2 years, 273 (18%) patients were from Asian countries (Hong Kong, Japan, Republic of Korea, Malaysia, Singapore, Thailand), while 1,234 (82%) patients were from countries outside of Asia. Supplementary Table 1 shows the comparison of demographics between Asian and non-Asian patients. Notably, Asian patients were younger, less likely to be female, had a lower BMI, had a lower incidence of hypertension, hyperlipidemia and atrial fibrillation, and were less likely to have had previous PCI, CABG and stroke. In addition to these patient demographic differences, the HBR profiles were noticeably different between groups (Supplementary Table 2), including fewer Asian patients with planned OAC use to continue after PCI and age ≥75 years.
To account for differences in patient demographics (Supplementary Table 1) and HBR differences (Supplementary Table 2), Asian and non-Asian patients were matched 1 : 1 (261 patients from each group) based on propensity scores using 21 different covariates, including 13 HBR covariates (see Methods). Although matched patient groups had fewer differences in demographics, Asian patients were significantly less likely to have hyperlipidemia and chronic obstructive pulmonary disease, but more likely to have diabetes (Table 1). Between matched cohorts, Asian and non-Asian patients had a similar mean number of HBR inclusion criteria (1.5±0.7 vs 1.5±0.8; P=0.77), and differences between individual HBR criterion were similar between the two groups (Table 2).
Table 1.
Baseline Characteristics of Propensity Score Matched Asian and Non-Asian Patients
| |
Asian patients
(n=261) |
Non-Asian patients
(n=261) |
P value |
| Age (years) |
71.3±10.9 |
72.3±10.6 |
0.30 |
| Female |
36.4 (95) |
39.5 (103) |
0.53 |
| Body mass index (kg/m2) |
24.8±4.0 |
25.3±4.6 |
0.13 |
| Hypertension |
73.9 (193) |
81.2 (212) |
0.059 |
| Hyperlipidemia |
49.0 (128) |
71.6 (187) |
<0.001 |
| Previous MI |
23.0 (60) |
21.5 (56) |
0.75 |
| Previous PCI |
13.4 (35) |
14.9 (39) |
0.71 |
| Previous CABG |
1.5 (4) |
1.9 (5) |
>0.99 |
| Atrial fibrillation |
19.2 (50) |
24.1 (63) |
0.20 |
| Chronic obstructive pulmonary disease |
3.4 (9) |
12.3 (32) |
<0.001 |
| Diabetes |
43.7 (114) |
31.0 (81) |
<0.001 |
| Stroke/TIA |
8.0 (21) |
13.0 (34) |
0.086 |
| Peripheral vascular disease |
2.7 (7) |
3.8 (10) |
0.62 |
| Multivessel disease (≥2) |
55.2 (144) |
53.3 (139) |
0.73 |
| Left ventricular ejection fraction ≤35% |
11.6 (24) |
11.7 (21) |
>0.99 |
| Clinical presentation |
| Silent ischemia |
10.6 (26) |
8.9 (22) |
0.55 |
| Myocardial infarction |
27.2 (67) |
31.9 (79) |
0.28 |
| NSTEMI |
21.5 (53) |
27.0 (67) |
0.17 |
| STEMI |
5.7 (14) |
4.8 (12) |
0.69 |
Data are presented as % (n) or mean±SD. Covariates for propensity score matching include 13 high bleeding risk criteria plus sex, body mass index, previous percutaneous coronary intervention (PCI), coronary artery bypass graft (CABG), peripheral vascular disease, maximum lesion length, multivessel coronary artery disease, potent P2Y12i use at discharge, and intracoronary imaging. MI, myocardial infarction; NSTEMI, non-ST-elevated myocardial infarction; STEMI, ST-elevated myocardial infarction; TIA, transient ischemic attack.
Table 2.
HBR Criteria Among Propensity Score Matched Asian and Non-Asian Patients
| HBR inclusion criteria |
Asian patients
(n=261) |
Non-Asian patients
(n=261) |
P value |
| Mean number of HBR criteria |
1.5±0.7 |
1.5±0.8 |
0.77 |
| Age ≥75 years |
47.9 (125) |
51.7 (135) |
0.43 |
| Oral anticoagulation to continue after PCI |
24.5 (64) |
27.6 (72) |
0.49 |
| Hgb <11 g/dL (or transfusion within 4 weeks before procedure) |
22.2 (58) |
21.1 (55) |
0.83 |
| Creatinine clearance <40 mL/min |
21.5 (56) |
18.4 (48) |
0.44 |
| Non-skin cancer diagnosed or treated within 3 years |
10.0 (26) |
9.6 (25) |
>0.99 |
| Planned surgery in next 12 months requiring interruption of DAPT |
6.1 (16) |
6.5 (17) |
>0.99 |
| Expected non-compliance to prolonged DAPT |
6.1 (16) |
5.7 (15) |
>0.99 |
| Hospital admission for major bleeding in prior 12 months |
3.8 (10) |
4.6 (12) |
0.83 |
| Stroke in previous 12 months |
2.3 (6) |
2.3 (6) |
>0.99 |
| NSAID (other than aspirin) or steroids for ≥30 days after PCI |
1.1 (3) |
1.1 (3) |
>0.99 |
| Prior intracerebral bleed |
2.7 (7) |
1.5 (4) |
0.54 |
| Thrombocytopenia (PLT <100,000/mm3) |
1.9 (5) |
1.9 (5) |
>0.99 |
| Severe chronic liver disease |
0.4 (1) |
0.4 (1) |
>0.99 |
Data are presented as % (n) or mean±SD. DAPT, dual antiplatelet therapy; HBR, high bleeding risk; Hgb, hemoglobin; NSAID, nonsteroidal anti-inflammatory drug; PCI, percutaneous coronary intervention.
Procedural characteristics on matched cohorts are provided in Table 3. There were fewer lesions treated with correspondingly fewer stents implanted among Asian patients compared with non-Asian patients. Asian patients were more likely to have the left anterior descending vessel treated and less likely to have the left circumflex vessel treated than non-Asian patients. In matched cohorts, Asian patients were more likely to have longer lesions, chronic total occlusion, moderate to severe calcification, and modified American College of Cardiology/American Heart Association (ACC/AHA) Lesion Class B2/C compared with non-Asian patients. The preprocedure reference vessel diameter and percent stenosis were also significantly greater in Asian patients than in non-Asian patients. Asian patients were more likely to have undergone intracoronary imaging by IVUS or OCT than non-Asian patients (39.2% vs. 13.3%; P<0.001). However, among matched cohorts, 38.3% of Asian patients and 29.5% of non-Asian patients underwent intravascular imaging during PCI (Table 3; P=0.042). Among Asian patients who underwent intracoronary imaging during PCI (n=102), the procedural success rate was 85%. Among non-Asian patients who underwent intracoronary imaging during PCI (n=164), the procedural success rate was 92%. By comparison, among patients who underwent angiography alone during PCI, the procedural success rate in Asian patients (n=163) was 83%, whereas in non-Asian patients (n=1,041) it was 89%. The interaction between intracoronary imaging and procedural success based on region was not statistically significant (P=0.64).
Table 3.
Procedural and Lesion Characteristics Among Propensity Score Matched Asian and Non-Asian Patients
| |
Asian patients
(n=261; 334 lesions) |
Non-Asian patients
(n=261; 378 lesions) |
P value |
| Radial access |
30.9 (84) |
27.5 (76) |
0.40 |
| Staged procedure performed |
4.2 (11) |
5.7 (15) |
0.55 |
| Target vessel |
| LAD |
70.1 (183) |
55.9 (146) |
0.001 |
| LCX |
17.6 (46) |
33.3 (87) |
<0.001 |
| RCA |
34.5 (90) |
38.7 (101) |
0.36 |
| Left main |
0.4 (1) |
0.4 (1) |
>0.99 |
| Bypass graft |
0.4 (1) |
0.0 (0) |
>0.99 |
| In-stent restenosis |
2.5 (9) |
1.6 (7) |
0.45 |
| Bifurcation |
10.8 (39) |
12.0 (51) |
0.65 |
| Moderate or severe calcification |
61.9 (205) |
49.2 (184) |
<0.001 |
| Chronic total occlusion |
4.7 (17) |
0.7 (3) |
<0.001 |
| B2/C lesion class |
90.4 (302) |
81.2 (307) |
<0.001 |
| Reference vessel diameter (mm) |
2.87±0.43 |
2.78±0.48 |
0.010 |
| Percent stenosis (%) |
71.0±14.1 |
67.7±12.6 |
0.001 |
| Lesion length (mm) |
26.71±13.57 |
22.60±14.34 |
<0.001 |
| No. treated lesions per patient |
1.3±0.5 |
1.4±0.7 |
0.003 |
| No. stents per patient |
1.7±1.0 |
1.9±1.3 |
0.040 |
| Total stent length per lesion (mm) |
33.5±16.7 |
26.2±13.5 |
<0.001 |
| Total stent length per patient (mm) |
46.5±28.9 |
42.6±30.6 |
0.141 |
| Lesion success |
92.9 (302) |
95.5 (358) |
0.19 |
| Device success |
92.3 (300) |
93.9 (352) |
0.46 |
| Procedure success |
85.4 (216) |
88.0 (227) |
0.44 |
| Post-procedure hospital length of stay (days) |
2.3±2.8 |
2.1±4.1 |
0.41 |
Data presented as % (n) or mean±SD. Includes procedural data for index and staged procedures. LAD, left anterior descending; LCX, left circumflex; RCA, right coronary artery; RVD, reference vessel diameter.
Antiplatelet therapy and oral anticoagulation usage between matched patient cohorts from the index procedure to 2 years are presented in Figure 1. Onyx ONE Clear protocol mandated patients transition from DAPT at 1 month after procedure to SAPT. After 1 month, the majority of patients transitioned to SAPT only; however, compared with Asian patients, non-Asian patients were more likely to transition to SAPT plus an OAC. Furthermore, 1 month after the procedure, a greater proportion of Asian patients were prescribed aspirin as SAPT, whereas a greater proportion of non-Asian patients were prescribed P2Y12i (Supplementary Figure 1). However, beyond 2 months after the procedure, trends between matched patient cohorts diverged. Among the Asian cohort, the proportion of patients prescribed only aspirin or P2Y12i was maintained to 2 years, with a small increase in transition to DAPT, while at 1 year after the procedure, a small proportion transitioned to OAC only. In comparison, among non-Asian patients, while aspirin only use was more or less maintained to 2 years, the proportion of patients prescribed P2Y12i steadily decreased 2 months after the procedure. Some non-Asian patients similarly transitioned to OAC only use at 1 year. However, the most notable difference in antiplatelet therapy was DAPT usage. At discharge, including OAC use, 99% of Asian patients were on DAPT compared with 94% of non-Asian patients (P=0.001). This trend persisted at 1 month after the procedure (P=0.001). A sizeable proportion of non-Asian patients transitioned back onto DAPT for the duration of the follow-up to 2 years. After the protocol-mandated cessation of DAPT at 1 month, few Asian and non-Asian patients were on DAPT (3% vs. 2%; P=0.58). However, even between propensity matched cohorts, significantly fewer Asian patients were on DAPT compared with non-Asian patients at 6 months (4% vs. 10%; P=0.016), 1 year (4% vs. 13%; P=0.002), and 2 years (4% vs. 9%; P=0.034). Potential reasons for the discrepancy in DAPT use to 2 years between cohorts, and the consequences of higher DAPT use in non-Asian patients, is explored further in the Discussion.

Clinical Outcomes to 2 Years
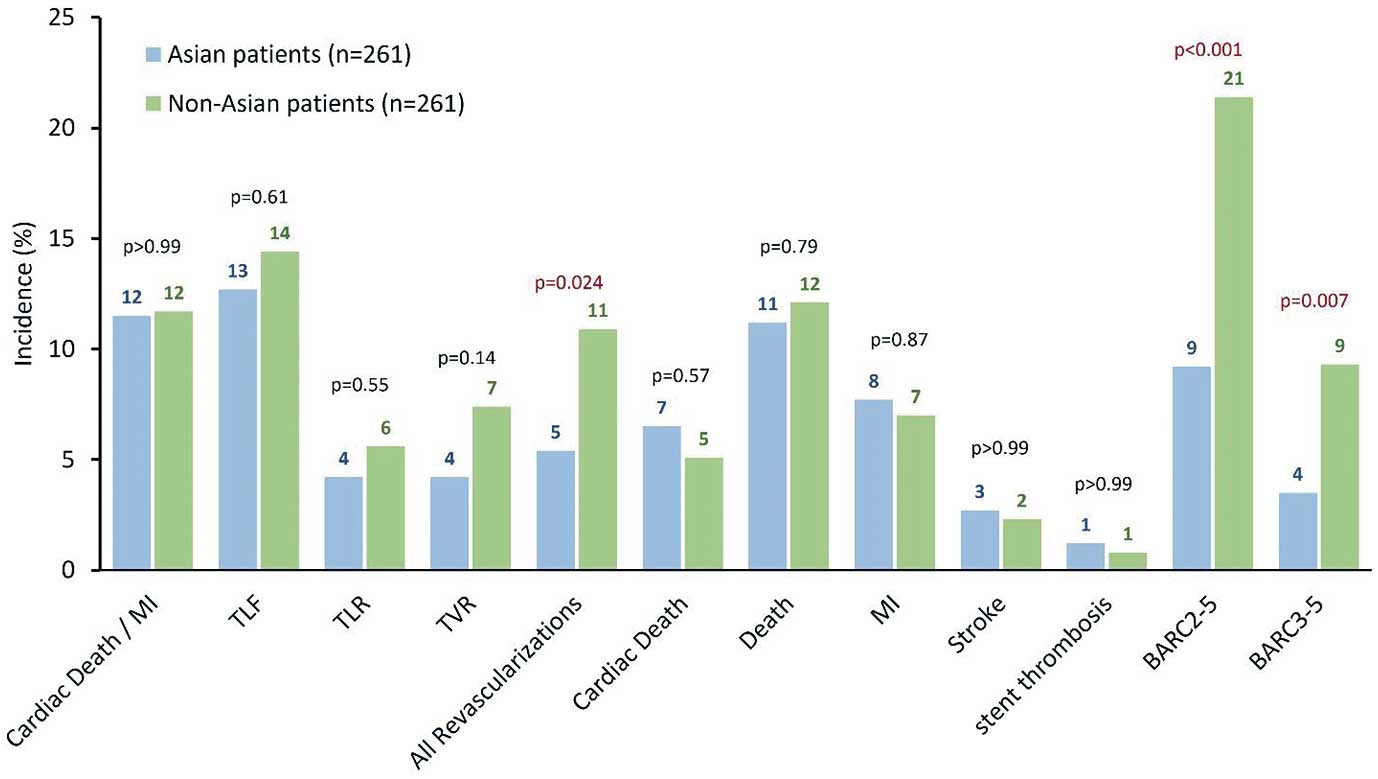
At 2 years, the primary safety endpoint of the composite incidence of CD and MI were comparable between propensity score matched Asian and non-Asian patients (Figure 2). Kaplan-Meier rate estimate of CD and MI between 1 month and 2 years was similar for Asian and non-Asian patients (Figure 3). Similarly, there were no significant differences in the incidence and Kaplan-Meier rate estimates between Asian and non-Asian patients for CD, TLF, clinically driven TLR, and non-clinically driven TLR (Figure 3).
Notably, the incidence of all revascularizations was significantly lower among Asian patients despite TLF and clinically driven TVR being not significantly different between groups (Figure 2). Interestingly, the incidence of bleeding (BARC types 2–5 and BARC types 3–5) was significantly lower among Asian patients compared with non-Asian patients (Figure 2), as well as by Kaplan-Meier rate estimate (Figure 3).
Discussion
Asian patients represented 18% of the total Onyx ONE Clear cohort of HBR patients. Asian patients had several baseline characteristic differences compared with non-Asian patients, including several important differences in HBR. Furthermore, one notable procedural difference between Asian and non-Asian cohorts was the higher use of IVUS or OCT during PCI in Asian patients vs. angiography-guided PCI alone. Thus, to compare outcomes from 1 month to 2 years between Asian and non-Asian cohorts, patients were matched 1 : 1 using propensity scores with 21 different covariates including the 13 HBR criteria and intracoronary imaging mentioned in the Methods. The incidence of ischemic events was generally low from 1 month to 2 years in both Asian and non-Asian patients. DAPT and OAC usage were lower among Asian patients, as was the incidence of coronary revascularization. Interestingly, and perhaps paradoxically, the incidence of bleeding events, including BARC types 3–5 events, was significantly lower in Asian patients compared with non-Asian patients.
Asian patients are commonly considered to be at higher risk for bleeding after PCI,8–11 and subsequent studies have confirmed this finding across East Asian geographies.18–20 High platelet reactivity has been postulated to be associated with cardiovascular events in Asian patients undergoing PCI.21 Reducing the dose of antiplatelet therapy has been found to reduce the risk of clinically serious bleeding events without compromising ischemic safety in Asian patients.22 However, an alternative strategy to appropriately balance ischemic and bleeding risks in HBR patients, including Asian patients, is to shorten the duration of DAPT after PCI before transitioning to SAPT.4
In the Onyx ONE Clear study, Asian patients had a significantly lower incidence of clinically serious bleeding events compared with non-Asian patients, even after propensity score matching. Moreover, Asian and non-Asian cohorts were similar in HBR criteria after propensity score matching. We hypothesize multiple factors contributed to this phenomena. First, the significantly higher overall revascularization rate in non-Asian patients and the higher prescription rate of DAPT likely underlies the higher rates of bleeding in that cohort. Additionally, the cumulative incidence of bleeding complications in HBR patients with or without any revascularization during the follow-up period also showed significantly higher bleeding events in those who received revascularization (Supplementary Figure 2). Revascularization correlates with the significantly greater use of DAPT in non-Asian patients, possibly increasing the incidence of BARC types 3–5 bleeding events. What explains the lower revascularization rate among Asian patients? One hypothesis is that Asians may have received more aggressive intervention at baseline, which led to lower rates of intervention during follow-up. From the results of the present study, we could see that the total stent length was longer at the index procedure in Asians. Also, during follow-up, clinically driven TLR was similar between Asian patients and non-Asian patients, while non-clinically driven TLR was the main factor for the overall higher revascularization rate in non-Asian patients. Second, Asian patients were prescribed with more ‘aspirin’ as SAPT, whereas non-Asian patients had more P2Y12
receptor antagonists (including potent P2Y12
inhibitors) as SAPT. This pattern may be associated with the current knowledge of the impact of CYP2C19 loss-of-function in East Asian patients.23 Third, the dose of antithrombotic medications could have been different between Asian and non-Asian populations, while Asian patients tend to use lower doses compared with non-Asian patients. This opens the possibility that the higher bleeding events in the matched non-Asian patients may be associated with the higher dose of antithrombotic medications in this population.
Study Limitations
Onyx ONE Clear was a single arm study in which all patients received 1-month DAPT. Thus, different DAPT durations were not assessed in this analysis. Patients only received the Resolute Onyx ZES, and results from this analysis may not be applicable to other drug-eluting stents. This post-hoc analysis was not powered to detect low frequency events, such as ST. Moreover, the present study was not designed to compare outcomes between Asian and non-Asian patients. As such, baseline and procedural characteristics were significantly different between unmatched Asian and non-Asian populations. Propensity score matching mitigates this limitation, in part; however, some characteristic differences remain between matched cohorts. Last, this post-hoc analysis compared results between patients in Asian vs. non-Asian countries; however, patient information on ethnicity and race could not be collected at certain sites. Thus, a few patients of Asian ethnicity may have been included in the non-Asian cohort and vice versa.
Conclusions
In Onyx ONE Clear, both Asian and non-Asian patients had relatively low incidences of ischemic and bleeding events, although Asian patients had a significantly lower incidence of bleeding events compared with non-Asian patients, possibly explained by a lower incidence of revascularization. Overall, however, these data support the safety and efficacy of the Resolute Onyx ZES followed by 1-month DAPT in HBR patients, including Asian HBR patients.
Clinical Perspectives
The ischemic and bleeding risks in patients after PCI with DES must be appropriately balanced. Asian patients present unique challenges, with greater bleeding risks but lower ischemic risks compared with non-Asian patients. Here, we evaluated outcomes between Asian and non-Asian patients from 1 month to 2 years using an abbreviated 1-month DAPT strategy after PCI. Both Asian and non-Asian patients were found to have low ischemic and bleeding risks in Onyx ONE Clear. This suggests abbreviated DAPT is an appropriate strategy post-PCI in Asian patients, who are more susceptible to bleeding events. Asian patients were found to have even lower bleeding risks than non-Asian patients in Onyx ONE Clear; however, this likely stems from differences in residual baseline and procedural characteristics and revascularization rates between propensity score matched patient groups.
Acknowledgments
Benjamin Woods, PhD, from Medtronic provided medical writing and editorial support.
Sources of Funding
The Onyx ONE Clear study was sponsored by Medtronic.
Disclosures
S.-H.H. receives research grants through Keimyung University Dongsan Hospital and from Terumo and Biotronik. He also receives consultation fees from Viatris.
C.C.T. is on the Medtronic Advisory Board.
D.E.K. reports institutional research/grant support from Biotronik, Boston Scientific, Cardiovascular Systems, Inc., Orbus Neich, Teleflex, Medtronic and Ablative Solutions, and personal consulting honoraria from Cardiovascular Systems, Inc., Medtronic, and Abbott Vascular.
A.J.K. reports institutional funding to Columbia University and/or the Cardiovascular Research Foundation from Medtronic, Boston Scientific, Abbott Vascular, Amgen, CSI, Siemens, Philips, ReCorMedical, Neurotronic, Biotronik. In addition to research grants, institutional funding includes fees paid to Columbia University and/or the Cardiovascular Research Foundation for consulting and/or speaking engagements in which A.J.K. controlled the content. He has also received consulting fees from IMDS, travel expenses/meals from Medtronic, Boston Scientific, Abbott Vascular, Abiomed, CSI, Siemens, Philips, ReCorMedical, Chiesi, OpSens, Zoll, and Regeneron.
A.L. reports being a consultant and/or on the advisory board of Medtronic, Abbott Vascular, and Boston Scientific.
T.-H.L. and S.-J.Y. are employees of Medtronic.
S.W. reports research, travel or educational grants to the institution without personal remuneration from Abbott, Abiomed, Amgen, Astra Zeneca, Bayer, Braun, Biotronik, Boehringer Ingelheim, Boston Scientific, Bristol Myers Squibb, Cardinal Health, CardioValve, Cordis Medical, Corflow Therapeutics, CSL Behring, Daiichi Sankyo, Edwards Lifesciences, Farapulse Inc. Fumedica, Guerbet, Idorsia, Inari Medical, InfraRedx, Janssen-Cilag, Johnson & Johnson, Medalliance, Medicure, Medtronic, Merck Sharp & Dohm, Miracor Medical, Novartis, Novo Nordisk, Organon, OrPha Suisse, Pharming Tech. Pfizer, Polares, Regeneron, Sanofi-Aventis, Servier, Sinomed, Terumo, Vifor, V-Wave. S.W. served as advisory board member and/or member of the steering/executive group of trials funded by Abbott, Abiomed, Amgen, Astra Zeneca, Bayer, Boston Scientific, Biotronik, Bristol Myers Squibb, Edwards Lifesciences, MedAlliance, Medtronic, Novartis, Polares, Recardio, Sinomed, Terumo, and V-Wave with payments to the institution but no personal payments. He is also member of the steering/executive committee group of several investigator-initiated trials that receive funding by industry without impact on his personal remuneration.
G.W.S. has received speaker honoraria from Medtronic, Pulnovo, Infraredx, Abiomed, Amgen, Boehringer Ingelheim, has served as a consultant to Abbott, Daiichi Sankyo, Ablative Solutions, CorFlow, Apollo Therapeutics, Cardiomech, Gore, Robocath, Miracor, Vectorious, Abiomed, Valfix, TherOx, HeartFlow, Neovasc, Ancora, Elucid Bio, Occlutech, Impulse Dynamics, Adona Medical, Millennia Biopharma, Oxitope, Cardiac Success, and HighLife, and has equity/options from Ancora, Cagent, Applied Therapeutics, Biostar family of funds, SpectraWave, Orchestra Biomed, Aria, Cardiac Success, Valfix, Xenter. G.W.S.’s employer, Mount Sinai Hospital, receives research grants from Abbott, Abiomed, Bioventrix, Cardiovascular Systems Inc, Phillips, Biosense-Webster, Shockwave, Vascular Dynamics, Pulnovo and V-wave. Family disclosure: G.W.S.’s daughter is an employee at IQVIA.
H.-S.K. received research grants through Seoul National University Hospital from Abbott, Medtronic, Biotronik, B Braun, and Daiichi Sankyo. He received fees for lecture and consultation from Edwards Lifesciences, Medtronic, Novartis, Pfizer, Sankyo, AmGen, Astrazeneca, and Beringer Ingelheim.
All other authors have no conflicts of interest to disclose.
IRB Information
The institutional review board of Seoul National University Hospital approved this study (Reference no. 1802-076-923).
Data Availability
Data from the Onyx ONE Clear study will not be shared.
Supplementary Files
Please find supplementary file(s);
https://doi.org/10.1253/circrep.CR-24-0037
References
- 1.
Urban P, Meredith IT, Abizaid A, Pocock SJ, Carrié D, Naber C, et al. Polymer-free drug-coated coronary stents in patients at high bleeding risk. N Engl J Med 2015; 373: 2038–2047.
- 2.
Windecker S, Latib A, Kedhi E, Kirtane AJ, Kandzari DE, Mehran R, et al. Polymer-based or polymer-free stents in patients at high bleeding risk. N Engl J Med 2020; 382: 1208–1218.
- 3.
Valgimigli M, Frigoli E, Heg D, Tijssen J, Jüni P, Vranckx P, et al. Dual antiplatelet therapy after PCI in patients at high bleeding risk. N Engl J Med 2021; 385: 1643–1655.
- 4.
Kandzari DE, Kirtane AJ, Windecker S, Latib A, Kedhi E, Mehran R, et al. One-month dual antiplatelet therapy following percutaneous coronary intervention with zotarolimus-eluting stents in high-bleeding-risk patients. Circ Cardiovasc Interv 2020; 13: e009565.
- 5.
Kandzari DE, Kirtane AJ, Mehran R, Price MJ, Simon DI, Latib A, et al. Clinical outcomes according to lesion complexity in high bleeding risk patients treated with 1-month dual antiplatelet therapy following PCI: Analysis from the Onyx ONE clear study. Catheter Cardiovasc Interv 2022; 99: 583–592.
- 6.
Moreno R, Kandzari DE, Kirtane AJ, Windecker S, Latib A, Kedhi E, et al. Coronary stenting in high bleeding risk patients with small coronary arteries followed by one-month dual antiplatelet therapy: Onyx ONE Clear. J Soc Cardiovasc Angiogr Interv 2022; 1: 100432.
- 7.
Kedhi E, Windecker S, Latib A, Kirtane AJ, Kandzari D, Mehran R, et al. Patients with diabetes at high bleeding risk with 1-month dual antiplatelet therapy: Onyx ONE Clear results. J Soc Cardiovasc Angiogr Interv 2022; 1: 100441.
- 8.
Levine GN, Jeong YH, Goto S, Anderson JL, Huo Y, Mega JL, et al. World Heart Federation expert consensus statement on antiplatelet therapy in East Asian patients with ACS or undergoing PCI. Nat Rev Cardiol 2014; 11: 597–606.
- 9.
Lee JH, Ahn SG, Park B, Park SW, Kang YS, Lee JW, et al. A pharmacodynamic study of the optimal P2Y12 inhibitor regimen for East Asian patients with acute coronary syndrome. Korean J Intern Med 2015; 30: 620–628.
- 10.
Jeong YH. “East Asian paradox”: Challenge for the current antiplatelet strategy of “one-guideline-fits-all races” in acute coronary syndrome. Curr Cardiol Rep 2014; 16: 485.
- 11.
Kang J, Park K, Palmerini T, Stone G, Lee M, Colombo A, et al. Racial differences in ischaemia/bleeding risk trade-off during anti-platelet therapy: Individual patient level landmark meta-analysis from seven RCTs. Thromb Haemost 2019; 119: 149–162.
- 12.
Marquis-Gravel G, Dalgaard F, Jones AD, Lokhnygina Y, James SK, Harrington RA, et al. Post-discharge bleeding and mortality following acute coronary syndromes with or without PCI. J Am Coll Cardiol 2020; 76: 162–171.
- 13.
Généreux P, Giustino G, Witzenbichler B, Weisz G, Stuckey TD, Rinaldi MJ, et al. Incidence, predictors, and impact of post-discharge bleeding after percutaneous coronary intervention. J Am Coll Cardiol 2015; 66: 1036–1045.
- 14.
Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD. Third universal definition of myocardial infarction. Circulation 2012; 126: 2020–2035.
- 15.
Cutlip DE, Windecker S, Mehran R, Boam A, Cohen DJ, van Es GA, et al. Clinical end points in coronary stent trials: A case for standardized definitions. Circulation 2007; 115: 2344–2351.
- 16.
Mehran R, Rao SV, Bhatt DL, Gibson CM, Caixeta A, Eikelboom J, et al. Standardized bleeding definitions for cardiovascular clinical trials: A consensus report from the Bleeding Academic Research Consortium. Circulation 2011; 123: 2736–2747.
- 17.
TIMI Study Group. The Thrombolysis in Myocardial Infarction (TIMI) trial: Phase I findings. N Engl J Med 1985; 312: 932–936.
- 18.
Nakamura M, Iijima R. Implications and characteristics of high bleeding risk in East Asian patients undergoing percutaneous coronary intervention: Start with what is right rather than what is acceptable. J Cardiol 2021; 78: 91–98.
- 19.
Nakamura M, Kadota K, Nakao K, Nakagawa Y, Shite J, Yokoi H, et al. High bleeding risk and clinical outcomes in East Asian patients undergoing percutaneous coronary intervention: The PENDULUM registry. EuroIntervention 2021; 16: 1154–1162.
- 20.
Ng AKY, Ng PY, Ip A, Ling IWH, Lam LT, Siu CW. Incidence, prediction, and outcomes of major bleeding after percutaneous coronary intervention in Chinese patients. JACC Asia 2022; 2: 341–350.
- 21.
Nakamura M, Kadota K, Takahashi A, Kanda J, Anzai H, Ishii Y, et al. Relationship between platelet reactivity and ischemic and bleeding events after percutaneous coronary intervention in East Asianp: 1-year results of the PENDULUM Registry. J Am Heart Assoc 2020; 9: e015439.
- 22.
Saito S, Isshiki T, Kimura T, Ogawa H, Yokoi H, Nanto S, et al. Efficacy and safety of adjusted-dose prasugrel compared with clopidogrel in Japanese patients with acute coronary syndrome: The PRASFIT-ACS Study. Circ J 2014; 78: 1684–1692.
- 23.
Sawayama Y, Tomita Y, Kohyama S, Higo Y, Kodama K, Asada K, et al. Clopidogrel use in CYP2C19 loss-of-function carriers with high bleeding risk after percutaneous coronary intervention. Circ J 2023; 87: 755–763.