2025 Volume 7 Issue 2 Pages 86-96
2025 Volume 7 Issue 2 Pages 86-96
Background: Constipation commonly coexists with heart failure (HF) and can increase blood pressure because of straining during defecation and accompanying mental stress. Daikenchuto, a Japanese herbal medicine to ameliorate gastrointestinal motility, may be effective as a complement to laxatives in improving outcomes in patients with HF and constipation.
Methods and Results: We used the Diagnosis Procedure Combination database to identify patients aged ≥65 years who were admitted for HF, had constipation, and were discharged alive between April 2016 and March 2022. We divided the 115,544 eligible patients into 2 groups according to the prescription of Daikenchuto in addition to laxatives at discharge and compared the incidence of 1-year HF readmission using 1 : 4 propensity score matching. Daikenchuto was prescribed at discharge in 3,315 (2.9%) patients. In the unmatched cohort, patients treated with Daikenchuto were more often male and had a higher prevalence of malignancy than those treated without Daikenchuto. In the 1 : 4 propensity score-matched cohort (3,311 and 13,243 patients with and without Daikenchuto, respectively), no significant difference was noted in 1-year HF readmission between the groups (22.2% vs. 21.9%; hazard ratio=1.02, 95% confidence interval=0.94–1.11). This result was consistent across clinically relevant subgroups except for renal disease.
Conclusions: Complementary use of Daikenchuto in combination with laxatives was not associated with a lower incidence of HF readmission in patients with HF and constipation.
In the context of the globally increased aging population, a “heart failure (HF) pandemic” has arisen, whereby the numbers of patients suffering from HF have been increasing annually.1–3 In Japan, the proportion of the older population aged ≥65 years reached 29% in the early 2020s, with >0.35 million new-onset HF cases every year.2 Therefore, it is essential to take measures to prevent repeated HF hospitalizations in patients with HF, because of the socioeconomic burden.4–7 Several novel medications, such as angiotensin-receptor–neprilysin inhibitors and sodium-glucose cotransporter 2 inhibitors, have been introduced into routine clinical practice over the past decade and have improved the prognosis of patients with HF.8,9 However, there is still unmet need in the complete management of HF, so the role of complementary and alternative medicine, such as traditional herbal medicines, has been attracting attention.10
Constipation is a common health issue in the older population,11 and is more prevalent in patients with HF than in those with other diseases,12 with an incidence of 20–30%.13,14 There are several possible reasons for this high prevalence in patients with HF, such as decreased intestinal peristalsis with mucosal edema, diuretic use with restricted body fluid intake, and limited physical activity.15 Furthermore, excretion can trigger acute decompensation of HF.16 Notably, constipation is a risk factor associated with incident HF readmission,13,14 presumably because constipation induces increased blood pressure variability owing to straining during defecation,15,17 as well as mental stress, resulting in increased sympathetic nervous activity.18 Because straining to defecate increases blood pressure, the Japanese Society of Hypertension Guidelines for the Management of Hypertension has recommended that guidance for the prevention of constipation should be given, and, if necessary, laxatives should be administered.19
Daikenchuto is a traditional Japanese herbal medicine composed of dried Japanese pepper, processed ginger, ginseng radix, and maltose powder and is commonly used in patients with HF in Japan,20 as well as in the general population.21 Although its exact mechanisms in the gastrointestinal system remain unknown, randomized trials have indicated that Daikenchuto is effective in improving gastrointestinal motility and constipation.22–24 Constipation may induce an imbalance in intestinal microbiota that leads to the progression of atherosclerosis.25 Research using experimental models suggests that Daikenchuto may improve the status of intestinal microbiota.26–28 Therefore, we hypothesized that Daikenchuto may decrease the risk of HF readmission through its effect on the gastrointestinal system among older patients with HF and constipation. The present study examined whether complementary use of Daikenchuto in addition to laxatives was associated with a lower risk of HF readmission in these patients.
This was a retrospective cohort analysis using the Diagnosis Procedure Combination (DPC) database managed by the DPC Study Group, one of the largest healthcare databases in Japan (Supplementary Methods).29,30 The diagnoses and procedures in the DPC database have been well validated with high accuracy.31,32 This study was approved by the Institutional Review Board of The University of Tokyo (approval number: 3501-[5]), and the requirement for informed consent from each patient was waived owing to the anonymized and de-identified nature of the data.
Study ParticipantsThe present study included patients aged ≥65 years who were admitted with the primary diagnosis of HF, had constipation, and were discharged alive between April 1, 2016 and March 31, 2022. The first hospitalization for HF during the study period was used as the index hospitalization. Constipation at discharge was defined by the prescription of laxatives (magnesium oxide, sennoside, picosulfate, bisacodyl, lubiprostone, linaclotide, elobixibat, polyethylene glycol, lactulose, and dioctyl sodium sulfosuccinate33), similar to the definition used in previous studies.13,34 The exclusion criteria were set with reference to an ongoing randomized trial of Kampo medication for HF:35 (1) receipt of coronary artery bypass grafting, valve surgery/intervention (e.g., surgical or transcatheter valve replacement or repair), or cardiac resynchronization therapy within 90 days prior to or during index hospitalization; (2) ventricular assist device recipient; (3) heart transplantation recipient; (4) congenital heart/vascular disease; (5) acute coronary syndrome; (6) end-stage renal failure; (7) receipt of mechanical circulatory support during index hospitalization (Supplementary Table 1); (8) receipt of palliative care within 90 days prior to or during index hospitalization; and (9) prescription of Mashiningan (a Kampo medicine with possible laxative effects) at discharge. The present study included patients with coronary artery disease or bradyarrhythmia (without acute coronary syndrome) who underwent percutaneous coronary intervention or permanent pacemaker implantation without cardiac resynchronization function.
ExposureThe exposure of interest was the prescription of Daikenchuto at discharge. We divided the eligible patients with HF and constipation into 2 groups: received Daikenchuto in addition to laxatives (Daikenchuto group) or received laxatives alone (no-Daikenchuto group).
Patients’ CharacteristicsWe identified 70 variables for covariates as potential confounders from the DPC database, based on current guidelines,8,36,37 previous administrative claims database studies,38,39 and clinical relevance in the field of HF (details in Supplementary Methods): age, sex, ambulance use for admission, body mass index, cognitive function, systolic blood pressure at admission, cardiac diseases, Charlson Comorbidity Index, specific comorbidities (Supplementary Table 1), in-hospital management for HF, laxatives and HF/other medications at discharge, discharge status (Supplementary Table 2), hospital characteristics, and fiscal year of discharge. In the Daikenchuto group, the exact discharge dose was unavailable in the database, but the approximate daily dose was calculated by dividing the total Daikenchuto dose by the total days of use during hospitalization, serving as a proxy for the daily dose after discharge.
End PointsThe primary end point was HF readmission within 1 year after discharge from the index HF hospitalization. The major secondary end point was a composite of HF readmission and death during any-cause readmission within 1 year after discharge. Other secondary end points were all-cause death during any-cause readmission, hospitalization for dehydration, and hospitalization for acute kidney injury (AKI). The end points of dehydration and AKI were examined because these events may occur more frequently as adverse events resulting from diarrhea, possibly caused by Daikenchuto in combination with laxatives. In the DPC database, we were able to detect readmissions to the index hospital (where a patient had been initially admitted for HF) and deaths occurring during the readmission using a unique identifier assigned to each patient at each hospital. However, we were unable to identify readmissions if a patient was readmitted to a different hospital. The follow-up period for each patient was until 1 year after discharge, death during any-cause readmission, or the last date of follow-up, whichever came first (Supplementary Methods).
Persistence of Constipation and Continued Use of DaikenchutoData on post-discharge constipation or medication continuation were unavailable. Instead, we extracted data on in-hospital medications during readmission for HF. In patients readmitted for HF, the use of laxatives during readmission was regarded as a proxy for persistent constipation. In the Daikenchuto group, the use of Daikenchuto during readmission was regarded as a proxy for its continued use.
Statistical AnalysisCategorical variables are presented as numbers and percentages. Continuous variables are presented as mean and standard deviation (SD) or median and interquartile range (IQR). Kaplan-Meier curves are presented to describe the cumulative incidence of end points with the log-rank test. We set a two-sided significance level of 0.05 and conducted all statistical analyses using Stata, version 18 (StataCorp, College Station, TX, USA).
Propensity Score Matching We conducted propensity score matching at a variable ratio (maximum 1 : 4) to balance patient characteristics between the Daikenchuto and no-Daikenchuto groups.40 We estimated propensity scores in a multivariable logistic regression model including the 70 variables as covariates (Table 1). Each patient in the Daikenchuto group was matched with a maximum of 4 patients in the no-Daikenchuto group, with replacement using the nearest neighbor method with a caliper width set at ≤0.2 of the pooled SD of logits of the propensity scores. This variable ratio-matching method is recommended when applicable in the statistical literature because it allows for higher precision (i.e., narrower confidence intervals [CIs]) than 1 : 1 matching, at the cost of a slight increase in bias.41,42 The covariate balance between the groups before and after propensity score matching was assessed using standardized mean difference, of which the absolute value <0.1 indicated a negligible difference between 2 groups.43 In the propensity score-matched cohort, a Cox proportional hazard model was applied to estimate the hazard ratios (HRs) and 95% CIs of complementary Daikenchuto use for the end points. Moreover, a Fine-Gray subdistribution hazard model was applied to account for a competing risk of death against HF readmission and other end points and estimate subdistribution HRs and 95% CIs.44
Characteristics of Patients With and Without Complementary Daikenchuto Use for Constipation
| Unmatched cohort | 1 : 4 propensity score-matched cohort | |||||
|---|---|---|---|---|---|---|
| Daikenchuto (n=3,315) |
No-Daikenchuto (n=112,229) |
SMD | Daikenchuto (n=3,311) |
No-Daikenchuto (n=13,243) |
SMD | |
| Age, years, mean (SD) | 84.2 (7.3) | 84.3 (7.6) | −0.011 | 84.2 (7.3) | 84.2 (7.5) | 0.002 |
| Male | 1,693 (51.1) | 50,776 (45.2) | 0.117 | 1,689 (51.0) | 6,779 (51.2) | −0.004 |
| Ambulance use for admission | 1,174 (35.4) | 40,930 (36.5) | −0.022 | 1,173 (35.4) | 4,796 (36.2) | −0.016 |
| BMI, kg/m2 | ||||||
| <18.5 | 609 (18.4) | 17,545 (15.6) | 0.073 | 608 (18.4) | 2,440 (18.4) | −0.002 |
| 18.5–24.9 | 1,819 (54.9) | 61,207 (54.5) | 0.007 | 1,817 (54.9) | 7,300 (55.1) | −0.005 |
| 25.0–29.9 | 532 (16.0) | 19,705 (17.6) | −0.040 | 532 (16.1) | 2,138 (16.1) | −0.002 |
| ≥30 | 125 (3.8) | 5,257 (4.7) | −0.045 | 124 (3.7) | 488 (3.7) | 0.003 |
| Missing data | 230 (6.9) | 8,515 (7.6) | −0.025 | 230 (6.9) | 877 (6.6) | 0.013 |
| Cognitive function | ||||||
| Normal | 2,044 (61.7) | 68,362 (60.9) | 0.015 | 2,040 (61.6) | 8,163 (61.6) | −0.001 |
| Mild dysfunction | 851 (25.7) | 28,365 (25.3) | 0.009 | 851 (25.7) | 3,498 (26.4) | −0.016 |
| Moderate/severe dysfunction | 420 (12.7) | 15,502 (13.8) | −0.034 | 420 (12.7) | 1,582 (11.9) | 0.022 |
| Systolic BP at admission, mmHg | ||||||
| >140 | 958 (28.9) | 35,095 (31.3) | −0.052 | 958 (28.9) | 3,899 (29.4) | −0.011 |
| 100–140 | 1,506 (45.4) | 48,367 (43.1) | 0.047 | 1,503 (45.4) | 5,943 (44.9) | 0.010 |
| <100 | 270 (8.1) | 8,677 (7.7) | 0.015 | 270 (8.2) | 1,132 (8.5) | −0.014 |
| Missing data | 581 (17.5) | 20,090 (17.9) | −0.010 | 580 (17.5) | 2,269 (17.1) | 0.010 |
| Cardiac disease | ||||||
| Atrial fibrillation | 1,314 (39.6) | 45,449 (40.5) | −0.018 | 1,313 (39.7) | 5,279 (39.9) | −0.004 |
| CAD | 905 (27.3) | 32,914 (29.3) | −0.045 | 904 (27.3) | 3,520 (26.6) | 0.016 |
| DCM | 49 (1.5) | 1,710 (1.5) | −0.004 | 49 (1.5) | 206 (1.6) | −0.006 |
| MR | 171 (5.2) | 6,049 (5.4) | −0.010 | 170 (5.1) | 711 (5.4) | −0.011 |
| AS | 158 (4.8) | 5,977 (5.3) | −0.026 | 158 (4.8) | 636 (4.8) | −0.001 |
| AR | 107 (3.2) | 2,850 (2.5) | 0.041 | 106 (3.2) | 422 (3.2) | 0.001 |
| Complete AV block | 50 (1.5) | 1,707 (1.5) | −0.001 | 50 (1.5) | 184 (1.4) | 0.010 |
| VT | 49 (1.5) | 2,250 (2.0) | −0.040 | 49 (1.5) | 193 (1.5) | 0.002 |
| Comorbidity | ||||||
| Charlson Comorbidity Index | ||||||
| 1 | 964 (29.1) | 34,234 (30.5) | −0.031 | 963 (29.1) | 3,956 (29.9) | −0.017 |
| 2 | 902 (27.2) | 32,101 (28.6) | −0.031 | 901 (27.2) | 3,556 (26.9) | 0.008 |
| 3 | 713 (21.5) | 23,651 (21.1) | 0.011 | 712 (21.5) | 2,817 (21.3) | 0.006 |
| ≥4 | 736 (22.2) | 22,243 (19.8) | 0.059 | 735 (22.2) | 2,914 (22.0) | 0.005 |
| Hypertension | 2,007 (60.5) | 70,131 (62.5) | −0.040 | 2,006 (60.6) | 8,015 (60.5) | 0.001 |
| Diabetes | 916 (27.6) | 31,556 (28.1) | −0.011 | 915 (27.6) | 3,758 (28.4) | −0.017 |
| Dyslipidemia | 871 (26.3) | 31,635 (28.2) | −0.043 | 870 (26.3) | 3,454 (26.1) | 0.004 |
| Prior stroke | 257 (7.8) | 9,043 (8.1) | −0.011 | 257 (7.8) | 1,042 (7.9) | −0.004 |
| Renal disease | 532 (16.0) | 17,034 (15.2) | 0.024 | 532 (16.1) | 2,026 (15.3) | 0.021 |
| Liver disease | 84 (2.5) | 3,434 (3.1) | −0.032 | 84 (2.5) | 303 (2.3) | 0.016 |
| Chronic pulmonary disease | 397 (12.0) | 12,662 (11.3) | 0.022 | 397 (12.0) | 1,592 (12.0) | −0.001 |
| Malignancy | 376 (11.3) | 8,327 (7.4) | 0.135 | 374 (11.3) | 1,456 (11.0) | 0.010 |
| Anemia | 643 (19.4) | 18,900 (16.8) | 0.066 | 643 (19.4) | 2,519 (19.0) | 0.010 |
| In-hospital management | ||||||
| ICU admission | 199 (6.0) | 5,849 (5.2) | 0.034 | 199 (6.0) | 829 (6.3) | −0.010 |
| HCU admission | 285 (8.6) | 9,533 (8.5) | 0.004 | 285 (8.6) | 1,152 (8.7) | −0.003 |
| Dobutamine | 312 (9.4) | 9,830 (8.8) | 0.023 | 312 (9.4) | 1,257 (9.5) | −0.002 |
| Dopamine | 152 (4.6) | 4,710 (4.2) | 0.019 | 152 (4.6) | 592 (4.5) | 0.006 |
| Noradrenaline | 115 (3.5) | 2,784 (2.5) | 0.058 | 115 (3.5) | 452 (3.4) | 0.003 |
| PDE III inhibitor | 23 (0.7) | 824 (0.7) | −0.005 | 23 (0.7) | 90 (0.7) | 0.002 |
| Carperitide | 732 (22.1) | 26,406 (23.5) | −0.034 | 732 (22.1) | 2,858 (21.6) | 0.013 |
| Intravenous nitrate | 795 (24.0) | 28,147 (25.1) | −0.026 | 793 (24.0) | 3,215 (24.3) | −0.008 |
| PCI | 75 (2.3) | 3,154 (2.8) | −0.035 | 75 (2.3) | 312 (2.4) | −0.006 |
| PPM/ICD implantation | 41 (1.2) | 1,484 (1.3) | −0.008 | 41 (1.2) | 163 (1.2) | 0.001 |
| Red cell transfusion | 320 (9.7) | 8,812 (7.9) | 0.064 | 320 (9.7) | 1,266 (9.6) | 0.004 |
| Cardiac rehabilitation | 1,691 (51.0) | 58,052 (51.7) | −0.014 | 1,689 (51.0) | 6,831 (51.6) | −0.011 |
| Concomitant laxatives at discharge | ||||||
| Magnesium oxide | 2,151 (64.9) | 71,119 (63.4) | 0.032 | 2,149 (64.9) | 8,651 (65.3) | −0.009 |
| Sennoside | 1,331 (40.2) | 42,024 (37.4) | 0.056 | 1,328 (40.1) | 5,347 (40.4) | −0.005 |
| Lubiprostone | 582 (17.6) | 11,331 (10.1) | 0.217 | 579 (17.5) | 2,260 (17.1) | 0.011 |
| Picosulfate | 250 (7.5) | 4,205 (3.7) | 0.165 | 247 (7.5) | 981 (7.4) | 0.002 |
| Lactulose | 77 (2.3) | 1,630 (1.5) | 0.064 | 77 (2.3) | 282 (2.1) | 0.013 |
| Linaclotide | 89 (2.7) | 1,622 (1.4) | 0.087 | 87 (2.6) | 394 (3.0) | −0.021 |
| Elobixibat | 95 (2.9) | 1,416 (1.3) | 0.113 | 92 (2.8) | 343 (2.6) | 0.012 |
| Bisacodyl | 37 (1.1) | 1,061 (0.9) | 0.017 | 37 (1.1) | 151 (1.1) | −0.002 |
| PEG | 40 (1.2) | 593 (0.5) | 0.073 | 38 (1.1) | 151 (1.1) | 0.001 |
| DSS | 12 (0.4) | 494 (0.4) | −0.012 | 12 (0.4) | 51 (0.4) | −0.004 |
| HF and other medications at discharge | ||||||
| ACE-I/ARB | 1,475 (44.5) | 53,392 (47.6) | −0.062 | 1,474 (44.5) | 5,927 (44.8) | −0.005 |
| ARNI | 50 (1.5) | 1,933 (1.7) | −0.017 | 50 (1.5) | 197 (1.5) | 0.002 |
| MRA | 1,300 (39.2) | 44,558 (39.7) | −0.010 | 1,296 (39.1) | 5,124 (38.7) | 0.009 |
| β-blocker | 1,592 (48.0) | 56,467 (50.3) | −0.046 | 1,589 (48.0) | 6,374 (48.1) | −0.003 |
| SGLT2 inhibitor | 203 (6.1) | 6,863 (6.1) | 0.000 | 202 (6.1) | 795 (6.0) | 0.004 |
| Loop diuretic | 2,716 (81.9) | 92,288 (82.2) | −0.008 | 2,712 (81.9) | 10,795 (81.5) | 0.010 |
| Thiazide | 185 (5.6) | 5,690 (5.1) | 0.023 | 185 (5.6) | 706 (5.3) | 0.011 |
| Tolvaptan | 882 (26.6) | 26,869 (23.9) | 0.061 | 881 (26.6) | 3,417 (25.8) | 0.018 |
| Digitalis | 132 (4.0) | 5,168 (4.6) | −0.031 | 132 (4.0) | 499 (3.8) | 0.011 |
| Ivabradine | 2 (0.1) | 118 (0.1) | −0.016 | 2 (0.1) | 8 (0.1) | 0.000 |
| Amiodarone | 187 (5.6) | 5,678 (5.1) | 0.026 | 186 (5.6) | 763 (5.8) | −0.006 |
| Calcium-channel blocker | 1,232 (37.2) | 41,018 (36.5) | 0.013 | 1,232 (37.2) | 4,960 (37.5) | −0.005 |
| DOAC | 1,087 (32.8) | 36,048 (32.1) | 0.014 | 1,085 (32.8) | 4,414 (33.3) | −0.012 |
| Warfarin | 497 (15.0) | 17,134 (15.3) | −0.008 | 497 (15.0) | 1,988 (15.0) | 0.000 |
| Aspirin | 754 (22.7) | 27,056 (24.1) | −0.032 | 752 (22.7) | 2,998 (22.6) | 0.002 |
| P2Y12 inhibitor | 416 (12.5) | 13,659 (12.2) | 0.011 | 415 (12.5) | 1,657 (12.5) | 0.001 |
| Statin | 907 (27.4) | 33,184 (29.6) | −0.049 | 904 (27.3) | 3,591 (27.1) | 0.004 |
| Discharge status | ||||||
| ADL at discharge | ||||||
| Independence | 1,198 (36.1) | 41,851 (37.3) | −0.024 | 1,197 (36.2) | 4,764 (36.0) | 0.004 |
| Partial dependence | 1,311 (39.5) | 42,460 (37.8) | 0.035 | 1,309 (39.5) | 5,195 (39.2) | 0.006 |
| Total dependence | 427 (12.9) | 15,132 (13.5) | −0.018 | 427 (12.9) | 1,760 (13.3) | −0.012 |
| Missing data | 379 (11.4) | 12,786 (11.4) | 0.001 | 378 (11.4) | 1,524 (11.5) | −0.003 |
| Length of hospital stay, days, median (IQR) |
21.0 (14.0–34.0) | 20.0 (14.0–31.0) | 0.057 | 21.0 (14.0–34.0) | 21.0 (14.0–32.0) | 0.008 |
| Discharge home | 2,615 (78.9) | 89,904 (80.1) | −0.030 | 2,611 (78.9) | 10,388 (78.4) | 0.010 |
| Home medical care after discharge | ||||||
| No | 2,808 (84.7) | 96,434 (85.9) | −0.034 | 2,804 (84.7) | 11,246 (84.9) | −0.006 |
| Yes | 471 (14.2) | 14,693 (13.1) | 0.033 | 471 (14.2) | 1,852 (14.0) | 0.007 |
| Missing data | 36 (1.1) | 1,102 (1.0) | 0.010 | 36 (1.1) | 145 (1.1) | −0.001 |
| Hospital characteristics | ||||||
| University hospital | 328 (9.9) | 7,689 (6.9) | 0.110 | 324 (9.8) | 1,303 (9.8) | −0.002 |
| Annualized hospital volume of eligible older patients with HF and constipation (cases/year) | ||||||
| Low (≤17) | 1,039 (31.3) | 36,727 (32.7) | −0.030 | 1,039 (31.4) | 4,097 (30.9) | 0.010 |
| Intermediate (18–28) | 1,164 (35.1) | 37,387 (33.3) | 0.038 | 1,162 (35.1) | 4,646 (35.1) | 0.000 |
| High (≥29) | 1,112 (33.5) | 38,115 (34.0) | −0.009 | 1,110 (33.5) | 4,500 (34.0) | −0.010 |
| Fiscal year | ||||||
| 2016 | 637 (19.2) | 23,236 (20.7) | −0.037 | 637 (19.2) | 2,466 (18.6) | 0.016 |
| 2017 | 604 (18.2) | 19,730 (17.6) | 0.017 | 604 (18.2) | 2,436 (18.4) | −0.004 |
| 2018 | 613 (18.5) | 18,647 (16.6) | 0.049 | 613 (18.5) | 2,444 (18.5) | 0.002 |
| 2019 | 449 (13.5) | 16,573 (14.8) | −0.035 | 447 (13.5) | 1,806 (13.6) | −0.004 |
| 2020 | 509 (15.4) | 17,746 (15.8) | −0.013 | 508 (15.3) | 2,061 (15.6) | −0.006 |
| 2021 | 503 (15.2) | 16,297 (14.5) | 0.018 | 502 (15.2) | 2,030 (15.3) | −0.005 |
Data are presented as n (%) unless indicated otherwise. ACE-I, angiotensin-converting enzyme inhibitor; ADL, activities of daily living; AR, aortic regurgitation; ARB, angiotensin-receptor blocker; ARNI, angiotensin-receptor-neprilysin inhibitor; AS, aortic stenosis; AV, atrioventricular; BMI, body mass index; BP, blood pressure; CAD, coronary artery disease; DCM, dilated cardiomyopathy; DOAC, direct oral anticoagulant; DSS, dioctyl sodium sulfosuccinate; HCU, high care unit; ICD, implantable cardioverter defibrillator; ICU, intensive care unit; IQR, interquartile range; MR, mitral regurgitation; MRA, mineralocorticoid receptor antagonist; PCI, percutaneous coronary intervention; PDE, phosphodiesterase; PEG, polyethylene glycol; PPM, permanent pacemaker; SD, standard deviation; SGLT2, sodium-glucose cotransporter 2; SMD, standardized mean difference; VT, ventricular tachycardia.
Subgroup Analysis Effect modifications were examined in the following clinically relevant subgroups of the propensity score-matched cohort: age, sex, systolic blood pressure at admission, atrial fibrillation, hypertension, and renal disease.
Sensitivity Analyses Three sensitivity analyses were performed. First, falsification end points were used to assess potential residual confounding. We selected hospitalizations for hip fracture and those for gastrointestinal bleeding (Supplementary Table 1), which are unlikely to be affected by Daikenchuto use and thus can serve as negative control events in the absence of residual confounding.
Second, E-values were calculated for the association between Daikenchuto use and 1-year HF readmission in the propensity score-matched cohort (Supplementary Methods).45 Although we performed propensity score matching to eliminate confounding bias from the measured factors, the analysis did not adjust for unmeasured confounding, which may have biased the estimates towards the null association in our main analysis for the primary end point in the propensity score-matched cohort. In other words, such unmeasured confounding may have masked the association between Daikenchuto use and a lower incidence of 1-year HF readmission. An E-value is defined as the minimum strength of association that an unmeasured confounder must have with both a treatment and end point to fully explain the association between the treatment and end point, conditional on the measured covariates.46,47
Lastly, a complete case analysis was conducted in patients without missing data to confirm the robustness of the results from our main analysis, where the category of “missing” was used for missing data in the following categorical variables: body mass index, systolic blood pressure at admission, activities of daily living (ADL) at discharge, and home medical care after discharge.
A total of 115,544 eligible patients (mean age 84.3 [SD 7.6] years; 45.4% male) were admitted for HF, had constipation, and were discharged alive (Figure 1). Among them, 3,315 (2.9%) patients received Daikenchuto in addition to laxatives (Daikenchuto group) and 112,229 (97.1%) received laxatives alone at discharge (no-Daikenchuto group). In 3,315 patients in the Daikenchuto group, the median dose of Daikenchuto during hospitalization was 7.50 (IQR 7.50–8.75) g/day. Patients in the Daikenchuto group were more often male (51.1% vs. 45.2%), had a higher prevalence of malignancy (11.3% vs. 7.4%), and were more likely to be treated at a university hospital (9.9% vs. 6.9%) than those in the no-Daikenchuto group (Table 1). There were no significant differences between the groups in terms of age (mean, 84.2 [SD 7.3] vs. 84.3 [SD 7.6] years), body mass index, cognitive dysfunction, systolic blood pressure at admission, and cardiac and non-cardiac comorbidities.

Flowchart of patient selection. CABG, coronary artery bypass grafting; CRT, cardiac resynchronization therapy; HF, heart failure; VAD, ventricular assist device.
In-hospital management for HF, such as the use of catecholamines, vasodilators, and invasive procedures, did not differ between the groups. Regarding the concomitant use of laxatives at discharge, patients in the Daikenchuto group received lubiprostone (17.6% vs. 10.1%), picosulfate (7.5% vs. 3.7%), and elobixibat (2.9% vs. 1.3%) more frequently than those in the no-Daikenchuto group. No significant differences were observed in HF and other medications at discharge between the groups. Moreover, discharge status, such as ADL and home medical care after discharge, did not differ between the groups.
The propensity score matching created a cohort of 3,311 and 13,243 patients with and without Daikenchuto, respectively, where the patient characteristics were well-balanced between the two groups (Table 1).
End PointsIn the propensity score-matched cohort, there were no significant differences between the Daikenchuto and no-Daikenchuto groups in terms of HF readmission (22.2% vs. 21.9%; HR=1.02, 95% CI=0.94–1.11) or the composite end point (25.5% vs. 25.1%; HR=1.02, 95% CI=0.95–1.10) (Figure 2, Table 2). Consistent results were observed in the Fine-Gray model accounting for competing risk of death. Daikenchuto use was significantly associated with a slightly higher incidence of hospitalization for dehydration, with no significant association with the other secondary end points.
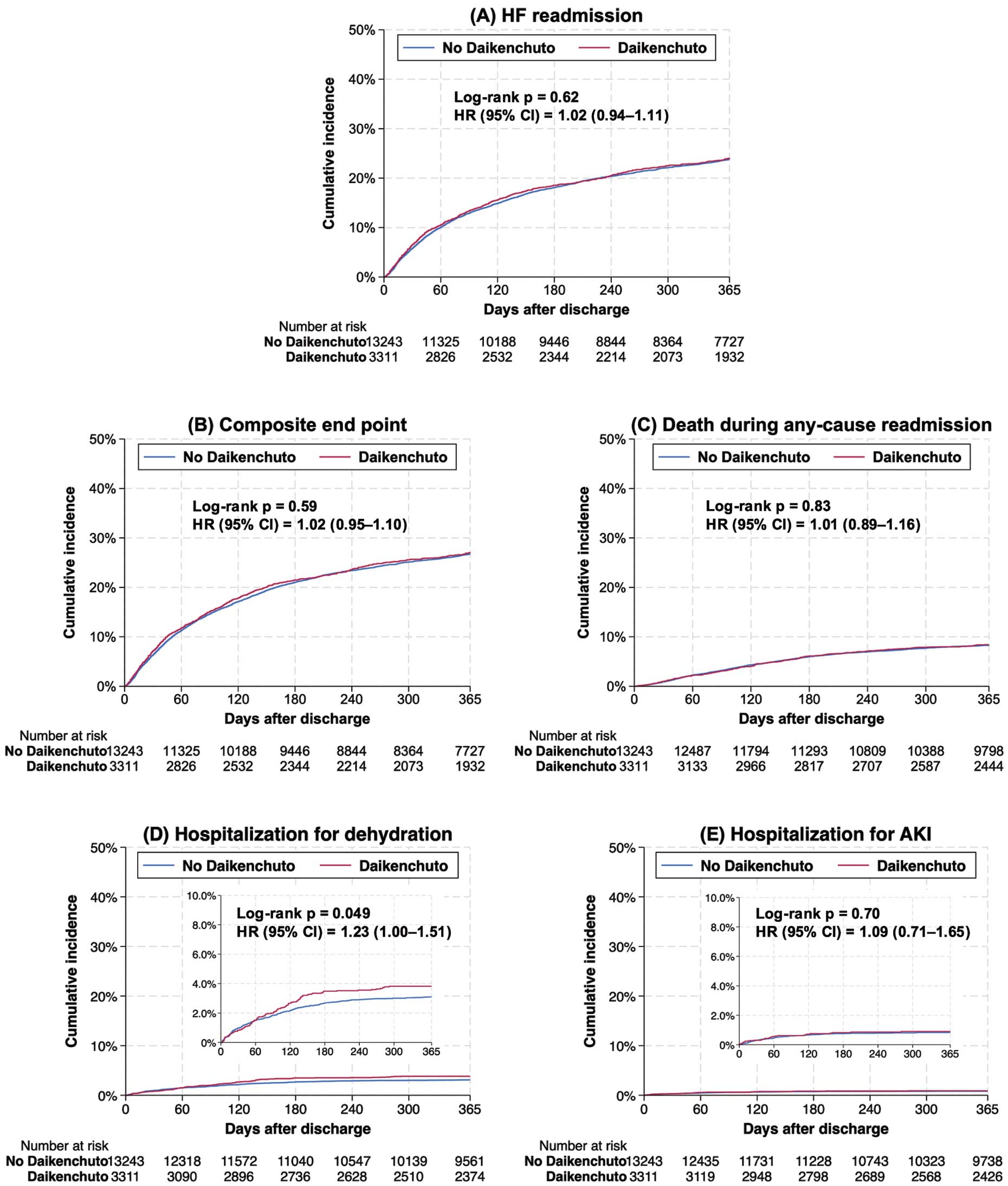
Primary and secondary end points between the Daikenchuto and no-Daikenchuto groups in the propensity score-matched cohort of older patients with heart failure (HF) and constipation. (A) HF readmission, (B) composite end point of HF readmission and death during any-cause readmission, (C) death during any-cause readmission, (D) hospitalization for dehydration, and (E) hospitalization for acute kidney injury (AKI). CI, confidence interval; HR, hazard ratio.
1-Year Outcomes in Patients With and Without Complementary Daikenchuto Use for Constipation
| Unmatched cohort | 1 : 4 propensity score- matched cohort |
Cox model | Fine–Gray model | |||||
|---|---|---|---|---|---|---|---|---|
| Daikenchuto (n=3,315) |
No- Daikenchuto (n=112,229) |
Daikenchuto (n=3,311) |
No- Daikenchuto (n=13,243) |
HR (95 CI) |
P value | sHR (95 CI) |
P value | |
| Primary end point | ||||||||
| HF readmission | 736 (22.2) | 25,158 (22.4) | 736 (22.2) | 2,895 (21.9) | 1.02 (0.94–1.11) |
0.62 | 1.02 (0.94–1.11) |
0.64 |
| Major secondary end point | ||||||||
| Composite end point |
845 (25.5) | 28,473 (25.4) | 845 (25.5) | 3,323 (25.1) | 1.02 (0.95–1.10) |
0.59 | NA | |
| Other secondary end points | ||||||||
| Death during any- cause readmission |
259 (7.8) | 8,458 (7.5) | 259 (7.8) | 1,019 (7.7) | 1.01 (0.89–1.16) |
0.83 | NA | |
| Hospitalization for dehydration |
117 (3.5) | 3,031 (2.7) | 117 (3.5) | 380 (2.9) | 1.23 (1.00–1.51) |
0.049 | 1.23 (1.00–1.51) |
0.049 |
| Hospitalization for AKI |
28 (0.8) | 919 (0.8) | 28 (0.8) | 103 (0.8) | 1.09 (0.71–1.65) |
0.70 | 1.09 (0.71–1.65) |
0.70 |
| Falsification end points | ||||||||
| Hospitalization for hip fracture |
21 (0.6) | 785 (0.7) | 21 (0.6) | 90 (0.7) | 0.93 (0.58–1.50) |
0.77 | 0.93 (0.58–1.50) |
0.77 |
| Hospitalization for GI bleeding |
34 (1.0) | 900 (0.8) | 33 (1.0) | 108 (0.8) | 1.22 (0.83–1.80) |
0.31 | 1.22 (0.83–1.80) |
0.31 |
Data are presented as n (%) unless indicated otherwise. AKI, acute kidney injury; CI, confidence interval; GI, gastrointestinal; HF, heart failure; HR, hazard ratio; NA, not applicable; sHR, subdistribution hazard ratio.
In the propensity score-matched cohort, 3,631 (21.9%) patients were readmitted for HF within 1 year (n=736 in the Daikenchuto group; n=2,895 in the No-Daikenchuto group; Table 2). During readmission, 88.8% of patients received laxatives (91.4% in the Daikenchuto group; 88.2% in the No-Daikenchuto group). In the Daikenchuto group, 75.5% of those readmitted for HF received Daikenchuto during readmission.
Subgroup AnalysisSubgroup analyses demonstrated no effect modifications in the association between Daikenchuto and HF readmission in the subgroups stratified by age, sex, systolic blood pressure at admission, atrial fibrillation, and hypertension, except for renal disease (P for interaction=0.031; Figure 3); Daikenchuto was associated with a numerically lower incidence of HF readmission among patients with renal disease, though the difference was not statistically significant (24.6% vs. 28.3%, HR=0.84, 95% CI=0.70–1.02).

Subgroup analysis of Daikenchuto use for heart failure readmission in the propensity score-matched cohort. BP, blood pressure; CI, confidence interval; HR, hazard ratio.
Sensitivity Analyses
The falsification end points of hip fracture and gastrointestinal bleeding demonstrated no significant difference between the 2 groups (Table 2). E-values were calculated under the assumption that the true HR of Daikenchuto use on a lower incidence of 1-year HF readmission ranged from 0.6 to 0.9 (Table 3). The smallest E-value to explain the null association between Daikenchuto use and 1-year HF readmission was 1.40. Such an unmeasured factor, so strongly associated with both Daikenchuto use and 1-year HF readmission, would be unlikely to exist in the propensity score-matched cohort. The complete case analysis demonstrated results consistent with those of the main analysis (Supplementary Tables 3,4; Supplementary Figure).
E-Value Analyses for the Association Between Daikenchuto Use and 1-Year HF Readmission
| Hypothetical estimates for possible true association between Daikenchuto use and 1-year HF readmission |
||||
|---|---|---|---|---|
| HR=0.6 | HR=0.7 | HR=0.8 | HR=0.9 | |
| E-value for point estimates | 2.24 | 1.92 | 1.65 | 1.40 |
| E-value for confidence interval | 2.07 | 1.75 | 1.48 | 1.21 |
The smallest E-value is 1.40, indicating that an unmeasured confounder has to be associated with both Daikenchuto use and 1-year HF readmission by 1.40-fold when the true HR of Daikenchuto use on the incidence of 1-year HF readmission is 0.9. HF, heart failure; HR, hazard ratio.
In the present nationwide study, complementary use of Daikenchuto was not significantly associated with a lower incidence of HF readmission within 1 year after the first episode of HF hospitalization. This association was consistent across clinically relevant subgroups, except for those with or without renal disease. Daikenchuto was significantly associated with a slightly higher incidence of hospitalization for dehydration.
In Japan, Daikenchuto use is covered by the national health insurance to treat constipation and gastrointestinal symptoms (e.g., abdominal bloating) in both outpatient21 and inpatient20 settings. A recent experimental study found that Daikenchuto enhanced colonic transit activity through its possible propulsive motor effect.48 Moreover, a recent randomized, placebo-controlled trial demonstrated that Daikenchuto significantly improved stool consistency and reduced gastrointestinal symptoms in patients with constipation.24 These effects may be helpful to promote gastrointestinal motility and prevent straining for defecation among patients with HF and constipation.15 However, the present study found that the prevalence of Daikenchuto use in patients with HF and constipation receiving laxatives was only 2.9%, suggesting that Daikenchuto is not commonly used in this patient group. This infrequent use of Daikenchuto may be due to the lack of evidence of a prognostic effect of Daikenchuto in patients with HF and coexistent constipation. Among patients readmitted for HF, approximately 90% received laxatives during readmission, suggesting that most continued to have constipation. Moreover, in the Daikenchuto group, three-quarters of patients readmitted for HF received Daikenchuto during readmission, suggesting that Daikenchuto was frequently continued after discharge from the index hospitalization.
To the best of our knowledge, this is the first study to assess the association between the complementary use of Daikenchuto and the incidence of 1-year HF readmission. Our results found no association between Daikenchuto use and the incidence of 1-year HF readmission. This finding does not seem to be explainable by potentially unmeasured confounding in light of our falsification end points and E-value analyses. Although constipation is reportedly associated with an increased risk of death, cardiovascular events, and chronic kidney disease,34,49 no evidence to our knowledge is available regarding the beneficial effects of laxative use on prognosis in patients with constipation. Rather, a recent observational study reported that laxative use was associated with increased deaths from cardiovascular disease.50 In this context, it may be reasonable that Daikenchuto, an agent affecting the gastrointestinal system, had no or little effect on reducing the risk of HF readmission. Interestingly, an effect modification was observed for the association between Daikenchuto and HF readmission in the subgroups stratified by renal disease, where Daikenchuto use was associated with a numerically (although not statistically significantly) lower incidence of HF readmission in patients with renal disease. The mechanism for the association in patients with HF, renal disease, and constipation is unclear, warranting further studies.
The present study also demonstrated an association between Daikenchuto use and a slightly higher risk of dehydration. Although data on the occurrence of diarrhea were unavailable, some patients may have developed dehydration associated with diarrhea (the most common adverse event in Daikenchuto users51) because of the excessive gastrointestinal motility induced by Daikenchuto. Nonetheless, given the potential effect of Daikenchuto on gastrointestinal symptoms and the large number of patients with HF and constipation, further studies are needed to examine the effectiveness of Daikenchuto on patient-reported outcomes (e.g., constipation-related symptoms) in patients with HF and constipation.
Study LimitationsFirst, the present study may be subject to inherent biases related to the retrospective study design using an administrative claims database. The diagnoses may have been affected by misclassification bias. Clinical information, such as patient’s past history, symptoms, laboratory and imaging findings, was unavailable in the DPC database, similar to other administrative databases. A history of laparotomy or ileus was not available in the database, which may have affected the choice of Daikenchuto use and its effect. Moreover, the absence of left ventricular ejection fraction data poses a considerable limitation in administrative database studies, including the present and previous studies.38,39 Although our propensity score-matched analysis adjusted for measured confounders to balance the patient status and severity of HF between the groups, the results may be affected by unmeasured confounders. However, our sensitivity analyses demonstrated that an unmeasured confounder would be unlikely to mask the association between Daikenchuto use and 1-year HF readmission. Second, this study lacked data on constipation status, such as the frequency of defecations, straining during defecation, and stool characteristics. As a result, constipation was defined by the prescription of laxatives at discharge. This definition excluded patients who may have had constipation but did not receive a laxative prescription upon discharge. It is reasonable to assume that these patients would likely have experienced relatively mild or transient constipation. Third, although we assessed the continuation of laxative use among patients readmitted for HF, the DPC database did not enable us to determine whether the overall study population continued to have constipation during the follow-up period. We also could not capture data on medication continuation, discontinuation, and adherence after discharge. Fourth, this study did not identify minor Kampo medicines other than Daikenchuto and Mashiningan that possibly affect the gastrointestinal system because we do not believe that they were confounders due to their infrequent use (e.g., Goresain used in <1% of the study patients) and the lack of evidence of effectiveness.52 Fifth, there may have been residual biases related to missing data because missing data were handled by creating a “missing” category in the main analysis. Nonetheless, the results were consistent with those of the complete case analysis. Finally, there may have been some patients who were readmitted for HF to hospitals that were different from the index hospital or did not participate in the database, resulting in the underestimation of readmissions. However, this underestimation would be unlikely to bias our results because we do not think that such readmissions would be more likely to occur in either group.
This nationwide cohort study demonstrated that Daikenchuto, a potential complement to laxatives, was not associated with a lower incidence of 1-year HF readmission in older patients with HF and constipation. Further investigations are warranted to explore the possibility of the benefits of gastrointestinal medications on the improvement of constipation-related adverse events in patients with HF.
T.I. received a grant from the Japan Kampo Medicines Manufacturers Association (Grant on Health Economics Research, 2023). A.O. received a grant from the Japan Kampo Medicines Manufacturers Association (Grant on Health Economics Research, 2024). H.Y. received grants from the Ministry of Health, Labour and Welfare, Japan (grant numbers: 23AA2003 and 24AA2006). The funding sources had no role in the design of the study; collection, analysis, and interpretation of data; writing of the report; or decision to submit the article for publication.
T.I. and A.M. have an academic affiliation with the Department of Health Services Research, which is a cooperative program between The University of Tokyo and Tsumura & Company. N.M. and H.M. had an academic affiliation with the Department of Health Services Research. A.O. has an academic affiliation with the Department of Prevention of Diabetes and Lifestyle-Related Diseases, which is a cooperative program between The University of Tokyo and the Asahi Mutual Life Insurance Company. Tsumura & Company and the Asahi Mutual Life Insurance Company played no roles in the design of the study; the collection, analysis, or interpretation of the data; the writing of the manuscript; or the decision to publish the results.
A.M. reports receiving grants from the Ministry of Health Labour and Welfare (Health Labour Sciences Research Grant 23AA2004), the General Incorporated Association Data for Social Transformation, the Health Care Science Institute, and the Japan Society for the Promotion of Science outside the submitted work; and receiving consulting fees from M3, Inc., Mitsubishi Tanabe Pharma, and Datack, Inc. outside the submitted work. The other authors declare no conflicts of interest.
This study was approved by the Institutional Review Board of The University of Tokyo (approval number: 3501-[5]).
The data used in the present study are not publicly available owing to contracts with hospitals that provide data to the database.
Please find supplementary file(s);
https://doi.org/10.1253/circrep.CR-24-0114