Article ID: CR-19-0128
Article ID: CR-19-0128
Background: The aim of this study was to identify factors of left ventricular filling pressure (LVFP) elevation following transcatheter atrial septal defect (ASD) closure.
Methods and Results: The study involved 97 adult patients with sinus rhythm who underwent both transcatheter ASD closure and transthoracic echocardiography. Elevated LVFP was diagnosed during the first month of follow-up according to the American Society of Echocardiography guidelines: that is, ratio of transmitral early filling to the lateral annular diastolic velocity (lateral E/e’) >13 was used to exclude the effect of the device on the atrial septum. Fifteen patients (15.5%) were diagnosed with increased LVFP during the 1-month follow-up period (median lateral E/e’: from 9.2, IQR, 6.6–10.8; to 15.5, IQR, 13.8–17.8; P<0.001). Independent predictors of LVFP elevation were left ventricular (LV) relative wall thickness, lateral E/e›, and peak tricuspid regurgitation pressure gradient (TRPG) at baseline (OR, 1.67; 95% CI: 1.04–2.69; OR, 1.52; 95% CI: 1.07–2.15; and OR, 1.14; 95% CI: 1.04–1.25; cut-offs: 0.42, 7.5, and 27.0 mmHg, respectively). Median lateral E/e› returned to baseline in most patients with LVFP elevation during 6 months of subsequent follow-up (1-month–6-month follow-up: 15.5, IQR, 13.8–17.8; 11.1, IQR, 8.8–14.8, respectively; P=0.001).
Conclusions: The increase in Doppler-estimated LVFP following transcatheter ASD closure may be related to LV hypertrophy, diastolic dysfunction, and peak TRPG in elderly patients.
Transcatheter closure is an effective procedure in most patients with atrial septal defect (ASD) of the secundum type.1–3 Right ventricular (RV) volume overload improves immediately after closure, and there is also subsequent marked improvement in left ventricular (LV) stroke volume.4,5 It has been reported, however, that LV filling pressure (LVFP) increases after ASD device closure in some patients.6–9 The determinants of LVFP elevation after transcatheter ASD closure have not been fully investigated. Thus, we analyzed the time course of LVFP after transcatheter ASD closure on Doppler echocardiography to determine the predictors of subsequent LVFP elevation.
Transcatheter ASD device closure was performed in 128 consecutive patients at the present institution from September 2011 to June 2016. Patients in this cohort were in sinus rhythm and had undergone transthoracic echocardiography (TTE) prior to transcatheter closure and during follow-up for up to 6 months afterward. We excluded one patient in whom the device could not successfully be deployed, 12 patients with atrial fibrillation, 13 patients who did not undergo TTE during the 1-month follow-up period, and 5 patients in whom sufficient echocardiography measurements could not be acquired. Thus, a total of 97 patients (mean age, 53±18 years; 69 women) were finally included in the study. All study subjects gave written informed consent.
Transcatheter ASD Device ClosurePrior to transcatheter closure, we performed transesophageal echocardiography in all patients to determine the appropriateness of the procedure by verifying all relevant anatomic characteristics (i.e., maximum defect diameter, condition of the surrounding septal rims, and the number of defects). Right heart catheterization was performed before transcatheter ASD closure to rule out other comorbid shunt disease and measure the pulmonary vascular resistance. Thereafter, transcatheter closures were performed using the Amplatzer Septal Occluder (Abbott Medical, Plymouth, MN, USA) or Occlutech Figulla Flex II ASD Occluder (Occlutech, Jena, Germany) in 120 and in 8 patients, respectively, via the conventional method.10 Appropriate occluder size was determined by comprehensively considering both pre-procedural and balloon measurements of the defect size.10 Transcatheter closure was successfully performed using a single device in 124 patients and 2 devices in 3 patients. The device could not be successfully deployed in 1 patient due to broad deficiency of the superior rim. One patient had a major complication, namely cardiac tamponade requiring pericardiocentesis.
EchocardiographyTTE was performed 4 times: prior to transcatheter ASD closure, and then 2 days, 1 month, and 6 months after closure. Comprehensive echocardiograms, including 2-D, pulsed-wave, color, and tissue Doppler images, were obtained in all subjects using a commercially available system (Vivid E9; GE Healthcare, Milwaukee, WI, USA). Pulsed-wave Doppler of transmitral flow and tissue Doppler of the lateral mitral annulus were performed according to the American Society of Echocardiography recommendations for echocardiographic evaluation of LV diastolic function, published in 2009 and 2016.11,12 The calculation of left atrial (LA) volume is affected by the portion of the implanted device protruding into the LA in patients who have undergone transcatheter ASD closure, and peak tricuspid regurgitation pressure gradient (TRPG) may be influenced by the underlying disease, that is, ASD. Therefore, LVFP was estimated based on the ratio of transmitral early filling to mitral annular diastolic velocity (E/e’), as recommended by the American Society of Echocardiography guidelines established in 2009.11 Furthermore, lateral E/e’ was used to exclude the direct effects of the implanted ASD device on mitral annular motion;13 therefore, LVFP elevation during the first month of follow-up was diagnosed when post-procedural lateral E/e’ increased >13, which is considered to be abnormal according to the 2016 guidelines.12 Based on lateral E/e’ obtained at 1 month after the procedure, patients were divided into 2 groups according to the presence of LVFP elevation. The baseline clinical characteristics and echocardiography parameters of these 2 groups were compared, and the predictors of LVFP elevation after transcatheter ASD closure were identified. LV mass index was calculated using the following formula: {0.8×1.04×[(interventricular septum+LV end-diastolic internal diameter+inferolateral wall thickness)3−LV end-diastolic internal diameter3]+0.6}/body surface area g/m2.14 LV mass was also normalized according to 2 different height scales in meters: LV mass/height2.7 and LV mass/height1.7.14–16 We also analyzed relative wall thickness (RWT) with the formula (2×inferolateral wall thickness)/(LV internal diameter at end-diastole).14 LA volume was calculated based on both apical 4- and 2-chamber views using the area-length technique.14 We estimated RV systolic function by percentage fractional area change, defined as (end-diastolic area−end-systolic area)/end-diastolic area×100, which was calculated based on the RV−focused apical 4-chamber view.17 The severity of mitral regurgitation (MR) and of tricuspid regurgitation (TR) was classified as follows: mild, grade I; moderate, grade II; moderately severe, grade III; and severe, grade IV. Echocardiography parameters, including LVFP, were recorded for up to 6 months after the procedure in all patients.
Plasma Brain Natriuretic Peptide (BNP)Plasma BNP was measured using a specific immunoradiometric assay (ARCHITECT BNP-JP, Abbott Japan, Tokyo, Japan) at the same time as the echocardiograms.
Statistical AnalysisAll normally distributed data are presented as mean±SD, and non-normally distributed data as median (IQR). The chi-squared test was applied for categorical variables. Repeated-measures analysis of variance and subsequent post-hoc analysis were applied for continuous variables. P<0.05 was considered statistically significant. Univariable logistic regression analysis was performed to test the association between dependent variables and LVFP elevation after transcatheter ASD closure. Multivariable logistic regression analysis using baseline echocardiography parameters was performed to determine the independent predictors of LVFP elevation. Receiver operating characteristic (ROC) analysis was used to identify the cut-off point for each index identified as independently significant for predicting LVFP elevation. Positive and negative predictive values were calculated for the determined cut-off points. Predictive accuracy was obtained the sum of true positive and true negative by total number. We classified 3 independent predictors into 2-step models, and analyzed them using the likelihood ratio test. The Hosmer-Lemeshow goodness-of-fit statistic was used to assess the adequacy of the final model. All statistical analysis was performed using IBM SPSS Statistics version 22.0 (IBM, Armonk, NY, USA).
Serial changes in RV end-diastolic area and LV end-diastolic diameter calculated on TTE are shown in Figure 1. Remodeling of both ventricles started immediately after ASD closure, and gradual changes continued through 6 months of follow-up, but no significant changes were observed between the 1-month and 6-month follow-up time points.
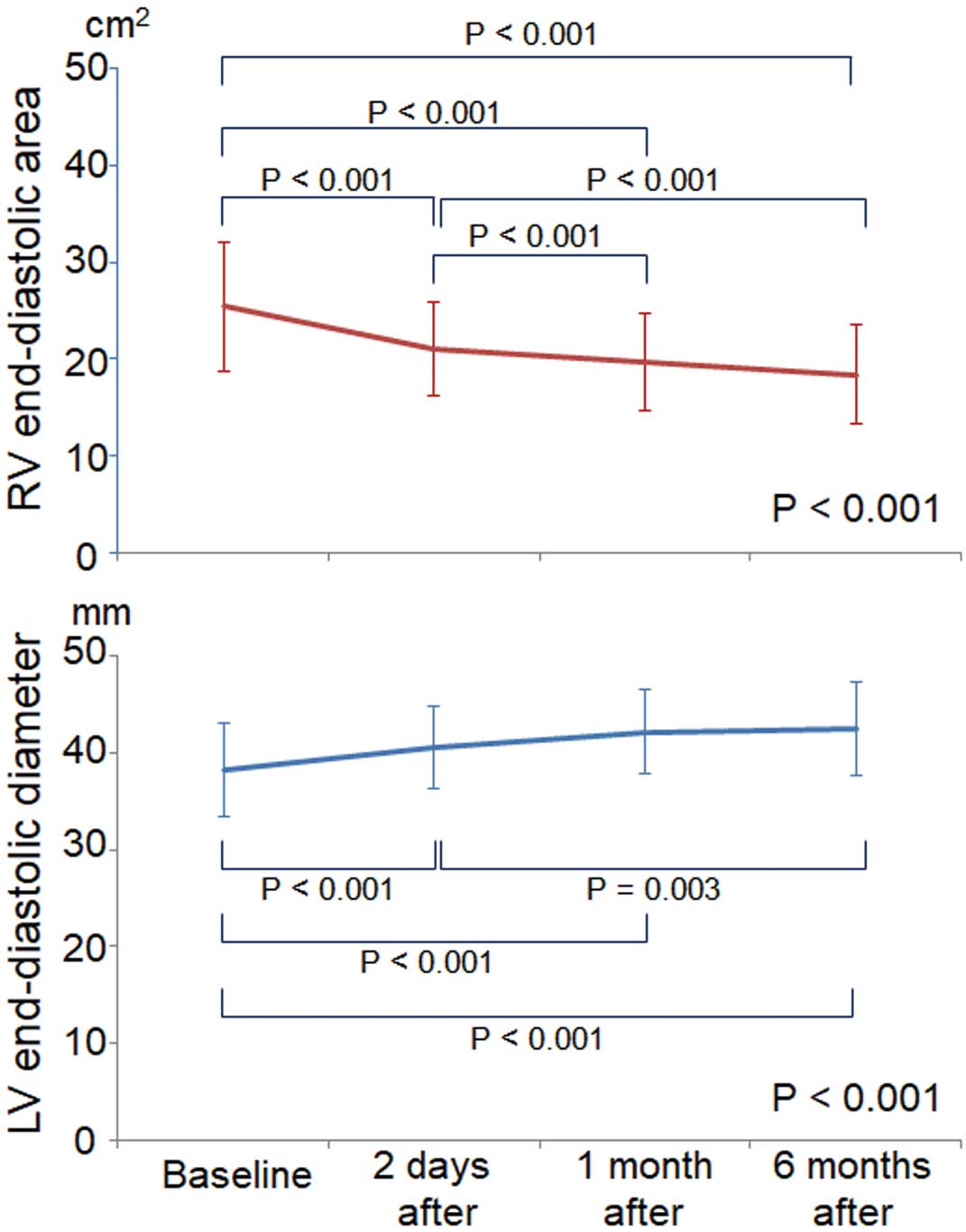
Change in right ventricular (RV) end-diastolic area and left ventricular (LV) end-diastolic diameter after transcatheter atrial septal defect closure. Data given as mean±SD.
Of 97 patients with sinus rhythm who underwent transcatheter ASD closure and who could be followed up for 6 months, 15 patients (15.5%) were diagnosed with LVFP elevation during the initial 1-month follow-up period. Baseline clinical characteristics and the parameters evaluated on TTE are listed in Tables 1,2. The patients with LVFP elevation were significantly older and had larger defect sizes than those without increased LVFP. LV mass index was not significantly different between the 2 groups. Also, neither LV mass/height2.7 nor LV mass/height1.7 at baseline were significantly different (patients with vs. without LVFP elevation: 25.9±6.4 vs. 22.7±7.3 g/height2.7, respectively, P=0.116; 40.5±9.9 vs. 36.4±12.2 g/height1.7, respectively, P=0.224). On comparison of echocardiography parameters, however, RWT was significantly greater in patients with LVFP elevation. The severity of TR significantly improved 1 month after the procedure in patients without LVFP elevation, but showed no marked change in patients with LVFP elevation. The severity of MR did not change significantly during the 1-month follow-up period in either group. Although pre-procedural lateral E/e’ was higher in patients with post-procedural LVFP elevation, there was only 1 patient in each group whose pre-procedural lateral E/e’ was >13. Pulmonary capillary wedge pressure (PCWP) at baseline in these patients with or without LVFP elevation was 15.2 mmHg and 11.0 mmHg, respectively. The former did not develop clinical heart failure after transcatheter ASD closure. PCWP was measured in 68 out of 97 patients who underwent right heart catheterization; it was >15 mmHg at baseline in 4 patients, and only 1 of these 4 patients had lateral E/e’ >13. Therefore, the sensitivity and specificity of increased lateral E/e’ (>13) for detecting significantly increased PCWP (>15 mmHg) at baseline were 0.25 and 0.98, respectively. The predictive accuracy was 0.94. As shown in Table 3, on logistic regression analysis the independent predictors of LVFP elevation after transcatheter ASD closure were LV RWT, lateral E/e’, and peak TRPG at baseline. The optimal cut-offs for predicting LVFP elevation were 0.42 for RWT, 7.5 for lateral E/e’, and 27.0 mmHg for peak TRPG. Measures of predictive accuracy, including ROC analysis, are listed in Table 4. The addition of lateral E/e’ and RWT to peak TRPG increased the predictive value of LVFP elevation following ASD closure (Hosmer-Lemeshow test, P=0.968).
| Variable | LVFP elevation (+) n=15 |
LVFP elevation (−) n=82 |
P-value |
|---|---|---|---|
| Age (years) | 64±9 | 50±18 | 0.005 |
| Female | 13 (87) | 56 (68) | 0.218 |
| Hypertension | 6 (40) | 14 (17) | 0.069 |
| Diabetes mellitus | 1 (7) | 3 (4) | 0.481 |
| β-blocker | 2 (13) | 3 (4) | 0.123 |
| ACEI/ARB | 1 (7) | 3 (4) | 0.590 |
| Diuretics | 1 (7) | 6 (7) | 0.919 |
| Maximum ASD size (TEE) (mm) | 18.0 (17.0–21.3) | 15.0 (12.0–19.0) | 0.011 |
| Device size (mm) | 24.0 (20.0–26.0) | 19.0 (14.0–24.0) | 0.001 |
| Residual shunt at 1 month after the procedure (TTE) | 6 (40) | 38 (46) | 0.650 |
| Cardiac catheterization data | |||
| Qp/Qs | 2.4 (2.2–2.6) | 2.0 (1.5–2.6) | 0.018 |
| PVR (Wood units) | 1.6 (1.1–2.6) | 1.0 (0.7–1.4) | 0.007 |
| Mean PAP (mmHg) | 20.0 (16.0–24.0) | 19.0 (16.0–20.0) | 0.497 |
| PCWP (mmHg) | 9.2 (6.6–10.8) | 6.5 (5.7–7.8) | 0.116 |
Data given as mean±SD, n (%) or median (IQR). ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; ASD, atrial septal defect; LVFP, left ventricular filling pressure; PAP, pulmonary arterial pressure; PCWP, pulmonary capillary wedge pressure; PVR, pulmonary vascular resistance; Qp/Qs, ratio of pulmonary blood flow to systemic blood flow; TEE, transesophageal echocardiography; TTE, transthoracic echocardiography.
| Variable | LVFP elevation (+) | LVFP elevation (−) | Baseline P-value |
1-month P-value |
||||
|---|---|---|---|---|---|---|---|---|
| Baseline | 1 month | P-value | Baseline | 1 month | P-value | |||
| Heart rate (beats/min) | 65±8 | 58±18 | <0.001 | 67±10 | 64±10 | 0.088 | 0.341 | 0.111 |
| LVEDD (mm) | 36.7±3.7 | 41.6±2.8 | <0.001 | 38.7±5.0 | 42.5±4.9 | <0.001 | 0.148 | 0.461 |
| LVMI (g/m2) | 57.0±13.0 | 70.8±18.6 | <0.001 | 51.7±16.2 | 59.0±15.2 | <0.001 | 0.230 | 0.009 |
| RWT | 0.47±0.06 | 0.40±0.06 | 0.003 | 0.39±0.09 | 0.35±0.08 | <0.001 | 0.001 | 0.012 |
| LVEF (%) | 67.8±6.8 | 69.8±5.1 | 0.396 | 64.1±6.9 | 67.3±5.8 | <0.001 | 0.060 | 0.133 |
| LAVI (mL/m2) | 43.3±8.0 | 46.4±9.5 | 0.331 | 34.2±9.8 | 33.1±10.2 | 0.563 | 0.001 | <0.001 |
| E (m/s) | 0.64±0.13 | 0.96±0.22 | <0.001 | 0.71±0.20 | 0.75±0.24 | 0.018 | 0.186 | 0.002 |
| E/A | 0.91±0.17 | 1.79±1.11 | 0.018 | 1.40±0.74 | 1.47±0.79 | 0.123 | <0.001 | 0.186 |
| DT (ms) | 197±36 | 170±22 | 0.019 | 195±39 | 202±48 | 0.141 | 0.844 | <0.001 |
| Lateral e’ (cm/s) | 7.8±2.6 | 6.0±1.3 | 0.002 | 11.0±3.4 | 9.6±3.5 | <0.001 | 0.001 | <0.001 |
| Lateral E/e’ | 9.2 (6.6–10.8) | 15.5 (13.8–17.8) | <0.001 | 6.5 (5.7–7.8) | 7.7 (6.4–9.9) | <0.001 | 0.005 | <0.001 |
| Peak TRPG (mmHg) | 33.6±8.9 | 30.0±4.9 | 0.061 | 24.8±7.6 | 20.1±5.9 | <0.001 | <0.001 | <0.001 |
| RVEDA (cm2) | 24.7±6.5 | 19.1±4.3 | 0.012 | 25.1±7.0 | 19.9±5.6 | <0.001 | 0.819 | 0.781 |
| RV FAC (%) | 43.4±6.5 | 36.6±9.5 | 0.014 | 43.8±7.5 | 37.2±8.0 | <0.001 | 0.616 | 0.718 |
| MAC | 2 (13) | – | – | 7 (9) | – | – | 0.556 | – |
| MR grade I/II | 15/0 | 13/2 | 0.164 | 81/1 | 80/2 | 0.320 | 1.000 | 0.112 |
| TR grade I/II/III/IV | 9/5/1/0 | 11/4/0/0 | 0.082 | 62/19/1/0 | 73/9/0/0 | 0.002 | 0.493 | 0.113 |
Data given as mean±SD, n (%) or median (IQR). A, late diastolic velocity; DT, deceleration time of transmitral early filling flow; E, early diastolic velocity; e’, early diastolic annular velocity; FAC, fractional area change; LAVI, left atrial volume index; LVEDD, left ventricular end-diastolic diameter; LVEF, left ventricular ejection fraction; LVFP, left ventricular filling pressure; LVMI, left ventricular mass index; MAC, mitral annular calcification; MR, mitral regurgitation; RV, right ventricular; RVEDA, right ventricular end-diastolic area; RWT, relative wall thickness; TR, tricuspid regurgitation; TRPG, tricuspid regurgitation pressure gradient; TTE, transthoracic echocardiography.
| Variable | Univariable analysis | Multiple logistic regression analysis | ||
|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | |
| Age | 1.06 (1.01–1.11) | 0.009 | – | – |
| Maximum ASD size | 1.19 (1.03–10.37) | 0.019 | – | – |
| RWT (for every 0.05 increase) | 1.56 (1.12–2.17) | 0.008 | 1.67 (1.04–2.69) | 0.034 |
| LVEF | 1.10 (1.00–1.21) | 0.059 | – | – |
| LAVI | 1.09 (1.03–1.16) | 0.003 | – | – |
| E/A | 0.09 (0.01–0.67) | 0.018 | – | – |
| Lateral e’ | 0.73 (0.59–0.90) | 0.003 | – | – |
| Lateral E/e’ | 1.52 (1.16–1.99) | 0.002 | 1.52 (1.07–2.15) | 0.019 |
| Peak TRPG | 1.12 (1.05–1.20) | 0.001 | 1.14 (1.04–1.25) | 0.004 |
Abbreviations as in Table 2.
| Variable | AUC (95% CI) |
P-value | Threshold† | Sensitivity | Specificity | PPV | NPV | Predictive accuracy |
|---|---|---|---|---|---|---|---|---|
| Univariate predictors | ||||||||
| Peak TRPG | 0.81 (0.69–0.93) | <0.001 | 27.0 mmHg | 0.87 | 0.69 | 0.33 | 0.97 | 0.72 |
| Lateral E/e’ | 0.73 (0.57–0.89) | 0.005 | 7.5 | 0.73 | 0.71 | 0.32 | 0.93 | 0.71 |
| RWT | 0.75 (0.64–0.86) | 0.002 | 0.42 | 0.87 | 0.66 | 0.32 | 0.96 | 0.69 |
| Chi-squared for LVFP elevation |
P-value for added terms |
|||||||
| Peak TRPG | 10.7 | |||||||
| Peak TRPG+lateral E/e’ | 16.9 | 0.013 | ||||||
| Peak TRPG+lateral E/e’+RWT | 22.2 | 0.021 | ||||||
†Value that best identified the patients with LVFP elevation with optimal sensitivity and specificity. AUC, area under the receiver operating characteristics curve; NPV, negative predictive value; PPV, positive predictive value. Other abbreviations as in Table 2.
The follow-up data of plasma BNP and lateral E/e’ are shown in Figure 2. BNP in patients with LVFP elevation increased during the first month after ASD closure and decreased, although non-significantly, from 1 month to 6 months after the procedure. In patients without LVFP elevation, BNP rose significantly 2 days after ASD closure and decreased significantly through 6 months after closure. Lateral E/e’ varied with BNP level in both groups, and decreased from 1-month follow-up to 6-month follow-up in patients with LVFP elevation.
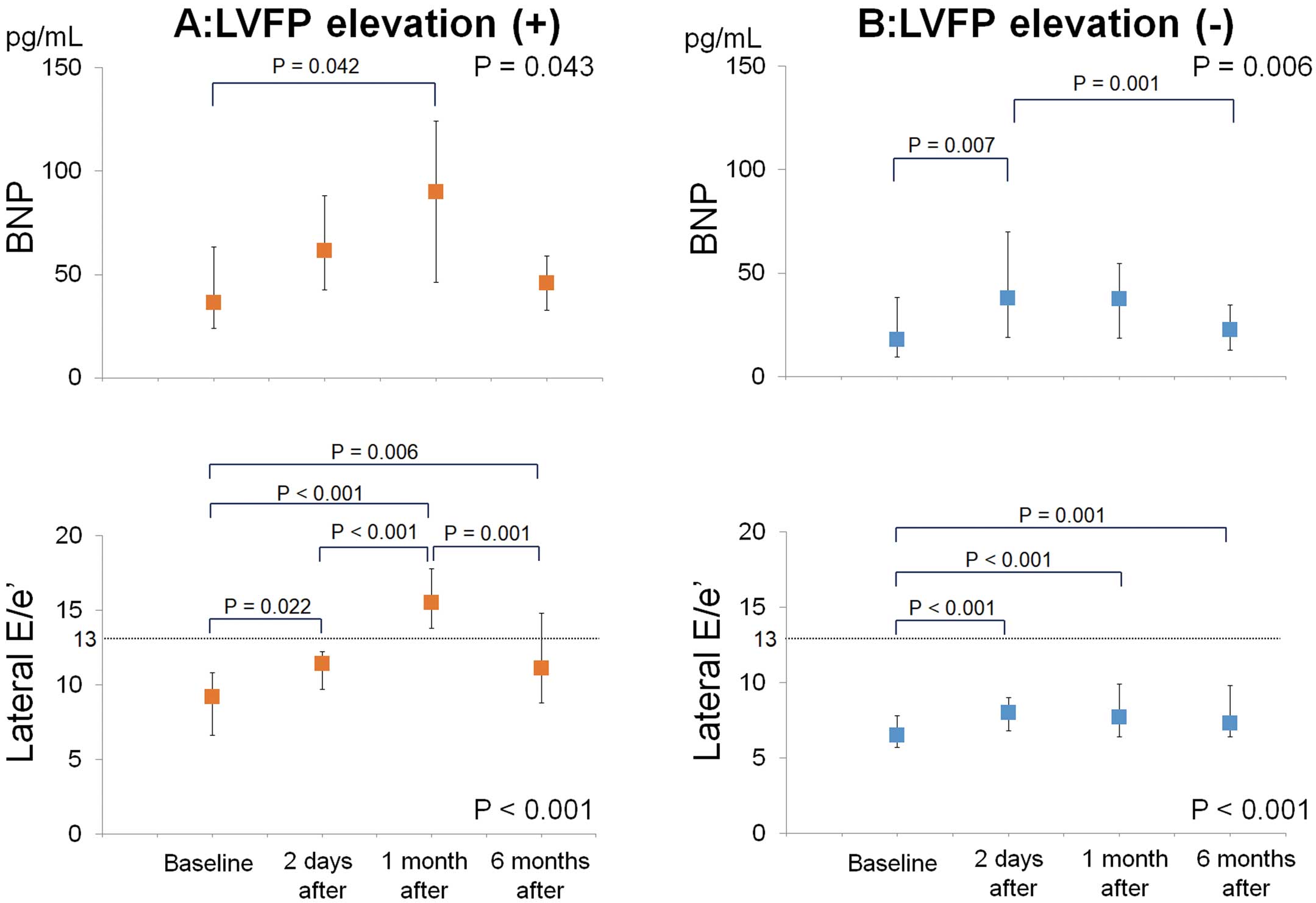
Plasma brain natriuretic peptide (BNP) and ratio of transmitral early filling to lateral annular diastolic velocity (lateral E/e’) in patients (A) with and (B) without left ventricular filling pressure (LVFP) elevation after transcatheter atrial septal defect closure. Data given as median and IQR.
Only 2 patients with LVFP elevation complained of exertional dyspnea after transcatheter ASD closure, necessitating the addition of diuretics alone or diuretics and isosorbide mononitrate. Thereafter, their symptoms were well-controlled for >2 years after closure. Figure 3 shows pulsed-wave Doppler imaging of transmitral flow, tissue Doppler examination of the lateral mitral annulus, and the plasma BNP of 3 patients who followed different clinical courses after transcatheter ASD closure. No significant increase of LVFP was observed in patient 1. The remaining 2 patients had LVFP elevation at 1 month after transcatheter ASD closure; in 1 of these patients, lateral E/e’ was decreased to normal range at 6 months after the procedure and plasma BNP level was improved. However, lateral E/e’ remained higher than normal range at the 6-month point in the other patient, though 2 parameters improved relative to the 1-month point.

Representative clinical courses in 3 patients after transcatheter atrial septal defect (ASD) closure according to (Top) pulsed-wave Doppler imaging of transmitral flow; (Middle) tissue Doppler imaging of the lateral mitral annulus; and (Bottom) plasma brain natriuretic peptide (BNP) and lateral E/e’ data. In patients 2 and 3, lateral E/e’ was increased at 1 month after transcatheter ASD closure. In patient 2, both this ratio and plasma BNP level were decreased at 6 months after the procedure, relative to the 1-month point, and lateral E/e’ decreased to normal range. On the other hand, lateral E/e’ in patient 3 remained higher than normal range at 6 months after the procedure.
Of 97 patients who underwent transcatheter ASD closure and who could be followed for up to 6 months, 15.5% were diagnosed with LVFP elevation at 1 month after the procedure. Independent predictors of this increase were LV RWT, lateral E/e’, and peak TRPG, all of which were calculated on echocardiography prior to the procedure. In all patients except 2 who developed heart failure symptoms, Doppler parameters indicated that LVFP returned to normal by 6 months after the procedure, without any need to titrate medical treatment.
As a result of the development of sophisticated closure devices and interventional techniques, as well as low complication rates, the number of elderly patients who undergo transcatheter ASD closure has continued to increase.3,18 Serious device-related complications are very rare,19 but some patients suffer from heart failure following ASD closure.6–8 Left-to-right inter-atrial shunting causes LV under-filling as a counterbalance to RV over-filling, which can prevent congestive heart failure due to intrinsic LV dysfunction by decreasing preload. The unmasking of this issue by ASD closure is generally considered to be the primary etiology of LVFP elevation following this procedure.6–8
Prediction of LVFP ElevationSchubert et al reported that hemodynamic measurement during complete balloon occlusion of ASD could be helpful for predicting LVFP elevation following closure.8 In their study, 15 of 59 patients (25%) had LV restriction defined as an increase of LA pressure >10 mmHg.8 Of these 15 patients, 13 received anti-heart failure pre-medication; subsequently, catheter ASD closure was successfully performed. The remaining 2 patients who underwent ASD closure without pre-medication had congestive heart failure requiring additional therapy.8 Baseline LA pressure and the prevalence of pulmonary hypertension and atrial fibrillation were higher in patients with LV restriction than in those without, but that study did not investigate pre-procedural LV function, including echocardiography parameters.8
LV Diastolic Dysfunction and LVFP ElevationIn the present study, patients with LVFP elevation had significantly larger ASD than those without. This is consistent with other variables, that is, greater maximum ASD size, device size used, and the ratio of pulmonary to systemic blood flow. Although in the Schubert et al study neither device size nor the ratio of pulmonary to systemic blood flow differed between the 2 patient groups,8 it is logical given that patients with larger ASD have greater post-procedural acute LV preload augmentation. On multivariate analysis for predicting post-procedural LVFP elevation, however, maximum ASD size was not selected as an independent predictor; rather, these factors were identified as pre-procedural LV RWT, lateral E/e’, and peak TRPG. Peak TRPG, used practically as an indicator of systolic pulmonary artery pressure, is determined by various factors such as pulmonary vasculature, left-to-right intra-cardiac shunt volume, RV function, mitral valve disease, and LV function. None of the subjects in this study had abnormally elevated pulmonary vascular resistance (range, 0.5–4.0 Wood units), reduced RV function (range of fractional area change, 34.7–67.0%),17 or significant organic mitral valve disease. Accordingly, peak TRPG in these subjects may have been influenced mainly by intrinsic LV dysfunction and large shunt volume. More specifically, LV dysfunction in the present cohort may have been determined by diastolic function, given that LV ejection fraction was >50% (mean, 64.6±7.0%) in all but 2 patients. Both of the other independent predictors of post-procedural LVFP elevation, namely E/e’ and LV RWT, were much more closely associated with LV diastolic function. Thus, the 3 independent predictors of LVFP elevation were all associated with LV diastolic function. LV RWT was an independent predictor of LVFP elevation after transcatheter ASD closure, whereas LV mass index and LV mass indexed by height were not. The LV of ASD patients were under-filled due to the intracardiac shunt flow at the atrial level. The under-filled LV morphology with hypertrophy may lead to this result.
Plasma BNP After Transcatheter ASD ClosureIn some reports, the increase in LVFP was estimated based on the increase in plasma BNP level.7,20 Masutani et al measured plasma BNP before and after defect closure and identified the predictors of increase after closure.7 They reported that age and lateral early diastolic mitral annular velocity before ASD closure independently predicted the increase of plasma BNP after closure.7 Although lateral early diastolic mitral annular velocity was also significantly impaired in patients with LVFP elevation in the present cohort, it was not an independent predictor. Plasma BNP after transcatheter ASD closure was also investigated as an outcome in some studies. In most of these, plasma BNP was measured immediately after closure or was analyzed without considering the patient baseline characteristics.5,7,20,21 In the present study, plasma BNP was measured immediately and up to 6 months after closure; furthermore, we demonstrated that the change in BNP level differed depending on whether or not considerable LVFP elevation was present after ASD closure. In patients without LVFP elevation, plasma BNP was slightly increased immediately after ASD closure and started to decrease in the first month after closure. In patients with LVFP elevation, however, BNP continued to increase during the first month and subsequently decreased through the 6 months after closure.
Biventricular Remodeling and LVFP ElevationLVFP elevation also may be affected by the remodeling of cardiac chambers following transcatheter ASD closure. Remodeling of both ventricles after transcatheter ASD closure started immediately after ASD closure, and gradual changes continued through 6 months of follow-up (Figure 1). The combination of gradual RV volume reduction and LV volume augmentation might offset the pathological increase of LVFP in most patients who undergo transcatheter ASD device closure. Adaptation, however, to the increased LV preload after closing the left-to-right shunt is estimated to take more time in patients with latent LV diastolic dysfunction.
After transcatheter ASD closure, 2 of 97 patients in this study had symptomatic heart failure. Masutani et al reported that 2 of 39 consecutive patients who underwent transcatheter ASD closure developed clinical heart failure.7 Another study divided 206 patients >60 years old into 3 groups depending on LV diastolic function and assessed outcomes after transcatheter ASD closure;22 more than half of the patients with severe diastolic dysfunction were prescribed diuretics prior to the procedure, and plasma BNP level did not increase after the procedure in these patients.22 Pre-procedural prescription of diuretics might prevent the development of clinical heart failure. Few studies with a relatively large number of patients have reported the incidence of heart failure after transcatheter ASD closure. It is also crucial to determine the predictive factors and clinical characteristics of LVFP elevation after this procedure.
Transcatheter ASD closure has been performed in patients of different ages, and the procedure is indicated for many elderly patients with impaired LV diastolic dysfunction. In such cases, LV wall thickening, LV diastolic function, and degree of pulmonary hypertension must be closely monitored to prevent clinical heart failure. According to the latest guidelines, ASD intervention is recommended for patients with significant RV volume overload or with paradoxical embolism.23 These guidelines, however, provide no relevant information regarding LV condition or intracardiac pressure. Considering the present results, ASD intervention may be performed earlier than has been previously recommended, that is, prior to the appearance of LV diastolic dysfunction.
Study LimitationsThis study had several limitations. First, we performed Doppler evaluation of LV diastolic dysfunction; given that we did not measure LV diastolic pressure directly under cardiac catheterization after transcatheter ASD closure in all patients, there may have been errors in estimating this parameter. We did, however, use currently accepted guidelines, and BNP fluctuations did not conflict with the Doppler-estimated LVFP. Regarding the diagnostic accuracy of increased lateral E/e’ (>13) for detecting significantly increased PCWP (>15 mmHg) at baseline, specificity and predictive accuracy were high. Therefore, this cut-off of lateral E/e› may accurately reflect significantly increased PCWP in the present study cohort. Second, 12 patients with atrial fibrillation were excluded from the cohort. The method for estimating LVFP in patients with atrial fibrillation has not been well established,12 and these patients may have complicated LV diastolic dysfunction. Therefore, further examination is needed to assess LV diastolic function in this population.
Increased LVFP was estimated on Doppler echocardiography after transcatheter ASD closure and was associated with LV wall thickening, impaired LV diastolic function, and increased peak TRPG. LVFP, however, decreased to the normal range by 6 months after closure in most patients.
We would like to express our gratitude to Dr. Tatsuya Kawasaki at Matsushita Memorial Hospital in Japan for his valuable advice regarding statistical analysis.
M.Y. and T.Y. designed the study and performed the analysis. M.Y., T.Y., and T.N. collected the data. M.Y. wrote the manuscript with support from T.Y., T.N., K.Z., H.S., T.S., and S.M. All authors revised the manuscript and approved the final version.
This research was not supported by grants from any funding agencies in the public, commercial, or not-for-profit sectors.