Article ID: CR-20-0081
Article ID: CR-20-0081
Background: The effect of the COVID-19 pandemic on the respiratory management strategy with regard to the use of non-invasive positive pressure ventilation (NPPV) and high-flow nasal cannula (HFNC) in patients with acute heart failure (AHF) in Japan is unclear.
Methods and Results: This cross-sectional study used a self-reported online questionnaire, with responses from 174 institutions across Japan. More than 60% of institutions responded that the treatment of AHF patients requiring respiratory management became fairly or very difficult during the COVID-19 pandemic than earlier, with institutions in alert areas considering such treatment significantly more difficult than those in non-alert areas (P=0.004). Overall, 61.7% and 58.8% of institutions changed their indications for NPPV and HFNC, respectively. Significantly more institutions in the alert area changed their practices for the use of NPPV and HFNC during the COVID-19 pandemic (P=0.004 and P=0.002, respectively). When there was insufficient time or information to determine whether AHF patients may have concomitant COVID-19, institutions in alert areas were significantly more likely to refrain from using NPPV and HFNC than institutions in non-alert areas.
Conclusions: The COVID-19 pandemic has compelled healthcare providers to change the respiratory management of AHF, especially in alert areas.
The global spread of COVID-19 has made it imperative to protect the health and safety of healthcare workers who are involved in the acute management of patients suspected of having COVID-19. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is primarily transmitted through direct contact and droplets, which are created during aerosol-generating procedures, although there are concerns that exhaled air dispersion during oxygenation therapy may increase the risk of virus transmission.
In patients presenting with acute heart failure (AHF), hypoxemia is very common, and supplemental oxygen is delivered via various interfaces. Compared with standard oxygen therapy that is administered via face masks and nasal cannulas, respiratory support with positive intrathoracic pressure is theoretically effective for patients with AHF because it can enhance the inspiratory tidal volume and maintain adequate ventilation.1,2 Therefore, non-invasive positive pressure ventilation (NPPV) has become the preferred primary modality for respiratory support in patients with AHF, and NPPV has been shown to have an overall benefit in reducing rates of in-hospital mortality, endotracheal intubation, and adverse events.3,4 High-flow nasal cannula (HFNC) oxygen delivery has increasingly received attention as an alternative means of respiratory support for AHF, especially when NPPV is inapplicable because of poor mask tolerance.5–7
However, during the COVID-19 pandemic, the use of NPPV or HFNC for patients with respiratory failure can be withheld because COVID-19 and AHF have respiratory failure as a common initial presentation, and the substantial amount of droplets exhaled during NPPV or HFNC can increase virus dispersion and subsequently increase the risk of nosocomial infection.8 In contrast, studies investigating the risks of transmission associated with the use of NPPV or non-invasive ventilation (NIV) in infected patients via the dispersion of exhaled air or related mechanisms have reported conflicting results.9–11 Indeed, a World Health Organization (WHO) guideline avers that NPPV and HFNC do not create widespread dispersion of exhaled air.12 Therefore, an ongoing clinical dilemma during the COVID-19 outbreak pertains to balancing the awareness of the increased risk of viral transmission from exhaled air during NPPV and HFNC against the need to provide appropriate oxygen therapy and avoid unnecessary intubation. However, it remains unclear whether healthcare providers (HCPs) are being forced to change their approach to the acute respiratory management of patients with AHF and respiratory failure during the COVID-19 pandemic.
Therefore, this study was conducted to ascertain the prevailing situation with regard to the respiratory management of AHF in Japan.
The present cross-sectional study on the effects of the COVID-19 pandemic on the acute respiratory management of patients with AHF was conducted by the Japanese Heart Failure Society. A nationwide survey was conducted using a self-reported online questionnaire that was developed using Google Forms and emailed to all members of the Japanese Heart Failure Society and the research registration facility of the Japanese Registry of Acute Decompensated Heart Failure (JROADHF)-NEXT on May 11, 2020. The response deadline was set for May 14, 2020. In cases where there were multiple respondents from the same facility, the earliest respondent was regarded as the appropriate representative for that facility. The main results of the survey have been reported on the official website of Japanese Heart Failure Society (http://www.asas.or.jp/jhfs/topics/20200519.html).
The questionnaire elicited information on the baseline characteristics of the institution and the number of confirmed COVID-19 cases, as well as how the COVID-19 pandemic has affected NPPV and HFNC use in patients with AHF (Table 1). It was assumed that the decision to use NPPV and HFNC in patients with AHF during the COVID-19 pandemic would depend on how likely it was that the patient would have a concomitant COVID-19 infection, and four conditions were assumed: (1) patients with AHF and confirmed COVID-19 infection; (2) patients with AHF and possible concomitant COVID-19 infection; (3) patients with AHF unlikely to have COVID-19; and (4) patients with AHF, but presenting under circumstances where physicians have insufficient time or information to gauge the possibility of COVID-19. Moreover, the Japanese government designated 13 prefectures (Hokkaido, Ibaraki, Tokyo, Kanagawa, Saitama, Chiba, Ishikawa, Gifu, Osaka, Aichi, Kyoto, Hyogo, and Fukuoka) as “special alert prefectures” based on several criteria, including a cumulative number of confirmed cases ≥400, and we evaluated whether the use of NPPV and HFNC differed between alert and non-alert areas.
| No. | Question | Possible responses |
|---|---|---|
| 1 | Before the COVID-19 pandemic (i.e., before December 2019), had you used non-invasive positive pressure ventilation (NIPPV) in patients with acute heart failure (AHF)? |
Yes/No |
| 2 | Have you changed the indications for NIPPV after the COVID-19 pandemic (i.e., after January 2020)? |
Yes/No |
| 3 | Before the COVID-19 pandemic (i.e., before December 2019), had you used a high-flow nasal cannula (HFNC) in patients with AHF? |
Yes/No |
| 4 | Have you changed the indications for HFNC after the onset of the COVID-19 pandemic (i.e., after January 2020)? |
Yes/No |
| 5 | Do you feel that it is more difficult to treat patients with AHF requiring NIPPV/HFNC now than it was before the COVID-19 pandemic? |
1. Not at all |
| 2. Not much | ||
| 3. Neither yes nor no | ||
| 4. A little | ||
| 5. Very much | ||
| 6 | Are you currently using or going to use NIPPV in patients with AHF and “confirmed” COVID-19? |
1. Yes, as same as other patients with AHF |
| 2. Yes, but some selected cases | ||
| 3. No, and I will never use them | ||
| 7 | Are you currently using or going to use HFNC in patients with AHF and “confirmed” COVID-19? |
1. Yes, as same as other patients with AHF |
| 2. Yes, but some selected cases | ||
| 3. No, and I will never use them | ||
| 8 | If you replied “Yes” to Q6 or Q7, please describe any specific indication, condition, or rule that is to be applied. |
(Free description) |
| 9 | Are you currently using or going to use NIPPV in patients with AHF and “suspected” of having COVID-19? |
1. Yes, similarly as in other patients with AHF |
| 2. Yes, but in some selected cases | ||
| 3. No, and I will never use it | ||
| 10 | Are you currently using or planning to use HFNC in patients with AHF and “suspected” of having COVID-19? |
1. Yes, similarly as in other patients with AHF |
| 2. Yes, but in some selected cases | ||
| 3. No, and I will never use it | ||
| 11 | Is there any patient initially suspected of having COVID-19 who was later confirmed to have COVID-19 after being treated with NIPPV or HFNC? |
Yes/No |
| 12 | If you replied “Yes” to Q9 or Q10, please describe any specific indication, condition, or rule that will be applied. |
(Free description) |
| 13 | Are you currently using or planning to use NIPPV in patients with AHF and “not suspected” of having COVID-19? |
1. Yes, similarly as in other patients with AHF |
| 2. Yes, but in some selected patients | ||
| 3. No, and I will never use it | ||
| 14 | Are you currently using or planning to use HFNC in patients with AHF and “not suspected” of having COVID-19? |
Yes/No |
| 15 | Is there any patient who was initially not suspected of having COVID-19 but was later confirmed to have COVID-19 after being treated with NIPPV or HFNC? |
Yes/No |
| 16 | If you replied “Yes” to Q13 or Q14, please describe any specific indication, condition, or rule that will be applied. |
(Free description) |
| 17 | Are you currently using or planning to use NIPPV in patients with AHF for whom there is “insufficient time/information to judge the possibility of” COVID-19? |
1. Yes, similarly as in other patients with AHF |
| 2. Yes, but in some selected patients | ||
| 3. No, and I will never use it | ||
| 18 | Are you currently using or planning to use HFNC in patients with AHF for whom there is “insufficient time/information to judge the possibility of” COVID-19? |
Yes/No |
| 19 | Is there any patient who initially presented for whom there was insufficient time/information to judge the possibility of COVID-19 and was later confirmed to have COVID-19 after being treated with NIPPV or HFNC? |
Yes/No |
| 20 | If you replied “Yes” to Q17 or Q18, please describe any specific indication, condition, or rule that will be applied. |
(Free description) |
| 21 | If you replied that the indications for NIPPV and HFNC have or will be changed, please select the answer which is the closest to your current practice. |
1. Use up to oxygen mask without a reservoir bag and if the patient’s oxygenation does not improve, intubate and mechanically ventilate the patient. |
| 2. Use up to oxygen mask with a reservoir bag and if the patient’s oxygenation does not improve, intubate and mechanically ventilate the patient. |
||
| 3. Neither of the above. (Free description) |
All respondents provided informed consent (written or verbal) for study participation. This study was approved by the Ethics Committee of Juntendo University (No. 2020095) and was conducted in accordance with the ethical principles of the Declaration of Helsinki and its later amendments. All authors take complete responsibility for the integrity of questionnaire creation, data collection, the study design, and the accuracy of the data analysis.
Statistical AnalysisData are expressed as the mean±SD for normally distributed variables and as the median with interquartile range (IQR) for non-normally distributed data. Categorical data are expressed as numbers and percentages. Intergroup differences (alert vs. non-alert areas) were evaluated using Student’s t-test or the Mann-Whitney U-test for continuous variables, and the Chi-squared or Fisher’s exact test for categorical variables. Statistical analyses were conducted using R version 3.5.2 (R Foundation for Statistical Computing, Vienna, Austria; http://www.R-project.org).
Responses were received from 174 institutions covering 43 prefectures in Japan. The characteristics of the institutions that responded are listed in Table 2. Overall, more than half of the institutions were located in the alert area, and almost all institutions had more than 100 beds and treated more than 100 patients with AHF annually. Compared with institutions in the non-alert area, those in the alert area had more beds and more staff cardiologists working with them. Moreover, these hospitals received more COVID-19 patients and patients with heart failure concomitant with COVID-19. Of the 131 institutions with experience in managing COVID-19, 45 (34.4%) had at least one case of COVID-19 in a heart failure patient. Furthermore, 17.5%, 21.2%, and 53.4% of hospitals with 1–4, 5–9, and ≥10 COVID-19 patients, respectively, had treated COVID-19 patients with concomitant heart failure requiring treatment (Ptrend <0.001).
| Variables | Overall (n=174) |
Non-alert area (n=74) |
Alert area (n=100) |
P-value |
|---|---|---|---|---|
| Institution in alert area | 100 (57.5) | |||
| No. beds | 0.030 | |||
| <100 | 4 (2.3) | 1 (1.4) | 3 (3.0) | |
| 100–499 | 75 (43.1) | 33 (44.6) | 42 (42.0) | |
| 500–799 | 60 (34.5) | 32 (43.2) | 28 (28.0) | |
| ≥800 | 35 (20.1) | 8 (10.8) | 27 (27.0) | |
| No. full-time cardiologists | 0.008 | |||
| 1–5 | 41 (23.6) | 26 (35.1) | 15 (15.0) | |
| 6–10 | 49 (28.2) | 17 (23.0) | 32 (32.0) | |
| 11–20 | 43 (24.7) | 19 (25.7) | 24 (24.0) | |
| 21–30 | 17 (9.8) | 8 (10.8) | 9 (9.0) | |
| 31–40 | 15 (8.6) | 3 (4.1) | 12 (12.0) | |
| ≥41 | 9 (5.2) | 1 (1.4) | 8 (8.0) | |
| Annual no. patients hospitalized with AHF | 0.453 | |||
| <100 | 24 (13.8) | 11 (14.9) | 13 (13.0) | |
| 100–199 | 58 (33.3) | 30 (40.5) | 28 (28.0) | |
| 200–299 | 50 (28.7) | 16 (21.6) | 34 (34.0) | |
| 300–399 | 28 (16.1) | 12 (16.2) | 16 (16.0) | |
| 400–499 | 9 (5.2) | 3 (4.1) | 6 (6.0) | |
| ≥500 | 5 (2.9) | 2 (2.7) | 3 (3.0) | |
| Treating COVID-19 patients | 135 (77.6) | 54 (73.0) | 81 (81.0) | 0.284 |
| No. COVID-19 patients treated | <0.001 | |||
| 0 | 43 (24.7) | 28 (37.8) | 15 (15.0) | |
| 1–4 | 40 (23.0) | 23 (31.1) | 17 (17.0) | |
| 5–9 | 33 (19.0) | 12 (16.2) | 21 (21.0) | |
| 10–29 | 40 (23.0) | 8 (10.8) | 32 (32.0) | |
| ≥30 | 18 (10.3) | 3 (4.1) | 15 (15.0) | |
| No. patients with COVID-19 concomitant with HF who were treated |
<0.001 | |||
| 0 | 129 (74.1) | 69 (93.2) | 60 (60.0) | |
| 1–4 | 39 (22.4) | 5 (6.8) | 34 (34.0) | |
| 5–9 | 3 (1.7) | 0 (0.0) | 3 (3.0) | |
| 10–29 | 2 (1.1) | 0 (0.0) | 2 (2.0) | |
| ≥30 | 1 (0.6) | 0 (0.0) | 1 (1.0) | |
| Procedures performed in AHF patients before the COVID-19 pandemic | ||||
| NIV | 167 (96.0) | 72 (97.3) | 95 (95.0) | 0.710 |
| HFNC | 131 (75.3) | 52 (70.3) | 79 (79.0) | 0.253 |
Unless indicated otherwise, data are presented as n (%). AHF, acute heart failure; HFNC, high-flow nasal cannula; NIV, non-invasive ventilation.
Figure 1 shows the perceived difficulty in the management of patients with AHF in whom NPPV or HFNC is indicated during the COVID-19 pandemic, graded using a five-point rating scale (not difficult at all, not very difficult, neither yes nor no, fairly difficult, and very difficult). More than 60% of institutions replied that the management of such patients during the pandemic is fairly or very difficult. Moreover, institutions in alert areas considered the management of such patients significantly more difficult than those in non-alert areas (P=0.004).

Distribution of responses to the question “How much more difficult is it to manage patients with acute heart failure with indication for NIV/HFNC compared with before the COVID-19 pandemic”? NIV, non-invasive ventilation; HFNC, high-flow nasal cannula.
Figure 2 shows the proportion of institutions that changed their clinical practice approach with regard to NPPV or HFNC use in patients with AHF during the COVID-19 pandemic. Overall, 61.7% and 58.8% of institutions changed their indications for NPPV and HFNC, respectively. Significantly more institutions in the alert area indicated that they had changed their practice of using NPPV and HFNC following the onset of the COVID-19 pandemic (P=0.004 and P=0.002, respectively). More specific policies in each institution regarding the use of NPPV and HFNC under different circumstances are shown in Figure 3. Approximately 80% and 60% of institutions responded that they would not use NPPV or HFNC, respectively, for patients with AHF who had confirmed or suspected SARS-CoV-2 infection. In cases where HCPs had insufficient time and/or information, approximately 40% of all institutions reported that they would not use NPPV or HFNC. However, approximately 50% and 40% of institutions responded that they would use NPPV and HFNC, respectively, for patients with AHF without suspected COVID-19. There were no significant intergroup differences in the use of NPPV and HFNC under any of the circumstances between institutions in alert and non-alert areas, except for cases where insufficient time or information was available. When there was insufficient time or information to determine whether patients with AHF may have concomitant COVID-19, institutions in the alert area were significantly more likely to refrain from using NPPV and HFNC than those in non-alert areas. One institution treated a patient with AHF suspected of having concomitant COVID-19 who later tested positive for SARS-CoV-2 after being treated with NPPV or HFNC, and three institutions received patients with AHF without suspected COVID-19 who subsequently tested positive for SRAS-CoV-2 after being treated with NPPV and HFNC. All four of these institutions were in the alert area.
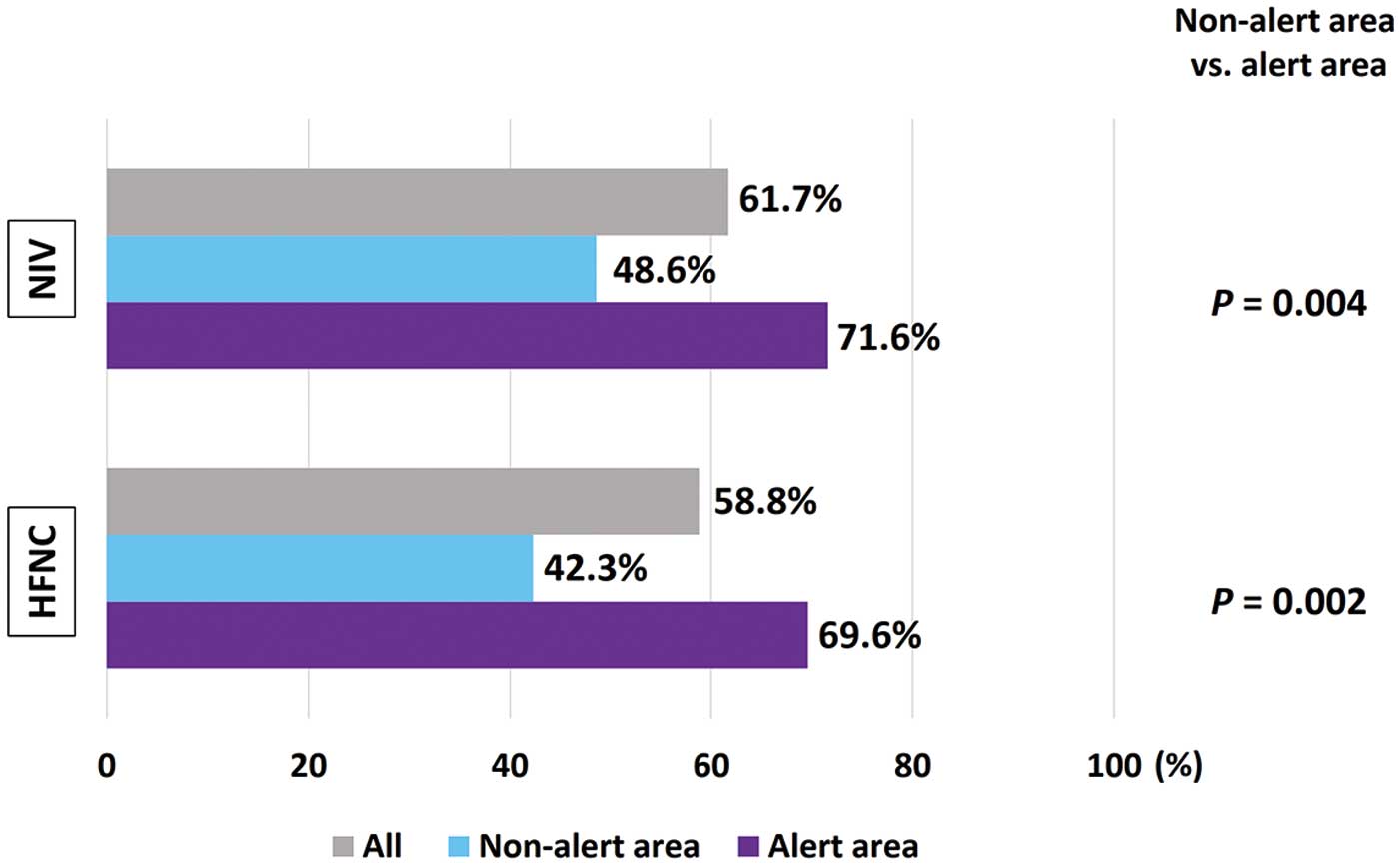
Proportion of institutions that changed their clinical practice with regard to the use of non-invasive ventilation (NIV) and high-flow nasal cannula (HFNC) in patients with acute heart failure after the outbreak of the COVID-19 pandemic, as well as a comparison of alert and non-alert areas.
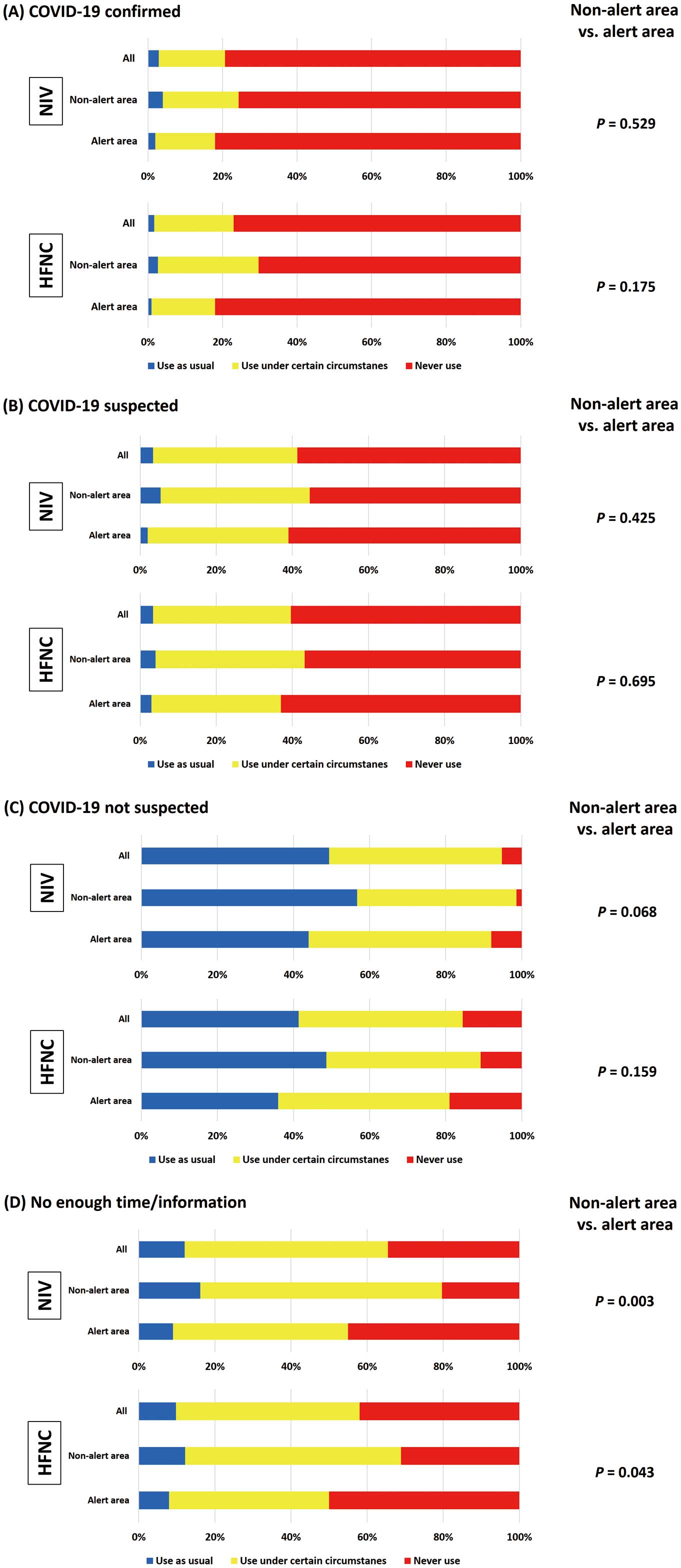
Policies regarding the use of non-invasive ventilation (NIV) and high-flow nasal cannula (HFNC) under four different scenarios: (A) acute heart failure (AHF) patients with confirmed COVID-19 infection; (B) AHF patients with possible concomitant COVID-19 infection; (C) AHF patients unlikely to have COVID-19; and (D) AHF patients presenting under circumstances where physicians have insufficient time or information to gauge the possibility of COVID-19 infection.
Of the 131 institutions with experience of COVID-19 patients, 45 (34.4%) had at least one COVID-19 patient with heart failure. In particular, institutions that received a greater number of COVID-19 patients were more likely to have experienced cases of patients with concomitant COVID-19 and heart failure. This finding implies that COVID-19 is not a rare complication for patients with heart failure, and this inference is supported by data from Italy, which indicates that 21.2% (21/99) of all patients with confirmed COVID-19 had concomitant heart failure.13 Moreover, the acute respiratory management of patients with AHF and potential concomitant COVID-19 is clinically important given that hypoxemic respiratory failure is a common presentation for both AHF and COVID-19.14 Indeed, a study among COVID-19 patients demonstrated that supplemental oxygen therapy was needed for 41% of hospitalized patients and 70% of patients with severe symptoms.15
Approximately half the institutions indicated that they had changed their approach to the use of NPPV and HFNC in patients with AHF following the outbreak of the COVID-19 pandemic. This is not surprising given that NPPV and HFNC are potentially aerosol-generating procedures that may create a serious biohazard when used in the emergency department and/or intensive care unit despite the uncertain likelihood of COVID-19. Generally, HFNC is considered a relatively low-risk procedure in terms of the risk for nosocomial infection compared with NPPV.16,17 The Society of Critical Care Medicine guidelines recommend HFNC over NPPV for patients with COVID-19 and acute hypoxemic respiratory failure.18 However, in the present study, a similar proportion of institutions reported changing the way they used NPPV and HFNC. Similarly, the use of NPPV and HFNC did not differ among the four different scenarios (i.e., AHF patients with confirmed, suspected, or unlikely COVID-19 infection, and in those with no time to determine information). This possibly highlights the fact that HCPs do not necessarily think that HFNC should be prioritized over NPPV in hypoxic patients with heart failure during the COVID-19 pandemic. However, the data in the present study are not sufficient to make recommendations regarding the use of HFNC and NPPV for COVID-19 patients, and future studies that focus on the safety and efficacy of these respiratory therapies are needed.
Geographical Differences in Respiratory Management of AHF During the COVID-19 PandemicIn this study, compared with hospitals in the non-alert zone, hospitals in the alert zone treated more COVID-19 patients and COVID-19 patients with heart failure, and they had to change their approach to the use of NPPV and HFNC during the COVID-19 pandemic. A higher proportion of institutions in the alert area was less likely to use NPPV and HFNC than in the non-alert area, whereas significantly more institutions in the alert area reported a hesitancy to use NPPV and HFNC if there was insufficient time to obtain patient information prior to treatment. This suggests that HCPs are changing their attitude towards NPPV and HFNC use in accordance with the severity of the pandemic in their area, especially when they do not have sufficient information or time to estimate the likelihood that the patient has COVID-19. This is reasonable behavior and, indeed, all four patients who were confirmed to have COVID-19 after being treated with NPPV or HFNC were from institutions in the alert area. However, this may imply that the institutions in the pandemic hotspots always hesitated to use NPPV and HFNC for most AHF patients given the absence of an established, quick diagnostic test or strategy with acceptable sensitivity to exclude the possibility of COVID-19 and the uncertainty associated with the safety of NPPV and HFNC use in these patients. Thus, the development of a rapid test that is capable of excluding the possibility of COVID-19 and/or clarifying the risk for nosocomial infection with NPPV and HFNC use in COVID-19 patients are important for the development of better treatment strategies for patients with hypoxic respiratory failure.
Study LimitationsThis study has several limitations that need to be acknowledged. First, the questionnaire was sent to a limited number of institutions, which could have introduced selection bias. Second, a response bias needs to be considered; the institutions that responded to the survey do not necessarily represent hospitals treating COVID-19 patients in Japan because institutions that are more actively treating COVID-19 patients may have been more likely to respond to the questionnaire or, in contrast, institutions may not have responded because they were busy with high patient loads and had no time.
The COVID-19 pandemic induced HCPs to change their respiratory management strategy for patients with AHF, and many HCPs encountered difficulties treating patients with AHF, even if the patients were not confirmed to have COVID-19.
We would like to express our special thanks of gratitude to the office staff of the Japanese Heart Failure Society for their technical support.
This work was supported in the conduct of medical writing and manuscript submission, which was funded by the Japanese Heart Failure Society.
Y.M. is affiliated with a department endowed by Philips Respironics, ResMed, and Fukuda Denshi, and has received remuneration from Otsuka Pharmaceutical Co.
Y.K. and K.Y. have received scholarship funds from Fukuda Denshi.
H.T. has acted as a consultant for Nippon Boehringer Ingelheim Co., Ltd., Bayer Yakuhin, Ltd., Novartis Pharma K.K., and Ono Pharmaceutical Co., Ltd. and has has received: remuneration from MSD K.K., Astellas Pharma Inc., Pfizer Japan Inc., Bristol-Myers Squibb Company, Otsuka Pharmaceutical Co., Ltd., Daiichi Sankyo Co., Ltd., Mitsubishi Tanabe Pharma Corporation, Nippon Boehringer Ingelheim Co., Ltd., Takeda Pharmaceutical Company Limited, Bayer Yakuhin, Ltd., Novartis Pharma K.K., Kowa Pharmaceutical Co. Ltd., and Teijin Pharma Ltd.; manuscript fees from Medical View and Nippon Rinsho; research funding from Actelion Pharmaceuticals Japan Ltd., Mitsubishi Tanabe Pharma Corporation, Nippon Boehringer Ingelheim Co., Ltd., Daiichi Sankyo Co., Ltd., IQVIA Services Japan, and Omron Healthcare; and scholarship funds from Astellas Pharma Inc., Novartis Pharma K.K., Daiichi Sankyo Co., Ltd., Takeda Pharmaceutical Company Limited, Mitsubishi Tanabe Pharma Corporation, Teijin Pharma Ltd., and MSD K.K.
The other authors have nothing to declare.
This study was approved by the Ethics Committee of Juntendo University (No. 2020095).
The deidentified participant data will not be shared because the data contain information on a sensitive issue (COVID-19).