Article ID: CR-21-0015
Article ID: CR-21-0015
Background: Abnormal diffuse coronary artery contraction is not easily diagnosed. In order to evaluate its true risk, we performed double left ventriculography (LVG) before and after intracoronary administration of isosorbide dinitrate (ISDN). We also investigated the relationship between changes in coronary lumen area and changes in left ventricular ejection fraction (LVEF) after ISDN.
Methods and Results: The study included 53 patients who underwent an acetylcholine (ACh) provocation test after coronary angiogram and LVG. The second LVG was performed after intracoronary ISDN administration. Coronary lumen area was measured by quantitative coronary arteriography (QCA). Simple and multiple regression analyses showed a significant correlation between changes in total QCA area before and after ISDN administration (pre-and post-total QCA area, respectively) and changes in LVEF. Using structural equation modeling, we observed a negative effect of pre-total QCA area and a positive effect of post-total QCA area on LVEF improvement. Importantly, LVEF improvement was similar between the ACh-positive and -negative groups on the coronary artery spasm test. Receiver operating characteristic curves indicated that the cut-off value at which changes in total QCA area affected changes in LVEF was 5%.
Conclusions: Performing double LVG tests before and after ISDN administration may detect myocardial ischemia caused by diffuse coronary artery contraction. The addition of this method to the conventional ACh provocation test may detect the presence of local and/or global myocardial ischemia.
Coronary artery spasm (CAS) is a condition that causes not only angina pectoris, but also myocardial infarction, arrhythmia, and sudden death.1–6 CAS is especially common among Asians, including Japanese.6,7 Although the diagnosis of CAS can be confirmed by determining changes in the electrocardiogram (ECG) during an attack, it is not always easy in actual clinical practice. However, as reported by Yasue et al, intracoronary administration of acetylcholine (ACh) in cardiac catheterization makes a definite diagnosis possible.8 Currently, there are detailed guidelines regarding the diagnosis of CAS following induction drugs such as ACh.9,10 These guidelines provide strictly defined diagnostic criteria for CAS; specifically, CAS is a transient total occlusion of the coronary arteries or subtotal occlusion exceeding 90% stenosis with signs of myocardial ischemia (anginal pain and ischemic ECG changes). A spasm that occurs in 1 isolated coronary segment is defined as focal CAS, whereas spasms occurring in 2 or more adjacent coronary segments are defined as diffuse CAS.9,10 However, there is no definite consensus as to the definition of ‘diffuse’ CAS.
Unmet Needs for Assessing Abnormal Diffuse Coronary Artery Contraction While Defining Diagnostic Criteria for CASAlthough the contraction has not reached 90% at any site in the coronary arteries, there are many cases in which the coronary artery is not normal and is mildly contracted diffusely. In such cases, coronary blood flow through all coronary arteries may decrease, but it is difficult to prove abnormal coronary artery contraction unless the ACh provocation test diagnoses CAS. We believe that the current diagnostic criteria based on the ACh provocation test alone in the current guidelines may underestimate some types of CASs. Therefore, a system is needed that evaluates the relationship between coronary artery contraction and cardiac ischemia from a new perspective.
Double Left Ventriculography (LVG) Before and After Intracoronary Administration of NitratesDiffuse coronary artery contractions that can induce ischemia probably impair cardiac function. It is presumed that the myocardium hibernates due to repeated coronary artery contraction.11–14 In such cases, nitrates may be effective in improving myocardial hibernation.15–18 This is because cardiac function can be expected to improve by supplying sufficient blood during long-term or repeated ischemic attacks. Based on these findings, we devised a double LVG method before and after intracoronary nitrate administration. In addition, we measured the quantitative coronary angiography (QCA) area before and after intracoronary nitrate administration to establish an association between the degree of coronary artery contraction and decreased cardiac function. We also specifically examined the significance of changes in left ventricular ejection fraction (LVEF) in the evaluation of ischemia. We hypothesized that the addition of the double LVG method to the conventional ACh provocation test may help obtain a more accurate diagnosis of abnormal coronary artery contractions that cause regional and/or global ischemia.
This study included 53 patients (35 men, 18 women) who were admitted to the Jikei University Hospital between July 2009 and July 2017. None of the patients had any significant coronary stenosis and all were tested for CAS with ACh. In addition, LVG was performed twice during cardiac catheterization according to the protocol described below.
This study was approved by the Institutional Review Board of the Jikei University School of Medicine (Tokyo, Japan; IRB no. 25-147[7282]) and complied with the routine ethical regulations of the institution. All clinical investigations were conducted in accordance with the Declaration of Helsinki. A notice about the study design with contact information was also posted in a public location at the Jikei University School of Medicine.
Patient Clinical Characteristics and Blood SamplingInformation regarding clinical characteristics (i.e., age, sex, height, weight, body mass index [BMI], smoking status, oral medication, and the frequency of chest pain during the month prior to the test) was obtained. BMI was calculated as body weight (kg) divided by height squared (m2). Hypertension, diabetes, and dyslipidemia were defined as described previously.19 Blood samples were collected from all patients before the study at the time of cardiac catheterization for determination of blood glucose, HbA1c, serum creatinine (Cr), B-type natriuretic peptide (BNP), and lipids (triglyceride, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol).
Cardiac Catheterization, ACh Provocation Test, and Double LVGIn all patients, coronary dilators were discontinued at least 48 h before the ACh provocation test.
Initially, control coronary angiography and the first LVG were performed, followed by the ACh provocation test for CAS based on the guidelines of the Japanese Circulation Society.10 During the ACh provocation test, ACh was injected in staged doses of 20 and 50 μg for the right coronary artery (RCA) and 20, 50, and 100 μg for the left coronary artery until CAS was provoked. In order to use less contrast medium, biplane coronary angiography was performed. Subsequently, isosorbide dinitrate (ISDN) was administered into each coronary artery, and angiography was re-evaluated while the coronary artery was maximally dilated. Finally, the second LVG was performed. Both the first and second LVG were performed in the 30° right anterior oblique (RAO) view and 60° left anterior oblique (LAO) view using 38–40 mL contrast medium injected at a rate of 8–10 mL/s. Left ventricular end-diastolic volume index (LVEDVI) and left ventricular end-systolic volume index (LVESVI) were measured using the method of Sandler and Dodge.20 The LVEF was calculated as follows:
LVEF = (LVEDV − LVESV) / LVEDV
Measurement of QCA AreaUsing coronary angiography findings at the same angles before and after the administration of the coronary dilator, QCA areas were measured at the same angles for the left anterior descending artery (LAD), left circumflex artery (LCX), and RCA using QAngio XA version 7.3 (Medis Medical Imaging Systems, Leiden, Netherlands). Based on the 15-segment American Heart Association classification,21 Segments 5–8 were defined as the LAD, Segments 11–14 were defined as the LCX, and Segments 1–3 were defined as the RCA because Segments 15 and 4 were absent in some cases. The change in the trace area was measured in the 40° LAO with 30° cranial angulation view (LAD, RCA) and 30° RAO with 30° caudal angulation view (LCX). The total QCA area was the sum of the QCA values for the LAD, LCX, and RCA.
Statistical AnalysisContinuous variables are expressed the mean±SD and categorical variables are expressed as percentages. Comparisons between groups were made using Pearson’s Chi-squared test for categorical variables and the Mann-Whitney U-test or Student’s t-test for continuous variables, as appropriate. To achieve a normal distribution, BNP values were log-transformed before analysis. To assess the dependent determinants of LVEF improvements, simple and multiple regression analyses were performed. Dependent variables were age, BMI, HbA1c, chest pain (times/month), current smoker (dichotomized; 0, ex or non-smokers; 1, current smokers), and a change from pre- to post-total QCA area. In all cases, 2-tailed P<0.05 was considered significant. Analyses were conducted using SPSS Statistics version 25.0 (SPSS Inc., Chicago, IL, USA).
In addition, structural equation modeling was performed in this study. The path diagram in structural equation modeling is devised according to the experience of the researcher and the purpose to be expressed. Strictly speaking, the path diagram does not show a true cause–effect relationship, but it does support the hypothesis of the researchers. In addition, confounding factors can almost be eliminated, and the effects among factors can be expressed as direct and indirect effects. In the present study, structural equation modeling was performed using IBM SPSS AMOS version 25 (Amos Development Corporation, Meadville, PA, USA). In addition, we applied Bayesian structural equation modeling in IBM SPSS AMOS version 25. Frequency polygons were described with the marginal posterior distributions of the estimands. The selected 2-dimensional contour line was used in this study because it is easily visualized.
Receiver operating characteristic (ROC) curves were used to determine the cut-off value (lowest line) for changes in LVEF at which changes in QCA area were significantly affected. To examine the cut-off value for percent change in LVEF, the data were simply stratified by 1%, 3%, 5%, 7%, and 10%. The area under the curve (AUC) was examined by plotting sensitivity and 1−specificity on the vertical and horizontal axes of the ROC curve, respectively.
Using the ACh provocation test, CASs were diagnosed in 36 or 53 patients. The clinical characteristics of patients with positive and negative responces to the ACh provocation test, currently recommended for the diagnosis of CAS10 are presented in Table 1.
| ACh-positive group | ACh-negative group | P value | |
|---|---|---|---|
| No. patients (%) | 36 (67.9) | 17 (32.1) | |
| Age (years) | 55.5±13.2 | 45.9±11.3 | <0.05 |
| Male sex (%) | 24 (66.7) | 11 (64.7) | NS |
| BMI (kg/m2) | 24.0±4.3 | 23.0±6.4 | NS |
| Chest pain attack (/month) | 7.9±12.1 (1.0) | 4.4±7.9 (0.0) | NS |
| Log[BNP] | 1.3±0.6 | 1.2±0.5 | NS |
| Serum creatinine (mg/dL) | 0.81±0.25 | 0.73±0.17 | NS |
| Hemoglobin (mg/dL) | 14.2±1.8 | 14.0±1.7 | NS |
| LDL-C (mg/dL) | 121.9±32.2 | 122.4±35.5 | NS |
| HDL-C (mg/dL) | 57.5±16.2 | 60.6±25.3 | NS |
| HbA1c (%) | 5.9±0.9 | 6.2±1.5 | NS |
| Current smoker (%) | 12 (33.3) | 1 (5.9) | <0.05 |
| Past + current smoker (%) | 22 (61.1) | 5 (29.4) | <0.05 |
| Comorbid diseases (%) | |||
| Diabetes | 6 (16.7) | 3 (17.6) | NS |
| Hypertension | 17 (47.2) | 4 (23.5) | NS |
| Dyslipidemia | 23 (63.9) | 7 (41.2) | NS |
| Medical therapy (%) | |||
| Statins | 12 (33.3) | 2 (11.8) | NS |
| CCB | 15 (41.7) | 3 (17.6) | NS |
| β-blocker | 7 (19.4) | 5 (29.4) | NS |
| AChE | 6 (16.7) | 2 (11.8) | NS |
| ARB | 8 (22.2) | 2 (11.8) | NS |
| Isosorbide dinitrate | 6 (16.7) | 1 (5.9) | NS |
| Nicorandil | 3 (8.3) | 0 (0) | NS |
Results are presented as the mean±SD or as n (%). ACh, acetylcholine; AChE, acetylcholine esterase; ARB, angiotensin receptor blocker; BMI, body mass index; BNP, B-type natriuretic peptide; CCB, calcium channel blocker; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol.
Table 2 presents QCA areas in ACh-positive and ACh-negative groups. The change from pre- to post-total QCA area was significantly larger in the ACh-positive than -negative group. There were no significant differences in pre- and post-total QCA area between the 2 groups.
| ACh-positive (n=36) | ACh-negative (n=17) | P value | |
|---|---|---|---|
| Pre-total QCA area (mm2) | 705.0±135.9 | 731.2±155.4 | NS |
| Post-total QCA area (mm2) | 887.2±166.2** | 864.3±166.9** | NS |
| Change from pre- to post-total QCA area (mm2) | 182.2±84.6 | 133.1±61.1 | <0.05 |
| Pre-total reference length (mm) | 309.3±40.7 | 299.2±37.2 | NS |
| Post-total reference length (mm) | 308.9±40.6 | 298.9±36.7 | NS |
| Change from pre- to post-total reference length (mm) | −0.43±1.9 | −0.27±1.1 | NS |
| Total volume ISDN administered (mL) | 3.8±1.3 | 3.9±1.2 | NS |
| Time from first to second LVG (min) | 48.6±12.1 | 50.5±9.3 | NS |
| Time from final ISDN administration to second LVG (min) |
12.2±5.0 | 12.8±4.5 | NS |
| Pre-LVEDVI (mL/m2) | 66.1±19.5 | 75.9±26.6 | NS |
| Post-LVEDVI (mL/m2) | 55.3±18.4** | 65.6±25.5** | NS |
| Pre-LVESVI (mL/m2) | 30.5±17.3 | 36.1±22.8 | NS |
| Post-LVESVI (mL/m2) | 24.0±17.3** | 30.1±19.8** | NS |
| HR Pre-LVG (/min) | 65.0±10.8 | 67.9±12.8 | NS |
| HR Post-LVG (/min) | 68.6±13.6 | 71.7±14.7 | NS |
| Pre-LVEDP (mmHg) | 17.8±5.6 | 17.1±4.4 | NS |
| Post-LVEDP (mmHg) | 18.6±6.9 | 19.4±8.3 | NS |
| Pre-LVEF (%) | 56.0±12.9 | 55.0±13.3 | NS |
| Post-LVEF (%) | 59.8±15.1** | 56.8±12.4** | NS |
| Post–Pre-LVEF (%) | 3.8±5.7 | 1.9±2.6 | NS |
| Pre-NBP-Sys (mmHg) | 136.8±19.8 | 134.5±19.9 | NS |
| Post-NBP-Sys (mmHg) | 120.7±20.8** | 115.8±12.0** | NS |
Results are expressed as the mean±SD. P values are for comparisons of ACh-positive and -negative groups. Asterisks next to values indicate results of comparisons with pre values (**P<0.01; NS, not significantly different). Total QCA area and total reference length were defined as the area and length, respectively, of the left anterior descending plus left circumflex plus right coronary arteries. ACh, acetylcholine; HR, heart rate; ISDN, isosorbide dinitrate; LVEDP, left ventricular end diastolic pressure; LVEDVI, left ventricular end diastolic volume index; LVEF, left ventricular ejection fraction; LVESVI, left ventricular end systolic volume index; LVG, left ventriculography; NBP, non-invasive blood pressure; QCA, quantitative coronary arteriography; Sys, systolic.
Table 2 also shows the LVG results in the ACh-positive and -negative groups. There were no significant differences in any LVG parameters between the ACh-positive and -negative groups. In particular, there was no significant difference in the change from pre- to post-LVEF between the 2 groups.
Univariate and Multivariate Analyses of Clinical Factors Affecting Changes in LVEFResults of univariate regression analysis of the degree of involvement of age, BMI, HbA1c, chest pain frequency, current smoking, and change from pre- to post-total QCA area as explanatory variables for changes in LVEF are summarized in Table 3. A significant correlation was only found between changes in pre- to post-LVEF and the change from pre- to post-total QCA area, as shown in Figure 1.
| Dependent variable | Univariate regression analysis | Multiple regression analysis (R2=0.252) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| β | St. β | 95% CI | P value | β | St. β | 95% CI | P value | VIF | |
| Age | 0.101 | 0.267 | −0.002, 0.203 | 0.054 | 0.071 | 0.188 | −0.029, 0.171 | 0.160 | 1.059 |
| Body mass index | −0.207 | −0.210 | −0.479, 0.065 | 0.132 | −0.141 | −0.143 | −0.418, 0.135 | 0.309 | 1.185 |
| HbA1c | −0.292 | −0.066 | −1.530, 0.945 | 0.637 | 0.221 | 0.050 | −1.034, 1.477 | 0.724 | 1.224 |
| Chest pain (/month) | 0.079 | 0.174 | −0.047, 0.205 | 0.212 | 0.022 | 0.048 | −0.102, 0.145 | 0.725 | 1.115 |
| Current smoker | 1.437 | 0.125 | −1.779, 4.653 | 0.374 | 1.267 | 0.110 | −1.727, 4.261 | 0.399 | 1.020 |
| Change from pre- to post-total QCA areaA |
0.027 | 0.429 | 0.011, 0.042 | 0.001 | 0.022 | 0.352 | 0.004, 0.040 | 0.018 | 1.257 |
| Constant | – | – | – | – | −2.589 | – | −12.807, 7.628 | 0.612 | |
ATotal quantitative coronary angiography (QCA) area was defined as the area of the left anterior descending plus left circumflex plus right coronary arteries. β, regression coefficient; CI, confidence interval; LVEF, left ventricular ejection fraction; St. β, standardized regression coefficient; VIF, variance inflation factor.

Correlation between the change from pre- to post-total quantitative coronary arteriography (QCA) area and the change from pre- to post-left ventricular ejection fraction (LVEF). Total QCA area was defined as the area of the left anterior descending (LAD) plus left circumflex (LCX) plus right coronary (RCA) arteries.
Multiple regression analysis of the same clinical factors also showed a significant correlation between the change from pre- to post-total QCA area and change from pre- to post-LVEF (Table 3).
Structural Equation Modeling Path Diagram and Bayesian Estimation AnalysisThe theoretical and simple path diagram we constructed is shown in Figure 2A. The relationship between pre- and post-total QCA areas and the effects of each value on the change from pre- to post-LVEF were included in the equation. Table 4 presents the results of structural equation modeling. Pre-total QCA area had a positive effect on post-total QCA area. Pre-total QCA area had a negative effect, whereas post-total QCA area had a positive effect, on LVEF improvement.
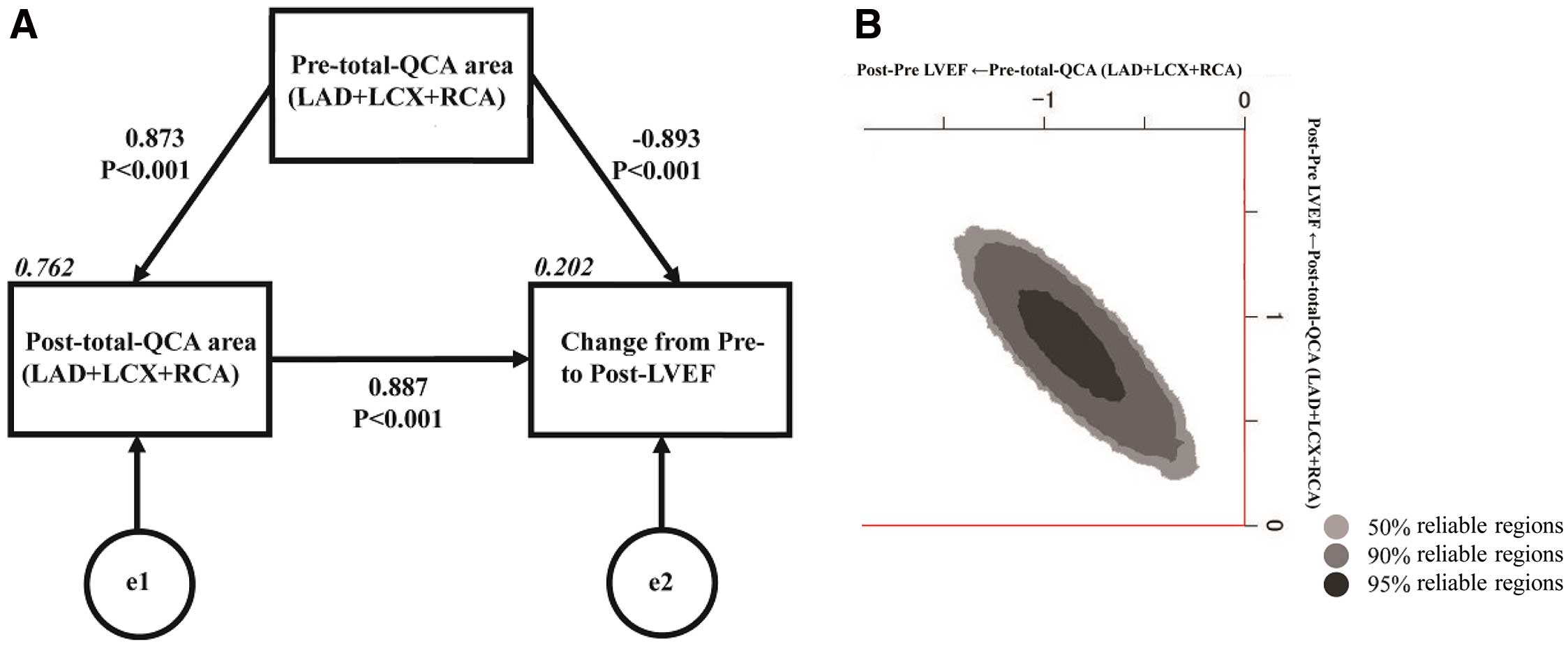
(A) Path model based on structural equation modeling and (B) Bayesian estimation in structural equation modeling. (A) An explanatory drawing of the possible cascade from the values of pre-total quantitative coronary arteriography (QCA) area and post-total QCA area to the change from pre- to post-left ventricular ejection fraction (LVEF). Each path has a coefficient showing the standardized coefficient of a regressing independent variable on a dependent variable of the relevant path. These variables indicate standardized regression coefficients (direct effect), squared multiple correlations (italics), and correlations among exogenous variables. Total QCA area was defined as the area of the left anterior descending (LAD) plus left circumflex (LCX) plus right coronary (RCA) arteries. (B) Frequency polygons described by marginal posterior distributions of the estimates. The 2-dimentional plot of the bivariate posterior density shows the relationship between the bivariate marginal posterior plots.
| Clinical factor | Estimate | SE | Test statistic |
P value | Standard regression coefficient | ||
|---|---|---|---|---|---|---|---|
| Direct effect |
Indirect effect |
Total effect |
|||||
| Pre-total QCA area | |||||||
| → Post-total QCA area | 1.019 | 0.079 | 12.894 | <0.001 | 0.873 | 0 | 0.873 |
| → Change from pre- to post-LVEF | −0.032 | 0.009 | −3.519 | <0.001 | −0.893 | 0.774 | −0.120 |
| Post-total QCA area | |||||||
| → Change from pre- to post-LVEF | 0.027 | 0.008 | 3.493 | <0.001 | 0.887 | 0 | 0.887 |
Total quantitative coronary angiography (QCA) area was defined as the area of the left anterior descending plus left circumflex plus right coronary arteries. LVEF, left ventricular ejection fraction.
In addition, we used Bayesian structure equation modeling and created frequency polygons with a marginal posterior distribution of the estimates. As can be seen from the 2-dimensional contour line (Figure 2B), pre- and post-total QCA area had a significant effect on the change from pre- to post-LVEF by reversing the positive and negative signs.
ROC Curve Analysis to Determine LVEF Cut-Off ValueThe results of ROC curve analysis (Figure 3) suggested that if the change from pre- to post-LVEF was >5%, the change from pre- to post-QCA may significantly affect the change from pre- to post-LVEF.

Determination of left ventricular ejection fraction (LVEF) cut-off values by receiver operating characteristic curve analysis. If the change in LVEF exceeds 5%, changes in quantitative coronary arteriography may have a significant effect on changes in LVEF. AUC, area under the curve.
In this study, the change from pre- to post-QCA was significantly correlated with the change from pre- to post-LVEF. This means that higher degree of basal tonus enhancement across the coronary arteries caused myocardial ischemia and reduced cardiac systolic function. Without the double LVG test, we may have missed the abnormal diffuse coronary constriction that needed treatment. Even if CAS as defined by the ACh provocation test in the guidelines is not proved, this phenomenon would be synonymous with the clinical significance of CAS.
Implications of Structural Equation Modeling and Bayesian EstimationThe path diagram constructed using structural equation modeling indicates that the pre- and post-total QCA areas independently affect the change from pre- to post-LVEF. Above all, it is clinically important that the pre-total QCA area alone affects the change from pre- to post-LVEF.
Clinical Significance of LVEF Improvement ≥5% After Intracoronary ISDNBecause the change from pre- to post-QCA area was significantly correlated with the change from pre- to post-LVEF, it is of clinical relevance to know the extent to which the change from pre- to post-LVEF is affected by the change from pre- to post-QCA area. As indicated by the AUC determined by ROC curve analysis, the cut-off change in LVEF was approximately 5%. That is, if the LVEF after ISDN administration exceeds 5%, it is possible that abnormal coronary artery contraction causing ischemia has occurred.
Classification Using Conventional ACh Provocation Test and Double LVG MethodBased on the results of the present study, the following 4 groups (A–D) can be defined combining the ACh provocation test and the proposed double LVG method (Figure 4):

Classification using the conventional acetylcholine (ACh) provocation test and the double left ventriculography (LVG) method. Four groups (A–D) for the diagnosis of coronary artery spasm (CAS) are formed by combing the results of the ACh provocation test and the proposed double LVG method. Of these groups, Group C should not be overlooked, and Group D is a severe CAS group.
• Group A: negative ACh provocation test and low (<5%) LVEF improvement. These findings suggest that coronary tonus is not so high locally or across all coronary arteries
• Group B: positive ACh provocation test and low (<5%) LVEF improvement. These findings indicate that only local CAS may be present; in this case, continuous treatment of CAS is required. In addition, the presence of another pathological condition should be considered. If there is a persistent decrease in wall motion that remains unchanged before and after the administration of nitroglycerin, strong or repeated CAS may have caused myocardial stunning.22–26 If so, immediate improvements in LVEF with the administration of nitrates cannot be expected
• Group C: negative ACh provocation test and large (>5%) improvement in LVEF. These results indicate that myocardial ischemia is caused by abnormal diffuse coronary artery contraction. Findings indicative of Group C are of great clinical importance. Although these findings do not meet the conventional CAS diagnostic criteria in the current guidelines, they do indicate dangerous coronary artery contraction causing widespread myocardial ischemia
• Group D: positive ACh provocation test and large (>5%) improvement in LVEF. These findings indicate that the presence of both abnormal diffuse coronary artery contraction and regional CAS should be considered. These patients are at risk of high CAS activity.
Study LimitationsThe influence of other factors on the outcomes needs to be discussed. First, coronary administration of ISDN affects systemic hemodynamics: the decrease in blood pressure may reduce afterload and improve LVEF. However, we think that this effect may be small because the amount of ISDN administered was very low and LVEF did not improve in all patients after intracoronary ISDN administration. Second, it is strongly suspected that myocardial ischemia, which decreases cardiac function, was involved in the group in which ISDN administration improved cardiac function, especially Group C (Figure 4). However, we did not conduct any additional testing to support our proposal, and this is an issue that needs to be considered in the future. Third, the present study was conducted on a relatively small number of patients. However, we think that this limitation was addressed, in part, by our use of Bayesian estimation. Nevertheless, it may be necessary to re-evaluate the results with more cases in the future.
Performing double LVG tests before and after intracoronary ISDN administration may detect myocardial ischemia caused by diffuse coronary artery contraction. With the addition of this method to the conventional ACh provocation test, the presence of local and/or global myocardial ischemia can be detected.
The authors thank Kumiko Nishiyama for her considerable contribution to data collection. The authors also thank Elsevier Language Editing Services for English language editing this manuscript.
This study did not receive any specific funding.
The authors declare that there is no conflict of interest to disclose directly related to this study. Outside this study, M.Y. reports lecture fees from Mochida Pharmaceutical Co., Ltd., Daiichi Sankyo Co., Ltd., Pfizer Japan Inc., and Kowa Co., Ltd., grants and lecture fees from Mitsubishi Tanabe Pharma Corporation, and grants from Teijin Pharma Ltd., Astellas Pharma Inc., and Shionogi & Co., Ltd. M.Y. is a member of Circulation Reports’ Editorial Team.
This study was approved by the Institutional Review Board of Jikei University School of Medicine (Tokyo, Japan; IRB no. 25-147[7282]).
The deidentified participant data will not be shared.