Abstract
Background:
Identifying risk factors for cancer therapeutics-related cardiac dysfunction (CTRCD) is essential for the early detection and prompt initiation of medial therapy for CTRCD. No study has investigated whether the sigmoid septum is a risk factor for anthracycline-induced CTRCD.
Methods and Results:
We enrolled 167 patients with malignant lymphoma who received a CHOP-like regimen from January 2008 to December 2017 and underwent both baseline and follow-up echocardiography. Patients with left ventricular ejection fraction (LVEF) ≤50% were excluded. CTRCD was defined as a ≥10% decline in LVEF and LVEF <50% after chemotherapy. The angle between the anterior wall of the aorta and the ventricular septal surface (ASA) was measured to quantify the sigmoid septum. CTRCD was observed in 36 patients (22%). Mean LVEF and global longitudinal strain (GLS) were lower, left ventricular mass index was higher, and ASA was smaller in patients with CTRCD. In a multivariable Cox proportional hazard analysis, GLS (hazard ratio [HR] per 1% decrease 1.20; 95% confidence interval [CI] 1.07–1.35) and ASA (HR per 1° increase 0.97; 95% CI 0.95–0.99) were identified as independent determinants of CTRCD. An integrated discrimination improvement evaluation confirmed the significant incremental value of ASA for developing CTRCD.
Conclusions:
Smaller ASA was an independent risk factor and had significant incremental value for CTRCD in patients with malignant lymphoma who received the CHOP-like regimen.
Anthracycline is an effective chemotherapeutic agent for several kinds of malignant tumors, particularly in patients with malignant lymphoma (ML) and breast cancer.1–3
In contrast, anthracycline-induced cancer therapeutics-related cardiac dysfunction (CTRCD), defined by a decline in left ventricular ejection fraction (LVEF), often causes poor clinical outcomes and low quality of life in patients with cancer.4–9
Identifying baseline risk factors for CTRCD is vital for the early detection of CTRCD and prompt initiation of medical therapy, which are essential for improving left ventricular (LV) contraction after CTRCD develops.10–13
Several baseline risk factors have been identified in previous studies, including baseline LVEF, global longitudinal strain (GLS), age, general cardiovascular risk factors, and a history of cardiovascular disease.4,11,14–16
Recently, LV hypertrophy was recognized as a risk factor for CTRCD in ML patients,17
suggesting LV morphologic remodeling also represents an accumulated myocardial injury that may result in vulnerability to CTRCD. Conversely, the sigmoid septum, known as a synonym of discrete upper septal thickening, is considered one type of LV morphologic remodeling. A previous study reported that the sigmoid septum was significantly related to LV diastolic dysfunction.18
However, no one has clarified the association of the sigmoid septum with the incidence of CTRCD.
In the present study we focused on the presence of the sigmoid septum and aimed to reveal its impact on anthracycline-induced CTRCD in patients with ML who were treated with a CHOP-like (cyclophosphamide + doxorubicin + vincristine+prednisolone±rituximab) regimen.
Methods
Study Population
Data for all ML patients who were treated with a CHOP-like regimen between January 2008 and December 2017 at Nagoya City University Hospital were reviewed retrospectively. The dosing and schedule for the CHOP-like regimen were standardized in this cohort. From this group, patients who underwent a baseline echocardiographic examination and ≥1 follow-up examination were enrolled in the study (Figure 1). Patients with an LVEF <50% at baseline but no echocardiographic images of adequate quality were excluded. The present study is a subanalysis of a previous study in which we described the clinical features of anthracycline-induced CTRCD.19
Study Protocol
Patients’ clinical characteristics, including age, sex, body mass index, general cardiovascular risk factors, past history, laboratory data, chemotherapeutic regimen, performance status, and echocardiographic parameters, were retrospectively collected from their medical records. The chemotherapeutic regimen, performance status, and dose of anthracycline were assessed by 2 hematologists. Patients with intravascular large B-cell lymphoma (IVL) and primary mediastinal large B cell lymphoma (PMLBCL) were enrolled in the present study as a subtype of diffuse large B-cell lymphoma (DLBCL). Echocardiographic parameters and the incidence of symptomatic heart failure and cardiovascular death were obtained and assessed by 2 cardiologists in a blinded manner. In the present study, CTRCD was defined as a decline in LVEF of ≥10% from baseline and LVEF <50% in the follow-up study.9
Cardiovascular risk factors were defined as hypertension, dyslipidemia, diabetes, and a history of smoking. Each cardiovascular risk factor was defined using the following criteria: hypertension was defined as a history of hypertension or being on antihypertensive therapy; dyslipidemia was defined as a history of dyslipidemia, serum low-density lipoprotein cholesterol ≥140 mg/dL, or being on cholesterol-lowering treatment; and diabetes was defined as a history of diabetes, HbA1c ≥6.5%, or being on blood glucose-lowering therapy. A history of ischemic heart disease was defined as a history of myocardial infarction, angina pectoris, or coronary artery revascularization.
This study was approved by the Ethics Committee of Nagoya City University (No. 60-19-125) and was performed in accordance with the principles of the Declaration of Helsinki.
Echocardiography
Echocardiographic data were measured according to the recommendations of the American Society of Echocardiography.20
However, avoiding the region of the sigmoid septum, the LV end-diastolic diameter (LVDd), interventricular septal wall thickness (IVSd), and posterior wall thickness (PWd) were measured at a mid-ventricular position. LVEF was calculated by the disk summation method using the TomTec Imaging System (Munich, Germany). To avoid a misdiagnosis of CTRCD, LVEF that had been measured as between 40% and 60% by one measurer was re-evaluated by a second measurer to assess the accuracy of diagnosis. LV wall thickness was defined as the mean IVSd and PWd. The LV mass index (LVMi) was calculated using the following formula:10
LVMi = 1.04 × [(LVDd + IVS + PWd)3 − LVDd3] × 0.8 + 0.6
Valvular diseases were graded according to the guidelines of the Japanese Circulation Society.21
As described in previous studies that investigated the sigmoid septum,18,22–26
we calculated the ratio of basal to mid-interventricular septal diameters (“B-M ratio”). We also measured the angle between the anterior wall of the aorta and the ventricular septal surface (i.e., aorto-septal angle [ASA]) during the end diastolic phase to quantify the sigmoid septum (Figure 2). To determine intra- and inter-rater reliability, the ASA was remeasured by the first rater (T.N.) and the second rater (J.Y.) in 30 patients who underwent baseline echocardiography between January 2016 and December 2017.
Statistical Analysis
Continuous variables are expressed as the mean±SD. For comparisons between groups of continuous variables, Welch’s test was used in the case of unequal variances and Student’s t-test was used in other cases. Categorical variables were analyzed using Pearson’s Chi-squared test. Cox proportional hazards analyses were performed to identify risk factors for the primary endpoint. The common logarithmic conversion was used when B-type natriuretic peptide was assessed in Cox proportional hazard analyses. Intra- and inter-rater reliabilities were evaluated with intraclass correlation coefficients (ICC), with an ICC ≥0.80 considered the preferred level of reliability.27
The primary endpoint was the incidence of CTRCD, and the initial time point for each survival analysis was the first day of the CHOP-like regimen. Follow-up censoring of participants with undetected CTRCD was the final echocardiographic examination. Clinical variables recognized as risk factors for CTRCD were analyzed using univariable Cox proportional hazards analyses. The GLS value was converted to an absolute value when assessed in Cox proportional hazard analyses, Kaplan-Meier analyses, and logistic regression analyses. In the first multivariable Cox proportional hazards analysis, echocardiographic parameters with P<0.05 in the univariable analyses were analyzed to clarify the independent echocardiography-derived determinants of the event (Multivariable Analysis 1). Next, the independent echocardiographic parameters were adjusted by the clinical parameters with P<0.05 in the univariable analyses as Multivariable Analysis 2. Multivariable Cox proportional hazard analyses were assessed by the forward stepwise method. The follow-up period varied among patients; therefore, we constructed time-dependent receiver operating characteristic (ROC) curves based on the incidence of the primary endpoint within 4 years from the initial time point, because <10% of patients were followed after 4 years. We used the inverse probability of censoring weighting technique to deal with censoring. SAS analytical software was used to estimate the time-dependent ROC curves and corresponding C-statistics. Youden’s index, derived from ROC curves, was used to determine the cut-off values for risk factors related to the primary endpoint. Event-free survival curves using the cut-off values were constructed by Kaplan-Meier analyses, and the log-rank test was used to assess the significance of differences between curves.
To assess the incremental value of newly recognized risk factors for CTRCD, we defined 2 categorized models: Model 1 included the risk factors that were already considered risk factors in a previous study and were recognized independently for CTRCD in the present study, whereas Model 2 had all independent risk factors identified in the present study, including newly recognized risk factors. First, we compared the C-statistics of each model using SAS. We calculated the area under the curve (AUC) to assess the utility of new risk factors. Second, we used the continuous-net reclassification index (cNRI) and integrated discrimination improvement (IDI) to evaluate the incremental value of risk factors newly identified in the present study.28,29
In these analyses, patients with a probability ≥0.5 in logistic regression analyses in each model were considered to have CTRCD. The R package, survIDINRI was used to estimate the IDI and cNRI to compare the predictive models for the incidence of primary endpoint within 4 years.
Two-sided P<0.05 was considered significant. Analyses were performed using SPSS ver. 26 (IBM Corp., Armonk, NY, USA), SAS ver. 9.4 (SAS Institute, Cary, NC, USA), and R ver. 4.1.0 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Study Population
Of the 443 patients initially enrolled in this study, 14 patients without baseline echocardiography data and 185 patients without follow-up echocardiography data were excluded. Fourteen patients with LVEF <50%, 62 patients whose GLS could not be quantified by tri-plane, and 1 patient without any echocardiographic images available for analysis were also excluded. In the comparison between patients who underwent GLS measurements and those who were excluded because they did not have GLS measurements, there were no significant differences in basic and laboratory data (Supplementary Table 1).
Finally, 167 patients were eligible for inclusion in the present study (Figure 1). CTRCD was observed in 36 patients (22%) during the follow-up period, which was a median of 305 days (interquartile range [IQR] 171–837 days). The mean and median number of follow-up echocardiographic examinations was 3.0±2.6 and 2 (IQR 1–4), respectively. The median interval between the baseline and follow-up echocardiographic studies was 112 days (IQR 50–238 days).
Baseline Characteristics
The baseline characteristics of eligible patients are summarized in
Table 1. No significant differences were found in baseline characteristics between patients with and without CTRCD, except for the prevalence of prior ischemic heart disease. Most patients (83%) received the R-CHOP regimen, with the remaining patients receiving the CHOP regimen. There were 111 patients with DLBCL (67%), including 4 and 2 patients with IVL and PMLBCL, respectively. The mean cumulative dose of doxorubicin was almost within the recommendation of a medical package insert of 291.5±95.3 mg/m2
(Table 1).
Table 1.
Baseline Characteristics
| Characteristic |
Total
(n=167) |
CTRCD |
P value |
Yes
(n=36; 22%) |
No
(n=131; 78%) |
| Basic data |
| Age (years) |
69.1±11.3 |
71.5±8.1 |
68.5±12.0 |
0.072 |
| Male sex |
91 (54) |
21 (58) |
70 (53) |
0.60 |
| BMI (kg/m2) |
20.9±2.3 |
21.2±1.9 |
20.8±2.4 |
0.19 |
| R-CHOP |
139 (83) |
27 (75) |
112 (85) |
0.14 |
| DLBCL |
111 (67) |
25 (69) |
86 (66) |
0.67 |
| Performance status |
|
|
|
0.88 |
| 0 |
33 (20) |
7 (19) |
26 (20) |
|
| 1 |
76 (46) |
14 (39) |
62 (47) |
|
| 2 |
37 (22) |
10 (28) |
27 (21) |
|
| 3 |
13 (8) |
3 (8) |
10 (8) |
|
| 4 |
8 (5) |
2 (6) |
6 (5) |
|
| Doxorubicin dose (mg/m2) |
291.5±95.3 |
298.7±97.1 |
289.5±95.1 |
0.95 |
| Cardiovascular risk factors |
| Hypertension |
58 (35) |
13 (36) |
45 (34) |
0.84 |
| Dyslipidemia |
50 (30) |
11 (31) |
39 (30) |
0.93 |
| Diabetes |
30 (18) |
6 (17) |
24 (18) |
0.82 |
| Smoking |
68 (41) |
19 (53) |
49 (37) |
0.10 |
| History of cardiovascular disease |
| Ischemic heart disease |
7 (4) |
5 (14) |
2 (2) |
0.001 |
| Heart failure |
5 (3) |
1 (3) |
4 (3) |
0.93 |
| Atrial fibrillation |
9 (5) |
2 (6) |
7 (5) |
0.96 |
| Cerebral infarction |
9 (5) |
0 (0) |
9 (7) |
0.11 |
| Laboratory measurements |
| Hemoglobin (g/dL) |
11.7±2.2 |
11.9±2.0 |
11.7±2.3 |
0.13 |
| Albumin (g/dL) |
3.5±0.8 |
3.5±0.7 |
3.5±0.8 |
0.21 |
| Serum sodium (mEq/L) |
139.2±3.9 |
139.2±3.4 |
139.2±4.1 |
0.42 |
| eGFR (mL/min/1.73 m2) |
70.8±22.0 |
66.2±22.1 |
72.1±21.8 |
0.67 |
| Total bilirubin (mg/dL) |
0.7±0.7 |
0.7±0.4 |
0.7±0.7 |
0.53 |
| BNP (pg/mL; n=147) |
61.2±109.2 |
81.5±171.2 |
54.9±80.8 |
0.06 |
| Echocardiography |
| LVEF (%) |
64.3±6.2 |
61.6±5.3 |
65.0±6.2 |
0.003 |
| GLS (%) |
−20.0±3.4 |
−18.7±2.9 |
−20.3±3.4 |
0.008 |
| LV diastolic diameter (mm) |
44.7±5.6 |
45.4±5.3 |
44.6±5.7 |
0.40 |
| Valvular disease ≥ moderate |
29 (18) |
7 (19) |
22 (17) |
0.71 |
| LV wall thickness (mm) |
9.5±1.4 |
9.8±1.2 |
9.5±1.4 |
0.23 |
| LV mass index (g/m2) |
94.6±22.4 |
101.1±18.5 |
92.8±23.1 |
0.047 |
| Left atrial diameter (mm) |
33.5±5.8 |
34.1±5.3 |
33.4±5.9 |
0.52 |
| Valsalva sinus diameter (mm) |
31.8±3.7 |
32.4±4.4 |
31.6±3.4 |
0.28 |
| E wave (cm/s) |
68.7±17.7 |
66.4±15.7 |
69.3±18.2 |
0.38 |
| Deceleration time (ms) |
230.1±61.9 |
232.1±67.1 |
229.6±60.6 |
0.83 |
| B-M ratio |
1.28±0.24 |
1.35±0.22 |
1.26±0.24 |
0.029 |
| ASA (°) |
117.8±13.9 |
112.1±14.2 |
119.4±13.4 |
0.005 |
Unless indicated otherwise, data are given as n (%) or the mean±SD. ASA, angle between the anterior-wall of the aorta and the ventricular septal surface; BMI, body mass index; B-M ratio, the ratio of basal to mid interventricular septal diameter; BNP, B-type natriuretic peptide; CTRCD, cancer therapeutics-related cardiac dysfunction; DLBCL, diffuse large B-cell lymphoma; eGFR, estimated glomerular filtration rate; GLS, global longitudinal strain; LV, left ventricular; LVEF, left ventricular ejection fraction; R-CHOP, rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone.
Echocardiographic parameters at baseline for the entire cohort are presented in
Table 1. Mean LVEF was 64.3±6.2% and 29 patients (18%) had moderate or greater valvular disease (aortic regurgitation, n=5; mitral regurgitation, n=6; tricuspid regurgitation, n=23; aortic stenosis, n=1). As a parameter of sigmoid septum, the B-M ratio and ASA were 1.28±0.24 and 117.8±13.9°, respectively (Table 1). The ICCs for the intra- and inter-rater reliabilities were 0.97 and 0.94, respectively.
We found significant differences between patients with and without CTRCD in LVEF (61.6±5.3% vs. 65.0±6.2%, respectively; P=0.003), GLS (18.7±2.9% vs. 20.3±3.4%, respectively; P=0.008), LVMi (101.1±18.5 vs. 92.8±23.1 g/m2, respectively; P=0.047), B-M ratio (1.35±0.22 vs. 1.26±0.24, respectively; P=0.029), and ASA (112.1±14.2° vs. 119.4±13.4°, respectively; P=0.005).
Risk Factors for CTRCD
In the univariable Cox proportional hazard analyses, of the baseline echocardiographic parameters, LVEF, GLS, LVMi, and ASA were significantly associated with the primary endpoint. With regard to clinical baseline characteristics, age, performance status ≥2, and a history of ischemic heart disease were associated with the primary endpoint (Table 2). In Multivariable Analysis 1, GLS (hazard ratio [HR] per 1% decrease 1.19; 95% confidence interval [CI] 1.06–1.33; P=0.003) and ASA (HR per 1° increase 0.97; 95% CI 0.95–0.99; P=0.004) were identified as independent risk factors. Multivariable Analysis 2 identified GLS (HR per 1% decrease 1.20; 95% CI 1.07–1.35; P=0.002), ASA (HR per 1° increase 0.97; 95% CI 0.95–0.99; P=0.003), and a history of ischemic heart disease (HR 5.13; 95% CI 1.94–13.56, P=0.001) as independent risk factors for the primary endpoint (Table 2).
Table 2.
Risk Factors for CTRCD
| Variable |
Univariable analysis |
Multivariable analysis 1 |
Multivariable analysis 2 |
| HR |
95% CI |
P value |
HR |
95% CI |
P value |
HR |
95% CI |
P value |
| Echocardiography |
| LVEF, per 10% decrease |
1.88 |
1.08–3.28 |
0.026 |
– |
– |
– |
|
|
|
| |GLS|, per 1% decrease |
1.17 |
1.05–1.30 |
0.005 |
1.19 |
1.06–1.33 |
0.003 |
1.20 |
1.07–1.35 |
0.002 |
| LV diastolic diameter |
1.01 |
0.94–1.07 |
0.88 |
|
|
|
|
|
|
Valvular disease ≥ moderate
vs. ≤ mild |
1.86 |
0.81–4.26 |
0.14 |
|
|
|
|
|
|
| LV wall thickness |
1.23 |
0.95–1.59 |
0.11 |
|
|
|
|
|
|
LV mass index, per 10-g/m2
increase |
1.17 |
1.00–1.37 |
0.045 |
– |
– |
– |
|
|
|
| Left atrial diameter |
1.02 |
0.96–1.09 |
0.45 |
|
|
|
|
|
|
| Valsalva sinus diameter |
1.02 |
0.93–1.13 |
0.65 |
|
|
|
|
|
|
| E wave, per 10-cm/s increase |
0.98 |
0.79–1.21 |
0.83 |
|
|
|
|
|
|
Deceleration time, per 10-ms
increase |
0.99 |
0.93–1.04 |
0.62 |
|
|
|
|
|
|
| B-M ratio, per 0.1 increase |
1.11 |
0.99–1.25 |
0.081 |
|
|
|
|
|
|
| ASA, per 1° increase |
0.97 |
0.95–0.99 |
0.007 |
0.97 |
0.95–0.99 |
0.004 |
0.97 |
0.95–0.99 |
0.003 |
| Basic data |
| Age, per 10-year increase |
1.46 |
1.01–2.09 |
0.042 |
|
|
|
– |
– |
– |
| Male vs. female |
1.14 |
0.59–2.22 |
0.70 |
|
|
|
|
|
|
| BMI |
1.06 |
0.93–1.22 |
0.39 |
|
|
|
|
|
|
| R-CHOP vs. CHOP |
0.48 |
0.23–1.03 |
0.059 |
|
|
|
|
|
|
| DLBCL vs. non-DLBCL |
1.29 |
0.63–2.62 |
0.49 |
|
|
|
|
|
|
| Performance status ≥2 vs. ≤1 |
1.96 |
1.01–3.82 |
0.047 |
|
|
|
– |
– |
– |
Doxorubicin dose, per
10-mg/m2 increase |
0.99 |
0.95–1.02 |
0.40 |
|
|
|
|
|
|
| Cardiovascular risk factors ≥2 vs. ≤1 |
1.54 |
0.79–3.01 |
0.21 |
|
|
|
|
|
|
History of ischemic heart
disease, yes vs. no |
4.59 |
1.75–12.00 |
0.002 |
|
|
|
5.13 |
1.94–13.56 |
0.001 |
| Laboratory measurements |
| Hemoglobin |
0.98 |
0.85–1.14 |
0.81 |
|
|
|
|
|
|
| Albumin |
0.90 |
0.59–1.39 |
0.64 |
|
|
|
|
|
|
| Serum sodium |
0.94 |
0.85–1.05 |
0.28 |
|
|
|
|
|
|
eGFR, per 10-mL/min/1.73 m2
increase |
0.88 |
0.76–1.02 |
0.081 |
|
|
|
|
|
|
| Total bilirubin |
1.37 |
0.73–2.57 |
0.33 |
|
|
|
|
|
|
| Log[BNP] (n=147) |
1.69 |
0.80–3.56 |
0.17 |
|
|
|
|
|
|
CHOP, cyclophosphamide, doxorubicin, vincristine, and prednisolone; CI, confidence interval; HR, hazard ratio. Other abbreviations as in Table 1.
We constructed ROC curves to determine the abilities of GLS and ASA to predict the incidence of CTRCD and to determine the cut-off value for each. Based on ROC curves, the cut-off values for GLS and ASA were −20.2% and 120°, respectively. The AUC, sensitivity, and specificity were 0.63, 0.73, and 0.60, respectively, for the GLS cut-off value and 0.76, 0.77, and 0.85, respectively, for the ASA cut-off value. The Kaplan-Meier estimate curves for GLS and ASA are shown in
Figure 3, with significant differences observed between each cut-off value.
Incremental Value of Newly Recognized Risk Factor
According to the independent risk factors for CTRCD in this study, we defined Model 1 as consistent with a history of ischemic heart disease and GLS, and Model 2 as consistent with a history of ischemic heart disease, GLS, and ASA. ROC curves for probability in Model 1 and Model 2 are shown in
Figure 4. The C-statistic (AUC) for Model 2 was 0.76, which was superior to the C-statistic of 0.67 for Model 1 (Model 1–Model 2=−0.089; 95% CI −0.21, 0.028). The reclassification tables for Model 1 and Model 2 are presented in
Table 3. The cNRI and IDI were calculated to compare Model 1 and Model 2. The cNRI was 0.50 (95% CI −0.046, 0.80; P=0.066) and the IDI was 0.096 (95% CI 0.005–0.257; P=0.027).
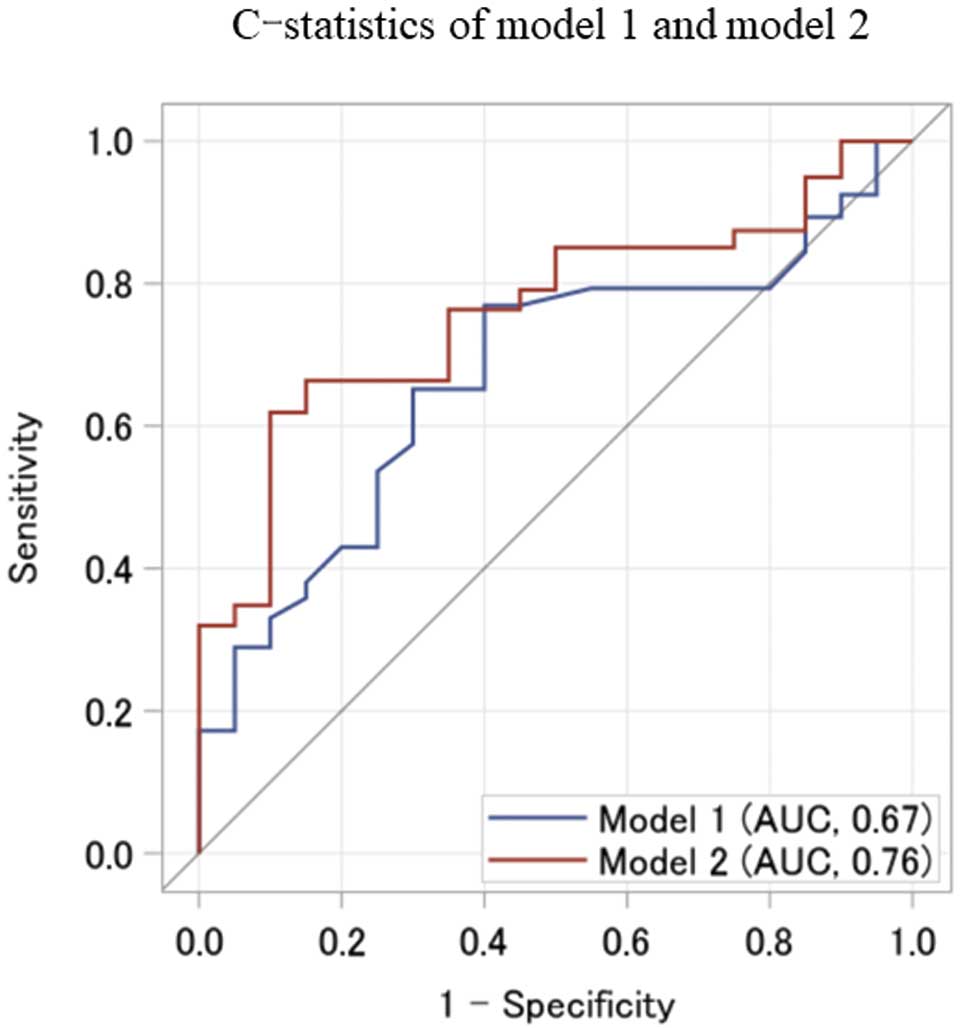
Table 3.
Estimated Reclassification Table From Model 1 and Model 2 at an Arbitrary Cut-off Value of 0.5
| |
Model 2 |
| ≥0.5 |
<0.5 |
Total |
| CTRCD (n=36) |
| Model 1 |
| ≥0.5 |
31.6 |
3.3 |
34.9 |
| <0.5 |
9.3 |
20.0 |
29.3 |
| Total |
40.9 |
23.3 |
64.2 |
| No CTRCD (n=131) |
| Model 1 |
| ≥0.5 |
16.4 |
11.7 |
28.1 |
| <0.5 |
10.7 |
64.0 |
74.7 |
| Total |
27.1 |
75.7 |
102.8 |
Patients with a model probability ≥0.5 are considered to have a high probability of developing cardiotoxicity. CTRCD, cancer therapeutics-related cardiac dysfunction.
Discussion
The present study demonstrated that ASA was an independent risk factor for CTRCD in patients with ML who were treated with a CHOP-like regimen, and that ASA had a significant incremental value to predict the development of CTRCD. Any other clinical characteristics at baseline and the dose of anthracycline were not independent risk factors for CTRCD. Furthermore, when excluding patients with moderate or greater valvular diseases from the analyses, ASA was still an independent risk factor for developing CTRCD (HR per 1° increase 0.95; 95% CI 0.93–0.98; P<0.001).
The relationship between almost all echocardiographic parameters, except LVEF and GLS, and CTRCD has not been examined previously. To the best of our knowledge, this study is the first to detect the impact of morphologic echocardiography parameters on CTRCD. In the present study, a history of ischemic heart disease and lower GLS were risk factors for CTRCD, consistent with previous studies.11,16,30,31
We also found that smaller ASA was a new risk factor for CTRCD.
Smaller ASA/Ventricular Sigmoid Septum
Several terms have been used to describe the sigmoid septum, including discrete upper septal thickening, subaortic ventricular septal bulge, and discrete upper septal knuckle. The ASA has been used previously to quantify the sigmoid septum,18,24–26
as has the B-M ratio.22,23
In the present study, the ICCs for intra- and inter-rater reliabilities were satisfactory, which indicates that the ASA measurement can be considered reliable and reproducible.
In general, the sigmoid septum is frequently observed in the elderly population,32–34
although some investigators have reported this geometric change as part of the spectrum of hypertrophic cardiomyopathy, because of LV outlet obstruction.35–37
Therefore, the sigmoid septum is considered to be the result of aging-related changes associated with aortic atherosclerosis, hypertension, or thickening and calcification of the aortic or mitral valve.38–40
In the present study, higher age and higher body mass index were independently associated with the presence of sigmoid septum, as defined by the cut-off value of the ROC curve for CTRCD, in the logistic regression analysis (Supplementary Table 2). In the present study, in univariable Cox regression analysis, age was one of the factors significantly associated with CTRCD, consistent with previous reports.11
However, after the multivariable analysis, ASA was proven as an independent risk factor for CTRCD, whereas age was not an independent determinant. This result may explain why a visible morphologic change due to aging was a more specific predictor for developing CTRCD than age alone.
Conversely, Cox regression analysis showed that the B-M ratio was not a significant predictor for developing CTRCD. However, Kaplan-Meier analysis with a cut-off value for the B-M ratio of 1.22, determined from a time-dependent ROC curve, demonstrated that patients with high B-M ratios had a significantly higher prevalence of CTRCD than patients with low B-M ratios (Supplementary Figure; log-rank P=0.002). Furthermore, when the B-M ratio was treated as a categorical variable (≥1.22), it was identified as an independent risk factor for developing CTRCD in both univariable analysis (HR 3.49; 95% CI 1.58–7.71; P=0.002) and multivariable analysis (HR 4.48; 95% CI 1.92–10.45; P=0.001). Nevertheless, a cut-off value of 1.22 for the B-M ratio could be arbitrary, because the source and adaptation groups were the same cohort. Therefore, we continued to use a continuous value for the B-M ratio in the main analyses of this study. However, the B-M ratio, treated as a continuous variable, was not significantly associated with CTRCD; this finding was probably due to the inadequate number of participants. Thus, a future study with a large number of participants or a prospective study with a B-M ratio cut-off of 1.22 are needed to confirm the utility of the B-M ratio.
Previous studies have not clarified the clinical or prognostic significance of the sigmoid septum. A large prospective observational trial could not show the effects of the sigmoid septum on exercise capacity in a healthy population.23
In addition, a large retrospective study reported that the sigmoid septum was not independently associated with adverse clinical outcomes.22
However, in those studies, due to the strict parameters, only a small number of patients met the criteria for a sigmoid septum. These parameters included an upper septal thickness ≥14 mm and a B-M ratio ≥1.3, with only 1.5% of patients meeting the criteria,22
or an upper septal thickness ≥13 mm in men or ≥12 mm in women and a B-M ratio ≥1.5, with 7% meeting these criteria.23
Applying these criteria to our cohort, only 14.3% and 12.6% of patients met the criteria, respectively. Furthermore, the Kaplan-Meier analyses revealed no significant differences in the prevalence of CTRCD between patients with and without a sigmoid septum based on these criteria (log-rank P=0.17 and P=0.66). In the previous studies, a mild-moderate sigmoid septum, with a B-M ratio ≥1.22, may have been categorized as normal. We speculate that those criteria may explain why the results from the present study seem to differ to those from previous studies. Consequently, more investigations are warranted to determine an adequate cut-off value for the B-M ratio. In recent years, imaging technology and 3-dimensional echocardiography diagnostic capabilities have evolved;41
these advances may contribute to setting more appropriate criteria for classifying a sigmoid septum.
Anthracycline-induced cardiotoxicity is considered cumulative. Consequently, the sum of baseline cardiomyocyte tissue damage and any additional tissue damage due to chemotherapy contributes to the development of CTRCD. The sigmoid septum is one of several visible morphologic changes associated with aging, aortic atherosclerosis, hypertension, and valvular thickening and calcification. These associations suggest that the presence of a sigmoid septum may indicate higher prior exposure to myocardial stress compared with the absence of a sigmoid septum. Furthermore, hemodynamically, the sigmoid septum was associated with LV diastolic dysfunction,18
increased central blood pressure, and increased aortic pressure wave reflection.25
Greater wave reflection is also an independent predictor of adverse cardiovascular events.42,43
These hemodynamic disadvantages may have affected the higher frequency of CTRCD in patients with a sigmoid septum. We speculate that these mechanisms may be associated with developing CTRCD in patients with smaller ASAs who undergo anthracycline-containing chemotherapy (Figure 5). A previous study showed that the sigmoid septum was a risk factor of stroke relapse in patients with hypertension.44
Future studies should investigate the impact of a sigmoid septum (defined adequately) on clinical outcomes in patients with heart diseases.

Lower LVEF and smaller GLS in patients with normal LVEF are already recognized as risk factors for anthracycline-induced CTRCD.11,14,15,19
In the present study, LVEF was not an independent determinant for anthracycline-induced CTRCD in the multivariable analysis, probably because GLS can detect myocardial injury more sensitively than LVEF.45,46
A history of ischemic heart disease is also considered a risk factor for CTRCD.31
Both decreased GLS and prior ischemic heart disease at baseline are generally considered to be associated with LV myocardial injury, even when LVEF is within normal limits. In the logistic regression analysis in the present study for GLS below the cut-off value, performance status ≥2, higher total bilirubin, and lower LVEF were independent determinants (Supplementary Table 3). Considering that a history of general risk factors of ischemic heart disease, including hypertension, hyperlipidemia, diabetes, and smoking, was not identified as a risk factor for CTRCD in the present study, a history of ischemic heart disease may indicate myocardial damage more directly.
Study Limitations
This study has some limitations. First, it was a single-center retrospective study, and enrollment volume was limited. Second, a relatively large number of patients who did not undergo baseline or follow-up echocardiography were excluded. In the comparison between the group enrolled in the study and the group excluded because of the absence of baseline or follow-up echocardiography, the enrolled group was older (69.1±11.3 vs. 64.9±13.9 years; P=0.002), suggesting the attending physicians ordered echocardiographic examinations more frequently for potentially high-risk patients, possibly resulting in the high prevalence of CTRCD in the present study. Third, the timing of follow-up echocardiography also varied depending on the attending physician. The median interval between the follow-up examinations was 112 days, suggesting relatively good-quality follow-up was performed. However, there was a possibility that the diagnosis of CTRCD was delayed or not made. Fourth, the sensitivity and specificity of the cut-off values evaluated in the same cohort used for derivation are biased due to over-optimism. Further studies are needed to assess the prognostic value of ASA in a prospective cohort.
Conclusions
In addition to lower GLS and a history of ischemic heart disease, decreased ASA was found to be a new independent risk factor for anthracycline-induced CTRCD in patients with ML treated with a CHOP-like regimen.
Acknowledgments
The authors thank the patients who participated in this study and their families, as well as the operations staff. The authors also thank Hiroshi Inagaki and Ayako Masaki (Nagoya City University Graduate School of Medical Sciences, Nagoya, Japan) for histologically diagnosing malignant lymphoma in this study.
Sources of Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosures
Y. Seo is a Fellow of the Japanese Circulation Society and a member of
Circulation Reports’ Editorial Team. The remaining authors declare no conflicts of interest associated with this study.
IRB Information
This study was approved by the Ethics Committee of Nagoya City University (No. 60-19-125).
Data Availability
The deidentified participant data will not be shared.
Supplementary Files
Please find supplementary file(s);
http://dx.doi.org/10.1253/circrep.CR-21-0145
References
- 1.
Coiffier B, Lepage E, Briere J, Herbrecht R, Tilly H, Bouabdallah R, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med 2002; 346: 235–242.
- 2.
Launchbury AP, Habboubi N. Epirubicin and doxorubicin: A comparison of their characteristics, therapeutic activity and toxicity. Cancer Treat Rev 1993; 19: 197–228.
- 3.
Fisher RI, Gaynor ER, Dahlberg S, Oken MM, Grogan TM, Mize EM, et al. Comparison of a standard regimen (CHOP) with three intensive chemotherapy regimens for advanced non-Hodgkin’s lymphoma. N Engl J Med 1993; 328: 1002–1006.
- 4.
Doroshow JH. Doxorubicin-induced cardiac toxicity. N Engl J Med 1991; 324: 843–845.
- 5.
Felker GM, Thompson RE, Hare JM, Hruban RH, Clemetson DE, Howard DL, et al. Underlying causes and long-term survival in patients with initially unexplained cardiomyopathy. N Engl J Med 2000; 342: 1077–1084.
- 6.
Henriksen PA. Anthracycline cardiotoxicity: An update on mechanisms, monitoring and prevention. Heart 2018; 104: 971–977.
- 7.
Armenian SH, Lacchetti C, Barac A, Carver J, Constine LS, Denduluri N, et al. Prevention and monitoring of cardiac dysfunction in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 2017; 35: 893–911.
- 8.
Campia U, Moslehi JJ, Amiri-Kordestani L, Barac A, Beckman JA, Chism DD, et al. Cardio-oncology: Vascular and metabolic perspectives: A scientific statement from the American Heart Association. Circulation 2019; 139: e579–e602.
- 9.
Curigliano G, Lenihan D, Fradley M, Ganatra S, Barac A, Blaes A, et al. Management of cardiac disease in cancer patients throughout oncological treatment: ESMO consensus recommendations. Ann Oncol 2020; 31: 171–190.
- 10.
Oliveira GH, Mukerji S, Hernandez AV, Qattan MY, Banchs J, Durand JB, et al. Incidence, predictors, and impact on survival of left ventricular systolic dysfunction and recovery in advanced cancer patients. Am J Cardiol 2014; 113: 1893–1898.
- 11.
Cardinale D, Colombo A, Bacchiani G, Tedeschi I, Meroni CA, Veglia F, et al. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation 2015; 131: 1981–1988.
- 12.
Ohtani K, Fujino T, Ide T, Funakoshi K, Sakamoto I, Hiasa KI, et al. Recovery from left ventricular dysfunction was associated with the early introduction of heart failure medical treatment in cancer patients with anthracycline-induced cardiotoxicity. Clin Res Cardiol 2019; 108: 600–611.
- 13.
Kadowaki H, Akazawa H, Ishida J, Komuro I. Cancer therapeutics-related cardiac dysfunction: Insights from bench and bedside of onco-cardiology. Circ J 2020; 84: 1446–1453.
- 14.
Ali MT, Yucel E, Bouras S, Wang L, Fei HW, Halpern EF, et al. Myocardial strain is associated with adverse clinical cardiac events in patients treated with anthracyclines. J Am Soc Echocardiogr 2016; 29: 522–527.e523.
- 15.
Hatazawa K, Tanaka H, Nonaka A, Takada H, Soga F, Hatani Y, et al. Baseline global longitudinal strain as a predictor of left ventricular dysfunction and hospitalization for heart failure of patients with malignant lymphoma after anthracycline therapy. Circ J 2018; 82: 2566–2574.
- 16.
Swain SM, Whaley FS, Ewer MS. Congestive heart failure in patients treated with doxorubicin: A retrospective analysis of three trials. Cancer 2003; 97: 2869–2879.
- 17.
Tanaka Y, Tanaka H, Hatazawa K, Yamashita K, Sumimoto K, Shono A, et al. Impact of hypertension on left ventricular function in patients after anthracycline chemotherapy for malignant lymphoma. Int J Cardiol 2021; 323: 126–132.
- 18.
Okada K, Mikami T, Kaga S, Nakabachi M, Abe A, Yokoyama S, et al. Decreased aorto-septal angle may contribute to left ventricular diastolic dysfunction in healthy subjects. J Clin Ultrasound 2014; 42: 341–347.
- 19.
Nakayama T, Oshima Y, Kusumoto S, Yamamoto J, Osaga S, Fujinami H, et al. Clinical features of anthracycline-induced cardiotoxicity in patients with malignant lymphoma who received a CHOP regimen with or without rituximab: A single-center, retrospective observational study. eJHaem 2020; 1: 498–506.
- 20.
Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015; 28: 1–39.e14.
- 21.
Izumi C, Eishi K, Ashihara K, Arita T, Otsuji Y, Kunihara T, et al. JCS/JSCS/JATS/JSVS 2020 guidelines on the management of valvular heart disease. Circ J 2020; 84: 2037–2119.
- 22.
Diaz T, Pencina MJ, Benjamin EJ, Aragam J, Fuller DL, Pencina KM, et al. Prevalence, clinical correlates, and prognosis of discrete upper septal thickening on echocardiography: The Framingham Heart Study. Echocardiography 2009; 26: 247–253.
- 23.
Canepa M, Malti O, David M, Al Ghatrif M, Strait JB, Ameri P, et al. Prevalence, clinical correlates, and functional impact of subaortic ventricular septal bulge (from the Baltimore Longitudinal Study of Aging). Am J Cardiol 2014; 114: 796–802.
- 24.
Bernstein RF, Tei C, Child JS, Shah PM. Angled interventricular septum on echocardiography: Anatomic anomaly or technical artifact? J Am Coll Cardiol 1983; 2: 297–304.
- 25.
Olafiranye O, Ibrahim M, Kamran H, Venner-Jones K, McFarlane SI, Salciccioli L, et al. Narrowed aortoseptal angle is related to increased central blood pressure and aortic pressure wave reflection. Cardiorenal Med 2012; 2: 177–183.
- 26.
Okada K, Kaga S, Tsujita K, Sakamoto Y, Masauzi N, Mikami T. Right ventricular basal inflow and outflow tract diameters overestimate right ventricular size in subjects with sigmoid-shaped interventricular septum: A study using three-dimensional echocardiography. Int J Cardiovasc Imaging 2019; 35: 1211–1219.
- 27.
Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977; 33: 159–174.
- 28.
Pencina MJ, D’Agostino RB Sr, D’Agostino RB Jr, Vasan RS. Evaluating the added predictive ability of a new marker: From area under the ROC curve to reclassification and beyond. Stat Med 2008; 27: 157–172.
- 29.
Pencina MJ, D’Agostino RB Sr, Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med 2011; 30: 11–21.
- 30.
Salz T, Zabor EC, de Nully Brown P, Dalton SO, Raghunathan NJ, Matasar MJ, et al. Preexisting cardiovascular risk and subsequent heart failure among non-Hodgkin lymphoma survivors. J Clin Oncol 2017; 35: 3837–3843.
- 31.
Wang L, Tan TC, Halpern EF, Neilan TG, Francis SA, Picard MH, et al. Major cardiac events and the value of echocardiographic evaluation in patients receiving anthracycline-based chemotherapy. Am J Cardiol 2015; 116: 442–446.
- 32.
Krasnow N. Subaortic septal bulge simulates hypertrophic cardiomyopathy by angulation of the septum with age, independent of focal hypertrophy: An echocardiographic study. J Am Soc Echocardiogr 1997; 10: 545–555.
- 33.
Swinne CJ, Shapiro EP, Jamart J, Fleg JL. Age-associated changes in left ventricular outflow tract geometry in normal subjects. Am J Cardiol 1996; 78: 1070–1073.
- 34.
Toth AB, Engel JA, McManus AM, McManus BM. Sigmoidity of the ventricular septum revisited: Progression in early adulthood, predominance in men, and independence from cardiac mass. Am J Cardiovasc Pathol 1988; 2: 211–223.
- 35.
Aslam F, Haque A, Foody J, Shirani J. The frequency and functional impact of overlapping hypertension on hypertrophic cardiomyopathy: A single-center experience. J Clin Hypertens (Greenwich) 2010; 12: 240–245.
- 36.
Shapiro LM. Hypertrophic cardiomyopathy in the elderly. Br Heart J 1990; 63: 265–266.
- 37.
Pearson AC. The evolution of basal septal hypertrophy: From benign and age-related normal variant to potentially obstructive and symptomatic cardiomyopathy. Echocardiography 2017; 34: 1062–1072.
- 38.
Ieki K, Imataka K, Sakurai S, Okamoto E, Ashida T, Fujii J. Differentiation of hypertrophic cardiomyopathy and hypertensive cardiac hypertrophy using the patterns of interventricular septum hypertrophy. J Cardiol 1996; 27: 309–314 (in Japanese).
- 39.
Chen-Tournoux A, Fifer MA, Picard MH, Hung J. Use of tissue Doppler to distinguish discrete upper ventricular septal hypertrophy from obstructive hypertrophic cardiomyopathy. Am J Cardiol 2008; 101: 1498–1503.
- 40.
Belenkie I, MacDonald RP, Smith ER. Localized septal hypertrophy: Part of the spectrum of hypertrophic cardiomyopathy or an incidental echocardiographic finding? Am Heart J 1988; 115: 385–390.
- 41.
Tanabe K. Three-dimensional echocardiography: Role in clinical practice and future directions. Circ J 2020; 84: 1047–1054.
- 42.
Nürnberger J, Keflioglu-Scheiber A, Opazo Saez AM, Wenzel RR, Philipp T, Schäfers RF. Augmentation index is associated with cardiovascular risk. J Hypertens 2002; 20: 2407–2414.
- 43.
Laurent S, Boutouyrie P, Asmar R, Gautier I, Laloux B, Guize L, et al. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension 2001; 37: 1236–1241.
- 44.
Manea P, Ghiuru R. Correlations between the presence of sigmoid interventricular septum and increased relapse risk of stroke in hypertensive patients. Rev Med Chir Soc Med Nat Iasi 2013; 117: 857–862.
- 45.
Plana JC, Galderisi M, Barac A, Ewer MS, Ky B, Scherrer-Crosbie M, et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: A report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2014; 27: 911–939.
- 46.
Zamorano JL, Lancellotti P, Rodriguez Muñoz D, Aboyans V, Asteggiano R, Galderisi M, et al. 2016 ESC position paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for Cancer Treatments and Cardiovascular Toxicity of the European Society of Cardiology (ESC). Eur Heart J 2016; 37: 2768–2801.






