Article ID: CR-24-0123
Article ID: CR-24-0123
Background: In the context of cardiovascular disease (CVD), iron metabolism assessment plays a pivotal role in the diagnosis of anemia and chronic inflammation. However, data regarding the prevalence of anemia, iron deficiency, and iron overload among outpatients in real-world clinical settings remain limited. Moreover, the influence of specific diseases on iron kinetics within the CVD spectrum has not been fully elucidated.
Methods and Results: We conducted a retrospective analysis of 260 patients attending a cardiology outpatient clinic who had undergone blood sampling for comprehensive evaluation of anemia and iron kinetics. The prevalence of anemia among these outpatients was 36.1%, but iron deficiency was observed in only 13.8% of patients (absolute iron deficiency: 1.5%). Notably, stored iron positively correlated with free iron in patients with sinus rhythm, but not in patients with atrial fibrillation (AF). Intriguingly, this relationship followed a similar pattern in the paroxysmal and longstanding AF subgroups. Moreover, multivariate regression analysis showed that iron dynamics significantly explained hemoglobin levels in patients with sinus rhythm but not in those with AF.
Conclusions: Although chronic inflammation may be a contributing factor, iron dynamics exhibited a distinct profile in patients with AF. The correlation between transferrin saturation and stored iron, evident in sinus rhythm patients, was abolished in AF, which supports the notion of chronic inflammation in patients with AF.
Heart failure is exacerbated by anemia,1 so comprehensive examination of anemia in outpatient cardiology clinics, which address various cardiovascular diseases (CVDs), is crucial. Studies have shown that chronic inflammation is a significant contributing factor to the development of CVD, including heart failure2–4 and atrial fibrillation (AF),5,6 Given the association of both anemia and chronic inflammation with CVD, their assessment, together with iron kinetics, is essential in the management of CVD.
Iron deficiency anemia, the most common form of anemia, is characterized by reduced levels of both free iron (Fe) and ferritin, whereas chronic inflammation is associated with decreased levels of Fe and increased iron stores.1 Therefore, a correlation between Fe and stored iron is expected in anemia but not in chronic inflammation.
In our previous study, we proposed that the FTL gene, responsible for ferritin, may be elevated in AF,7 so patients with AF are likely to have elevated ferritin levels and decreased Fe concentrations during cardiac overload. Moreover, the chronic inflammation associated with cardiac overload is more prevalent in patients with AF.8
Although studies of general outpatient populations of older patients with anemia have shown nutritional deficiencies to be the most common cause, followed by anemia of chronic inflammation and unexplained anemia,9 to our knowledge, there are no studies that have specifically examined anemia and iron kinetics in older patients with CVD.
Therefore, in the present study we aimed to investigate (1) the prevalence of anemia and iron deficiency in outpatients with CVD, (2) the correlation between Fe and iron stores in this patient population, and (3) how to identify patient groups affected by altered iron kinetics.
By addressing these objectives, it was hoped that this study would contribute to a deeper understanding of anemia and iron dynamics in older patients with CVD, thus providing valuable insights for clinical management.
The National Center for Geriatrics and Gerontology Ethics Committee approved this study (no. 1761; date of decision: November 14, 2023). Patient consent for this study was obtained via an opt-out process, which is used for obtaining participant data for research purposes.
Study ProtocolBetween July and October 2023, this cross-sectional study enrolled 260 patients who visited the outpatient clinic of a cardiologist and an arrhythmia specialist at the National Center for Geriatrics and Gerontology. Iron-related blood indices included ferritin, Fe, and transferrin saturation (TSAT). The TSAT, an index that considers both plasma iron and its main transport protein, is an important biochemical marker of body iron status,1 Patients who did not undergo iron-related blood examinations were excluded. To ensure the accuracy and reliability of the data, patients with acute overt inflammatory conditions were also excluded from the review of medical records (Figure 1A). The primary diagnosis for each of the 260 patients was indeterminate due to the retrospective study design, and the prevalence of different diseases is presented instead.

(A) Flow chart of patient enrollment. Patients without iron-related blood examination results were excluded. (B) Iron kinetics in CVD patients with and without anemia. A direct comparison of hemoglobin, ferritin, and transferrin saturation (TSAT) in the standard hemoglobin group (Hb >13 g/dL in men and >12 g/dL in women) and anemic group (<13 g/dL in men and <12 g/dL in women) was performed using Student’s t-test as the statistical analysis. Although Hb levels were lower in the anemic group, the figure shows its distribution. TSAT was calculated with ferritin in the denominator and free iron in the numerator. (C) Proportion of anemic patients (males Hb <13 g/dL, females <12 g/dL), (D) percentage of patients with absolute iron deficiency (ferritin <12 ng/mL), and (E) percentage of patients with relative iron deficiency in the outpatient CVD clinic. The percentages of patients with ferritin <100 ng/mL and transferrin saturation <20% in the CVD outpatient setting are shown. (F) Percentage of patients with iron overload (ferritin >500 ng/mL) in the CVD outpatient setting. CVD, cardiovascular disease; JCS, Japanese Circulation Society; JHRS, Japan Heart Rhythm Society.
Statistical Analysis
Statistical analysis and bar chart creation were performed using GraphPad Prism Version 9.5.0. Statistical significance was considered at P<0.05. Student’s t-test was used to directly compare hemoglobin (Hb), ferritin, and TSAT levels between the standard Hb group (>13 g/dL in men, >12 g/dL in women) and the anemic group (<13 g/dL in men, <12 g/dL in women). The normality test was the D’Agostino & Pearson test. Principal component analysis was conducted for Hb and iron dynamics (free and stored iron). Simple regression analyses were used to examine the relationships between ferritin and TSAT. Multiple regression analyses were performed, with Hb as the dependent variable, to assess erythropoiesis and iron kinetics across different heart rhythms.
Table 1 shows the clinical backgrounds of the patients in this study. Most were older (mean age, 78 years), and a significant proportion had hypertension. The mean creatinine level was 1.45 mg/dL, likely attributable to the characteristics of this patient population. We evaluated iron kinetics with and without anemia. As shown in Figure 1B, the direct comparison of Hb, ferritin, and TSAT in the standard Hb and anemic groups revealed the Hb distribution and showed that Hb levels were lower in the anemic group. The proportion of anemic patients, and the percentages of patients with absolute iron deficiency (ferritin <12 ng/mL), relative iron deficiency (ferritin <100 ng/mL and TSAT <20%), and iron overload (ferritin >500 ng/mL) are shown in Figure 1C–F.
Clinical Characteristics of the Study Population
| Factor | Cases | Factor | Number (SD) | |
|---|---|---|---|---|
| Male (%) | 151 (58.1) | SBP, mmHg | 130.01 (18.32) | |
| Age (years) | 78 (10.65) | DBP, mmHg | 71.57 (11.99) | |
| BMI | 23.37 (3.86) | Heart rate, bpm | 76.19 (48.65) | |
| HT (%) | 181 (70.7) | LVDd, mm | 45.61 (5.76) | |
| HL (%) | 141 (55.1) | LVDs, mm | 30.76 (5.83) | |
| DM (%) | 66 (25.8) | LVEF, % | 60.69 (9.17) | |
| Coronary artery disease (%) | 72 (28.1) | LAD, mm | 39.21 (7.88) | |
| Coronary artery bypass graft (%) | 7 (2.7) | Serum iron, μg/dL | 89.19 (34.77) | |
| Valve surgery (%) | 3 (1.2) | TSAT, % | 29.71 (11.49) | |
| Alcohol (%) | 27 (10.6) | TIBC, μg/dL | 307.82 (55.94) | |
| Smoker (%) | Ferritin | 156.59 (194.14) | ||
| Current | 19 (7.5) | Hb, g/dL | 12.98 (1.75) | |
| Past | 48 (18.8) | HbA1c, % | 6.20 (0.76) | |
| Cancer (%) | 14 (5.4) | Cre, mg/dL | 1.45 (7.48) | |
| COPD (%) | 15 (5.9) | BNP, pg/mL | 99.07 (149.10) | |
| Dementia (%) | 10 (3.8) | HDL, mg/dL | 55.97 (15.45) | |
| Stroke (%) | 21 (8.2) | LDL, mg/dL | 97.08 (29.03) | |
| Arrhythmia (%) | TG, mg/dL | 131.92 (66.88) | ||
| SR | 174 (66.9) | CRP, mg/dL | 0.31 (0.92) | |
| PAF | 22 (8.5) | Albumin, g/dL | 4.00 (0.42) | |
| Persistent AF | 4 (1.5) | |||
| Longstanding AF | 38 (14.6) | |||
| Other | 22 (8.5) |
AF, atrial fibrillation; BMI, body mass index; BNP, B-type natriuretic peptide; COPD, chronic obstructive pulmonary disease; Cre, creatinine; CRP, C-reactive protein; DBP, diastolic blood pressure; DM, diabetes mellitus; Hb, hemoglobin; HL, hyperlipidemia; HT, hypertension; HDL, high density lipoprotein; LAD, left atrial diameter; LDL, low density lipoprotein; LVDd, left ventricular end-diastolic diameter; LVDs, eft ventricular end-systolic diameter; LVEF, left ventricular ejection fraction; SBP, systolic blood pressure; SR, sinus rhythm; TG, triglyceride; TIBC, total iron binding capacity; TSAT, transferrin saturation.
The relationship between free and stored iron was evaluated. Under normal conditions, Fe should increase as iron stores increase (i.e., ferritin and TSAT should correlate). In this study, ferritin and TSAT did correlate in most patients (Figure 2A). The results of principal component analysis to extract the possible explanatory items for Hb and iron kinetics and determine their correlation coefficients demonstrated that both Hb and iron dynamics were consistent with the hypothesis that increased iron levels lead to elevated Hb levels (Supplementary Figure A). Fe, stored iron, and Hb strongly influenced Principal Component 1 (Supplementary Figure B). However, patients with AF often deviated from the predictions in the simple regression analysis of ferritin and TSAT (Figure 2B). Because iron kinetics are affected by cardiac rhythm, we divided the patients into those with sinus rhythm (SR) or AF. In patients with SR, Fe and TSAT increased as stored ferritin increased (Figure 3A), but in patients with AF, Fe and iron stores did not correlate (Figure 3B). Subsequently, we examined whether iron kinetics changed according to paroxysmal (PAF i.e., the patient is in SR most of the time and develops AF occasionally) or longstanding AF. Unlike in patients with SR, Fe and iron stores did not correlate in patients with PAF and furthermore, Fe decreased despite iron stores increasing (Figure 3C). Similarly, Fe did not correlate with iron stores in patients with longstanding AF, but no trend was observed toward an increase in iron stores leading to a decrease in Fe (Figure 3D).
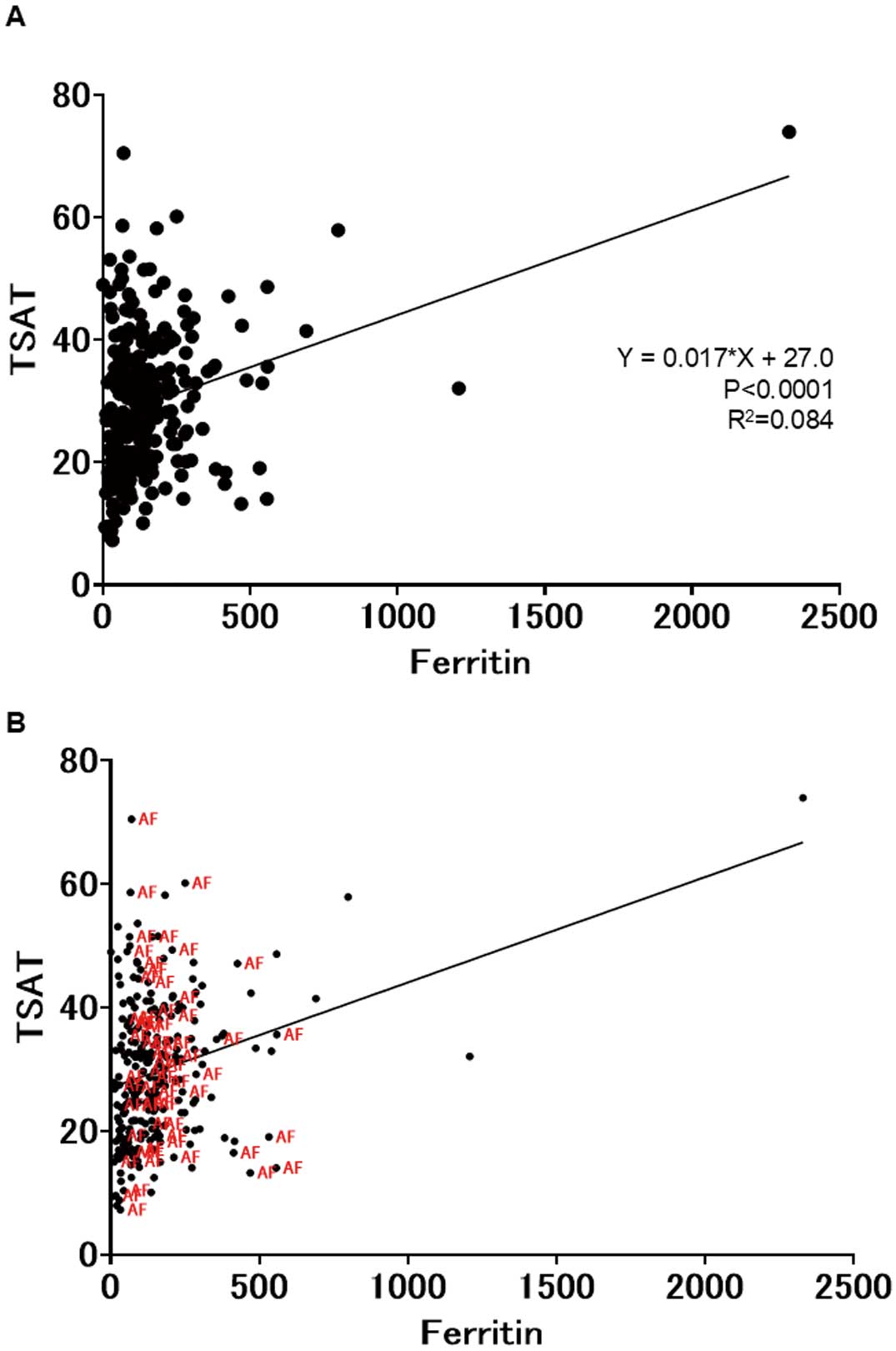
(A) Relationship between free and stored iron in the cardiovascular clinic outpatients. Under normal conditions, free iron should increase as iron stores increase (i.e., ferritin and transferrin saturation (TSAT) should correlate). Ferritin and TSAT correlated in most patients. (B) Iron kinetics in patients with atrial fibrillation deviated from predictions in the simple regression analysis of ferritin and TSAT.

Free iron and ferritin correlated in (A) patients with sinus rhythm, but not in (B) patients with atrial fibrillation (AF), (C) patients with paroxysmal atrial fibrillation (PAF), in whom free iron decreased although ferritin increased or in (D) patients with longstanding AF, but unlike patients with PAF, no trend was observed toward an increase in ferritin leading to a decrease in free iron.
The results are summarized as follows.
SR: TSAT = 0.022 × ferritin + 25.3 (P<0.0001, R2=0.16) (Figure 3A)
AF: TSAT = −0.0075 × ferritin + 32.3 (P=0.56, R2=0.056) (Figure 3B)
PAF: TSAT = −0.021 × ferritin + 37.6 (P=0.37, R2=0.040) (Figure 3C)
Longstanding AF: TSAT = −0.0036 × ferritin + 30.5 (P=0.81, R2=0.015) (Figure 3D)
Finally, we performed multiple regression analysis, with Hb as the dependent variable, to evaluate erythropoiesis and iron kinetics in each heart rhythm. Ferritin and TSAT were statistically significant explanatory variables for Hb in SR (Table 2), but not in AF, PAF (Table 3), or longstanding AF (Table 4). Figure 4 shows the residual, homoscedasticity, and quantile–quantile plots in the multiple regression analyses for each cardiac rhythm. Because we checked the normality of each multiple regression analysis, our multiple regression analysis is credible.
Multiple Regression Analysis for Hb in Patients With SR
| β | Factor | Estimated | Standard error |
95% CI | |t| | P value | VIF | R2 by other variables |
|---|---|---|---|---|---|---|---|---|
| β0 | 14.40 | 1.75 | 10.93~17.87 | 8.21 | <0.0001 | |||
| β1 | Age | −0.043 | 0.015 | −0.071~−0.014 | 2.92 | 0.0041 | 1.61 | 0.38 |
| β2 | Female | −1.02 | 0.26 | −1.528~−0.5100 | 3.96 | 0.00010 | 1.12 | 0.10 |
| β3 | BMI | 0.08 | 0.043 | −0.0045~0.17 | 1.87 | 0.06 | 2.04 | 0.51 |
| β4 | Ferritin | −0.0014 | 0.00069 | −0.0028~−4.95×10−005 | 2.05 | 0.04 | 1.38 | 0.28 |
| β5 | TSAT | 0.039 | 0.014 | 0.012~0.065 | 2.87 | 0.0048 | 1.37 | 0.27 |
| β6 | BNP | 0.0000 | 0.0014 | −0.0027~0.0027 | 0.02 | 0.99 | 1.78 | 0.44 |
| β7 | CRP | −0.25 | 0.19 | −0.62~0.12 | 1.33 | 0.19 | 1.08 | 0.07 |
| β8 | Cre | −0.0043 | 0.013 | −0.030~0.022 | 0.33 | 0.74 | 1.11 | 0.10 |
| β9 | HT | −0.25 | 0.32 | −0.88~0.38 | 0.78 | 0.44 | 1.36 | 0.27 |
| β10 | DM | 0.38 | 0.29 | −0.19~0.96 | 1.32 | 0.19 | 1.13 | 0.11 |
| β11 | HL | 0.29 | 0.29 | −0.29~0.87 | 0.99 | 0.32 | 1.31 | 0.24 |
| β12 | LVEF | 0.0017 | 0.015 | −0.028~0.032 | 0.11 | 0.91 | 1.39 | 0.28 |
| β13 | LAD | −0.018 | 0.031 | −0.079~0.043 | 0.60 | 0.55 | 2.08 | 0.52 |
“Hb = β0 + β1 × Age + β2 × Gender (Female) + β3 × BMI + β4 × Ferritin + β5 × TSAT + β6 × BNP + β7 × CRP + β8 × Cre + β9 × HT + β10 × DM + β11 × HL + β12 × LVEF + β13 × LAD (R2=0.33)” VIF, Variance Inflation Factor. Other abbreviations as in Table 1.
Multiple Regression Analysis for Hb in Patients With Paroxysmal AF
| β | Factor | Estimated | Standard error |
95% CI | |t| | P value | VIF | R2 by other variables |
|---|---|---|---|---|---|---|---|---|
| β0 | 8.94 | 3.83 | 1.16~16.71 | 2.33 | 0.026 | |||
| β1 | Age | −0.039 | 0.029 | −0.097~0.020 | 1.35 | 0.19 | 1.69 | 0.41 |
| β2 | Female | −1.79 | 0.75 | −3.30~−0.27 | 2.40 | 0.022 | 2.21 | 0.55 |
| β3 | BMI | 0.10 | 0.086 | −0.077~0.27 | 1.13 | 0.27 | 2.08 | 0.52 |
| β4 | Ferritin | 0.0034 | 0.0039 | −0.0045~0.011 | 0.88 | 0.39 | 2.66 | 0.62 |
| β5 | TSAT | 0.016 | 0.022 | −0.029~0.062 | 0.72 | 0.48 | 1.53 | 0.35 |
| β6 | BNP | 0.0035 | 0.0038 | −0.0041~0.011 | 0.93 | 0.36 | 2.28 | 0.56 |
| β7 | CRP | −0.18 | 0.25 | −0.68~0.33 | 0.72 | 0.48 | 2.63 | 0.62 |
| β8 | Cre | 0.0004 | 0.017 | −0.034~0.034 | 0.025 | 0.98 | 1.31 | 0.24 |
| β9 | HT | −0.06 | 0.68 | −1.44~1.32 | 0.093 | 0.93 | 1.65 | 0.39 |
| β10 | DM | 0.23 | 0.71 | −1.23~1.68 | 0.32 | 0.75 | 1.13 | 0.12 |
| β11 | HL | 0.29 | 0.61 | −0.94~1.52 | 0.47 | 0.64 | 1.48 | 0.33 |
| β12 | LVEF | 0.026 | 0.039 | −0.053~0.11 | 0.67 | 0.51 | 2.54 | 0.61 |
| β13 | LAD | 0.074 | 0.059 | −0.046~0.19 | 1.25 | 0.22 | 1.80 | 0.44 |
“Hb = β0 + β1 × Age + β2 × Gender (Female) + β3 × BMI + β4 × Ferritin + β5 × TSAT + β6 × BNP + β7 × CRP + β8 × Cre + β9 × HT + β10 × DM + β11 × HL + β12 × LVEF + β13 × LAD (R2=0.40)” Abbreviations as in Table 1.
Multiple Regression Analysis for Hb in Patients With Longstanding AF
| β | Factor | Estimated | Standard error |
95% CI | |t| | P value | VIF | R2 by other variables |
|---|---|---|---|---|---|---|---|---|
| β0 | 12.34 | 3.94 | 4.43~20.26 | 3.13 | 0.0029 | |||
| β1 | Age | −0.042 | 0.032 | −0.11~0.022 | 1.32 | 0.19 | 1.94 | 0.48 |
| β2 | Female | −0.75 | 0.55 | −1.86~0.35 | 1.37 | 0.18 | 1.43 | 0.30 |
| β3 | BMI | 0.063 | 0.085 | −0.11~0.23 | 0.74 | 0.46 | 2.64 | 0.62 |
| β4 | Ferritin | 0.0019 | 0.0026 | −0.0033~0.0070 | 0.74 | 0.46 | 1.37 | 0.27 |
| β5 | TSAT | 0.035 | 0.022 | −0.0083~0.078 | 1.62 | 0.11 | 1.47 | 0.32 |
| β6 | BNP | 0.0007 | 0.0015 | −0.0022~0.0036 | 0.47 | 0.64 | 1.45 | 0.31 |
| β7 | CRP | −0.53 | 0.90 | −2.34~1.29 | 0.58 | 0.56 | 1.19 | 0.16 |
| β8 | Cre | 0.0041 | 0.018 | −0.031~0.039 | 0.23 | 0.82 | 1.35 | 0.26 |
| β9 | HT | −0.64 | 0.56 | −1.76~0.49 | 1.14 | 0.26 | 1.33 | 0.25 |
| β10 | DM | 0.35 | 0.50 | −0.65~1.36 | 0.71 | 0.48 | 1.06 | 0.057 |
| β11 | HL | 0.91 | 0.55 | −0.19~2.01 | 1.66 | 0.10 | 1.45 | 0.31 |
| β12 | LVEF | 0.00026 | 0.027 | −0.054~0.055 | 0.010 | 0.99 | 1.19 | 0.16 |
| β13 | LAD | 0.025 | 0.038 | −0.050~0.10 | 0.67 | 0.50 | 1.89 | 0.47 |
“Hb = β0 + β1 × Age + β2 × Gender (Female) + β3 × BMI + β4 × Ferritin + β5 × TSAT + β6 × BNP + β7 × CRP + β8 × Cre + β9 × HT + β10 × DM + β11 × HL + β12 × LVEF + β13 × LAD (R2=0.33)” Abbreviations as in Table 1.

Residual, homoscedasticity, and quantile–quantile plots in multiple regression analysis of patients with (A) sinus rhythm, (B) paroxysmal atrial fibrillation (PAF) and (C) longstanding AF. (A–C) The normality of each multiple regression analysis was verified.
Transferrin transports iron that has not been utilized for erythropoiesis to storage pools.2 The role of ferritin as a soluble and effective storage compartment is important to ensure iron is readily available to meet the body’s demand; serum ferritin levels are proportional to systemic stores.3,4 Furthermore, iron levels and TSAT are also important markers that show decreased levels in iron deficiency and many chronic diseases.
Chronic inflammation also results in impaired iron metabolism. In individuals with chronic inflammation, serum iron and TSAT levels are typically decreased, whereas ferritin levels remain within the normal to high range.1
The results of this study indicated that chronic inflammation may be a contributing factor in patients with AF, who exhibited a distinct profile of iron dynamics.
CVD and Anemia/Iron DynamicsThe mean age of the patients in this study was 78 years, with 70.7%, 55.1%, and 25.8% having hypertension, hyperlipidemia, and diabetes mellitus, respectively. The prevalence of coronary artery disease was 28.1%, and the mean creatinine level was 1.45 mg/dL (Table 1). This outpatient group was consistent with the prevailing view of a CVD outpatient clinic focusing on older patients.
After including all patients with CVD, the anemic group showed lower TSAT (Figure 1A), consistent with the anemia being associated with reduced Fe. However, the ferritin values were not significantly different between the anemic and non-anemic groups: 36.1% of the outpatients met the criteria for anemia, yet only 1.5% exhibited absolute iron deficiency with a notable decline in ferritin levels. Furthermore, only 13.8% had relative anemia with reduced TSAT and ferritin levels. Conversely, 1.5% of patients had iron overload, with ferritin >500 (Figure 1B–E).
To elucidate the rationale behind the absence of a notable decline in ferritin levels in the anemic group, we investigated the factors that might have contributed to the observed discrepancies from the regression model. Among the identified variables, AF emerged as a prominent factor (Figure 2B). Although not shown, removing outliers did not significantly alter the results. Therefore, raw data were presented to maintain data integrity. Upon dividing the groups by SR and AF, the relationship between ferritin and TSAT exhibited notable differences between rhythm groups (Figure 3A,B). In AF patients, increased ferritin levels were not significantly associated with increased TSAT, but ferritin and TSAT correlated significantly in those with SR. PAF patients also demonstrated different iron kinetics than those with SR (Figure 3C), indicating that TSAT and ferritin were not correlated in either PAF or longstanding AF (Figure 3C,D).
Multiple Regression Analysis of Hb and Iron Dynamics and Chronic InflammationWe performed multiple regression analysis of Hb and iron dynamics in the SR, PAF, and longstanding AF groups. We also included C-reactive protein (CRP), a common marker of inflammation, and creatinine, a measure of renal function. However, the results showed that TSAT and ferritin were the only significant explanatory variables in SR. Furthermore, even when the patient exhibited PAF, neither TSAT nor ferritin was identified as important explanatory variables (Tables 2–4).
Interpretation of ResultsPatients with AF exhibited distinctive iron kinetics, which is not inconsistent with previous results of iron deficiency in 60% of patients with some types of CVD.5 However the number of patients with iron deficiency in the present study was smaller, which suggests that the background status of the outpatients was not of a significantly poor prognosis. The prevalence of hematologic diseases is established to be relatively low,9 and none of the study patients was subsequently diagnosed with a hematologic disorder.
The iron kinetics were distinctly different between patients with SR and those with AF. Notably, the correlation between ferritin and Fe was not affected by AF duration, even in cases of paroxysmal AF.
Generally, increased ferritin without increased Fe indicates chronic inflammation or renal anemia. However, in our study, TSAT and ferritin were not identified as explanatory variables for Hb in AF, although creatinine and CRP were included as explanatory variables in the multiple regression analysis. Furthermore, we incorporated creatinine and CRP into the model as additional explanatory variables to exclude the possibility of renal anemia and apparent inflammation or infection.
In patients with SR, iron stores and Fe were variables that defined Hb. In a normal iron-available environment, the amount of iron is related to the amount of Hb. However, in patients with AF, TSAT and ferritin were not variables of Hb, because of inflammation, alterations to iron dynamics, and impaired iron utilization, indicating that iron dynamics is not an explanatory variable for Hb in AF.
Although numerous studies have addressed the potential correlation between various inflammatory conditions and AF,8,10–12 and even if they had been included, multiple regression analysis would have incorporated CRP as an indicator, and CRP should have been associated with lower Hb levels, but this was not observed.
Possible Mechanisms Other Than InflammationThe most important pathophysiology in CVD is cell death, and ferroptosis is a newly described form of regulated cell death in CVD (including AF), involving iron regulation, metabolic mechanism, and lipid peroxidation.13
An additional potential mechanism is autophagy, a bulk degradation/recycling system that is essential for maintaining cellular homeostasis. Cardiac autophagy decreases with age, and the incidence of AF also increases with age.14,15 Cellular iron is frequently stored within lysosomes, which play a crucial role in the final stages of autophagy and other forms of autodigestion.7,16 Thus, the distinctive iron metabolism observed in AF patients may be attributed to an anomaly in lysosomes as the concluding stage of autophagy. Additional evidence suggests the involvement of NLRP3 inflammasome activation in the pathogenesis and progression of AF,17–19 and some studies suggest that lysosomes are fundamentally involved in the inflammasome, not just in the process of digestion.20
Study LimitationsThe results do not permit the determination of whether AF causes inflammation and altered iron dynamics or whether patients with inflammation are more susceptible to developing AF and exhibiting abnormal iron dynamics. Thus, we should monitor a cohort of patients without AF but with altered iron dynamics. Furthermore, although this study assessed iron kinetics, which can be extensively evaluated in the clinical setting, we were unable to assess well-established inflammatory markers such as interleukins.21,22
Clinical RelevanceDespite its limitations, we believe the results are significant. Many markers are associated with chronic inflammation, and the absence of an increase in any single marker does not preclude the existence of chronic inflammation. CRP is a suitable illustration. It is widely acknowledged that CRP levels remain unelevated even in the presence of systemic disorders such as systemic lupus erythematosus.23 However, iron kinetics are sensitive to changes in chronic inflammation and widely used in collagen disease clinics to evaluate chronic inflammation. The level of evidence for the usefulness of iron kinetics is high.24,25 Notably, the iron kinetics in AF indicated chronic inflammation and were also altered in PAF, suggesting the occurrence of chronic inflammation even with occasional AF.
In the outpatient setting, approximately 36% of the study patients had anemia, but only approximately 15% showed iron deficiency. Chronic inflammation may be a contributing factor, but the iron dynamics in patients with AF were notably distinct. In patients with SR, TSAT correlated with iron stores but not in those with AF, indicating the presence of chronic inflammation in patients with AF.
The authors extend their appreciation to the staff who supported this study and, in particular, to Ms. Shihoko Matsuda (National Center for Geriatrics and Gerontology) for laboratory assistance.
This study was supported by JSPS KAKENHI Grant Number 23K19602 (to T.K.).
None of the authors have any competing interests regarding this study.
The authors declare they have no conflicts of interest.
The protocol for the research project was approved by the Ethics Committee of the National Center for Geriatrics and Gerontology (no. 1761). The current study was performed in accordance with the provisions of the Declaration of Helsinki (as revised in Tokyo 2004).
Please find supplementary file(s);
https://doi.org/10.1253/circrep.CR-24-0123