Article ID: CR-24-0175
Article ID: CR-24-0175
Background: Angiotensin-receptor-neprilysin inhibitors (ARNIs) improve outcomes in patients with chronic heart failure (CHF). However, neprilysin is a major amyloid beta (Aβ)-degrading enzyme in the brain and although previous studies suggest that ARNI use does not induce neurocognitive dysfunction in CHF patients, data in Japanese patients are limited.
Methods and Results: This single-center, prospective, observational study enrolled 15 CHF patients: 6 who were being treated with ARNI (ARNI) and 9 who were not (non-ARNI). Cognitive assessments and blood biomarkers were evaluated at baseline and 1-year follow-up. Participants from the Parkinson’s and Alzheimer’s Disease Dimensional Neuroimaging Initiative cohort, comprising 7 patients with cerebral Aβ deposition (Aβ-positive) and 7 patients without deposition (Aβ-negative), were used for comparison. Despite the small sample size, significant differences in the Japanese version of Montreal Cognitive Assessment score and tau phosphorylated at threonine 181 level were observed between the Aβ-negative and Aβ-positive groups. In contrast, no significant difference in cognitive function or blood biomarkers were found between the non-ARNI and ARNI groups at baseline or after 1 year of follow-up.
Conclusions: In this pilot-scale study, ARNI use was not associated with cognitive impairment or elevated blood biomarkers related to cognitive dysfunction in Japanese patients with CHF. Due to the limited sample size and follow-up, further validation in larger, long-term trials is warranted.
Cognitive dysfunction is a common comorbidity in patients with chronic heart failure (CHF).1,2 Compared with the general population, patients with CHF have multiple vascular risk factors, which increase the likelihood of vascular dementia. In addition, several studies have shown that CHF is associated with an increased risk and worsened prognosis of Alzheimer’s disease (AD),3,4 which comprises the largest number of patients with dementia. Because cognitive dysfunction is associated with poor prognosis in patients with CHF, assessing and managing cognitive function is crucial for better medical outcomes in these patients.5
Neprilysin is responsible for breaking down various peptides, including the natriuretic peptides (atrial, B- and C-type: ANP, BNP, and CNP, respectively), which have multiple cardioprotective effects, including natriuretic, antihyperopic, and antifibrotic effects.6 Inhibition of neprilysin reduces NP breakdown, leading to an increase in circulating NP levels, and thus has been developed for the treatment of CHF. Multiple clinical trials have shown that treatment with a combination of neprilysin inhibitors and angiotensin-receptor blockers (angiotensin-receptorneprilysin inhibitors: ARNI), such as sacubitril/valsartan, improves mortality and morbidity in patients with CHF.7–9 However, neprilysin is also a major amyloid beta (Aβ)-degrading enzyme in the brain, and neprilysin deficiency has been reported to increase Aβ accumulation in the brain.10 Because brain Aβ accumulation is closely linked to AD pathogenesis,11 there is a concern that the use of ARNI may increase the risk of AD. Although several clinical observational studies have demonstrated that ARNI use is not associated with cognitive impairment,12,13 specific data from clinical trials and observational studies focusing on cognitive outcomes in CHF patients using ARNI are limited, particularly in Japanese populations.
In this study, we aimed to examine whether ARNI use is correlated with cognitive impairment in Japanese patients with CHF using cognitive assessments and blood biomarker tests.
This single-center, prospective, observational study was conducted between April 2021 and November 2023. The study adhered to the Declaration of Helsinki, and ethical approval was granted by the Institutional Review Board of the Kyoto Prefectural University of Medicine. Patients with stable CHF (NYHA classes II–III) were enrolled from the HF outpatient clinic of the University Hospital, Kyoto Prefectural University of Medicine. Inclusion criteria included age ≥18 years and the ability and willingness to provide written informed consent. The exclusion criteria were renal replacement therapy and pregnancy. Blood samples and clinical data of patients with or without cerebral Aβ deposition, assessed by Aβ positron emission tomography (Aβ-PET) scans, were acquired from the Parkinson’s and Alzheimer’s disease Dimensional Neuroimaging Initiative (PADNI) cohort (https://padni.org/).14
Data CollectionThe following data were collected at enrollment as baseline and at 1-year follow-up visits: clinical characteristics, medical history, laboratory results, echocardiographic data, current medications, and device-based therapy. The concentrations of plasma neurofilament light chain (NfL) and tau phosphorylated at threonine 181 (p-tau 181) were measured using a Simoa HD-X analyzer as described previously.15 Cognitive function was assessed by the same certified neuropsychologist using the Mini-Mental State Examination Japanese (MMSE-J) and the Japanese version of Montreal Cognitive Assessment (MoCA-J).16,17
Statistical AnalysisData are presented as mean±standard error of the mean (SEM). All statistical analyses were performed using GraphPad Prism 10 software. Statistical significance was set at P<0.05. Briefly, the normality of the data was tested using the Shapiro-Wilk test. Normally distributed data were analyzed by one-way ANOVA followed by multiple comparison test for >2 group comparisons and Student’s test for 2 group comparison. For data that did not pass the Shapiro-Wilk normality test, the Kruskal-Wallis test with post hoc Dunn test was used for >2 group comparisons and the Mann-Whitney U test for 2 group comparison.
A total of 15 patients with CHF were recruited during the study period. At enrollment, 9 patients (3 female, 6 male) were not being treated with ARNI (non-ARNI group) and 6 patients (1 female, 5 male) were being treated with ARNI (ARNI group) (Figure 1A). The baseline clinical characteristics of the patients are shown in the Table. The mean age of the non-ARNI and ARNI groups was 77.8±2.4 and 79.8±2.2 years, respectively (P=0.71) and the mean body mass index was 23.7±1.3 and 22.0±0.6, respectively (P=0.30). Medical history, including dyslipidemia (non-ARNI vs. ARNI: 77.8% vs. 66.7%, P>0.99), diabetes mellitus (44.4% vs. 33.3%, P>0.99), and atrial fibrillation (77.8% vs. 66.7%, P>0.99), was similar between the 2 groups, and there was a trend toward a higher prevalence of chronic kidney disease in the ARNI group (77.8% vs. 100%, P=0.49), and of hypertension (66.7% vs. 16.7%, P=0.12) and previous stroke (33.3% vs. 16.7%, P=0.60) in the non-ARNI group. In the non-ARNI group, 4 patients (44.4%) were being treated with angiotensin-converting enzyme inhibitors (ACEI) or angiotensin II receptor blockers (ARB). The ARNI group showed a trend toward higher left ventricular ejection fraction (LVEF: 37.3±3.9% vs. 46.8±6.5%, P=0.20), lower plasma BNP levels (238.1±73.6 vs. 184.6±55.8 pg/mL, P=0.60), lower creatinine levels (1.60±0.20 vs. 1.38±0.17 mg/dL, P=0.45), and higher estimated glomerular filtration rate (34.0±4.9 vs. 39.6±3.9 mL/min/1.73 m2, P=0.27) than the non-ARNI group.
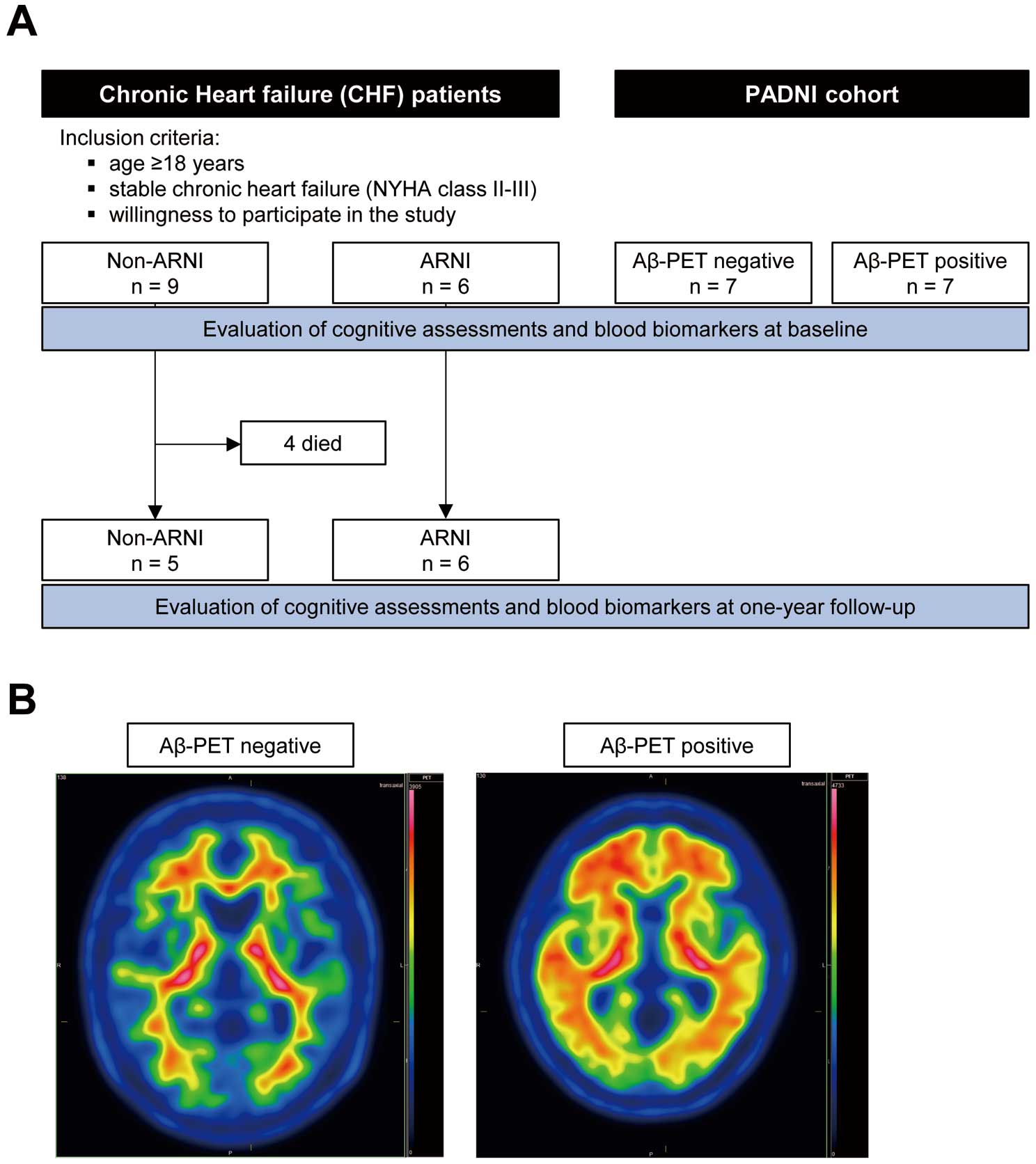
Study design and patient population. (A) Flow chart of patient inclusion and follow-up. Non-ARNI, patients untreated with ARNI; ARNI, patients treated with ARNI. (B) Representative axial amyloid-β (Aβ) PET images from Aβ-negative and Aβ-positive patients in the PADNI cohort. The image from an Aβ-positive patient shows abnormal deposition of amyloid in the brain. ARNI, angiotensin-receptor-neprilysin inhibitors; PET, positron emission tomography.
Baseline Characteristics of the Patients With Chronic Heart Failure and Controls
| Characteristics | CHF patients | P value | PADNI cohort | ||
|---|---|---|---|---|---|
| Non-ARNI | ARNI | Aβ-negative | Aβ-positive | ||
| Overall | 9 | 6 | 7 | 7 | |
| Female sex, n (%) | 3 (33.3) | 1 (16.7) | 0.60 | 4 (57.1) | 4 (57.1) |
| Age, years | 77.8±2.4 | 79.8±2.2 | 0.71 | 73.4±2.8 | 76.6±2.1 |
| Body mass index | 23.7±1.3 | 22.0±0.6 | 0.30 | 23.7±1.8 | 21.6±1.1 |
| Medical history, n (%) | |||||
| Hypertension | 6 (66.7) | 1 (16.7) | 0.12 | 3 (42.9) | 1 (14.3) |
| Dyslipidemia | 7 (77.8) | 4 (66.7) | >0.99 | 3 (42.9) | 1 (14.3) |
| Diabetes mellitus | 4 (44.4) | 2 (33.3) | >0.99 | 0 | 0 |
| Chronic kidney disease | 7 (77.8) | 6 (100) | 0.49 | 0 | 0 |
| Atrial fibrillation | 7 (77.8) | 4 (66.7) | >0.99 | 1 (14.3) | 0 |
| Stroke | 3 (33.3) | 1 (16.7) | 0.60 | 0 | 0 |
| Monotherapy with ACEI or ARB | 4 (44.4) | – | – | – | |
| Laboratory data | |||||
| BNP, pg/mL | 238.1±73.6 | 184.6±55.8 | 0.60 | – | – |
| Creatinine, mg/dL | 1.60±0.20 | 1.38±0.17 | 0.45 | 0.78±0.08 | 0.81±0.06 |
| eGFR, mL/min/1.73 m2 | 34.0±4.9 | 39.6±3.9 | 0.27 | 67.5±7.3 | 61.1±4.3 |
| Ejection fraction | 37.3±3.9 | 46.8±6.5 | 0.20 | – | – |
Data were presented as mean±SEM. P values were calculated using unpaired t tests. ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin-receptor blocker; ARNI, angiotensin-receptor-neprilysin inhibitors; BNP, B-type natriuretic peptide; eGFR, estimated glomerular filtration rate; NfL, neurofilament light chain; p-tau 181, tau phosphorylated at threonine 181.
Cognitive Assessments and Blood Biomarker Tests for Cognitive Impairment at Baseline
To assess the robustness of the cognitive assessments and blood biomarkers in the small sample size, age-matched participants in the PADNI cohort (Aβ-negative group: n=7; Aβ-positive group: n=7) were used as control groups (Figure 1A,B; Table). The Aβ-positive group showed a trend toward lower MMSE-J scores (Aβ-negative vs. Aβ-positive: 27.7±1.2 vs. 23.9±2.1, P=0.16, Figure 2A) and significantly lower MoCA-J scores (24.7±1.7 vs. 17.1±1.6, P<0.01, Figure 2B) compared with the Aβ-negative group. In contrast, there was no difference in MMSE-J scores (non-ARNI vs. ARNI: 26.4±1.0 vs. 27.5±1.0, P=0.91, Figure 2A) or MoCA-J scores (22.3±1.2 vs. 23.2±0.8, P>0.99, Figure 2B) between the non-ARNI and ARNI groups. Blood biomarker analysis revealed that the Aβ-positive group showed significantly higher p-tau181 levels than the Aβ-negative group (Aβ-negative vs. Aβ-positive: 2.64±0.54 vs. 7.45±1.20 pg/mL, P<0.01, Figure 2C). There was no difference in p-tau181 level between the non-ARNI and ARNI groups; instead, the ARNI group showed a trend lower p-tau181 level than the non-ARNI group (non-ARNI vs. ARNI: 7.38±1.45 vs. 4.27±0.52 pg/mL, P=0.20, Figure 2C). The differences in NfL levels were not observed between the Aβ-negative and positive groups (24.3±3.08 vs. 34.5±3.96 pg/mL, P=0.55, Figure 2D). Although it did not reach statistical significance, the ARNI group showed a trend toward lower NfL levels than non-ARNI group (61.6±10.0 vs. 37.9±5.18 pg/mL, P=0.05, Figure 2D).

Cognitive assessments and blood biomarkers for cognitive impairment in patients with chronic heart failure and control subjects at baseline. MMSE-J (A) and MoCA-J (B) scores for each group. Plasma p-tau181 (C) and NfL (D) levels in each group. Data are presented as mean±SEM. P values were calculated using one-way ANOVA followed by the Holm-Sidak multiple comparisons test. ARNI, angiotensin-receptor–neprilysin inhibitors; MMSE-J, Mini-Mental State Examination Japanese; MoCA-J, Japanese version of Montreal Cognitive Assessment; NfL, neurofilament light chain; p-tau 181, tau phosphorylated at threonine 181.
Cognitive Assessments and Blood Biomarkers Test for Cognitive Impairment at 1-Year Follow-up
During the follow-up period, 4 patients in the non-ARNI group had a cardiovascular death. Consequently, cognitive assessments and blood biomarker tests were performed for the remaining 11 patients at the 1-year follow-up. There was no difference in changes of LVEF (non-ARNI vs. ARNI: 0.60±4.80 vs. 0.67±1.58, P=0.99, Supplementary Figure 1A) or BNP level (−36.8±60.4 vs. −40.2±31.6, P=0.96, Supplementary Figure 1B) between the non-ARNI and ARNI groups. There was no difference in LVEF or BNP level between baseline and 1-year follow-up in either the non-ARNI or ARNI group (baseline vs. 1-year follow-up: LVEF in non-ARNI 37.8±5.86% vs. 38.4±5.38%, P=0.91, LVEF in ARNI 46.8±6.54% vs. 47.5±6.54%, P=0.69, Supplementary Figure 1C; BNP in non-ARNI 337.2±113.3 vs. 300.4±148.6 pg/mL, P=0.58, BNP in ARNI 184.6±55.8 vs. 144.4±30.9 pg/mL, P=0.26, Supplementary Figure 1D). There were no significant differences in MMSE-J (27.0±1.22 vs. 26.8±1.19, P=0.93, Figure 3A) or MoCA-J scores (22.5±1.76 vs. 23.8±1.51, P=0.59, Figure 3B) between the non-ARNI and ARNI groups. In addition, there were no significant differences between them in the changes in MMSE-J score (non-ARNI vs. ARNI: 0.75±1.49 vs. −0.67±1.33, P=0.56, Figure 3C) and MoCA-J scores (−0.50±1.04 vs. 0.67±0.76, P=0.38, Figure 3D) from baseline. Moreover, there was no significant difference in p-tau181 level between the non-ARNI and ARNI groups, and the ARNI group maintained a trend toward lower p-tau181 level (7.47±2.21 vs. 4.77±0.65, P=0.23, Figure 4A). There was no difference in NfL levels between the non-ARNI and ARNI groups (53.6±12.5 vs. 47.7±3.66 pg/mL, P=0.63, Figure 4B). In addition, a difference in the change of p-tau181 level from baseline was not observed between the non-ARNI and ARNI groups (−0.81±1.0 vs. 0.49±0.38 pg/mL, P=0.22, Figure 4C). A difference in the change of NfL level from baseline was also not observed between the non-ARNI and ARNI groups (2.19±4.72 vs. 9.86±4.26, P=0.18, Figure 4D). There was no difference in p-tau181 or NfL level between baseline and 1-year follow-up in either the non-ARNI or ARNI group (baseline vs. 1-year follow-up: p-tau181 in non-ARNI 8.28±2.61 vs. 7.47±2.21 pg/mL, P=0.46, p-tau181 in ARNI; 4.27±0.52 vs. 4.77±0.65 pg/mL, P=0.25, Supplementary Figure 2A; NfL in non-ARNI 37.8±5.18 vs. 47.7±3.66 pg/mL, P=0.07, NfL in ARNI 51.4±13.9 vs. 53.6±12.5 pg/mL, P=0.67, Supplementary Figure 2B).

Cognitive assessments in patients with chronic heart failure at 1-year follow-up. MMSE-J (A) and MoCA-J (B) scores for each group. Changes in MMSE-J (C) and MoCA-J (D) scores from baseline in each group. Dead patients were excluded from the analysis. Data are presented as mean±SEM. P values were calculated using unpaired t-tests. ARNI, angiotensin-receptor-neprilysin inhibitors; MMSE-J, Mini-Mental State Examination Japanese; MoCA-J, Japanese version of Montreal Cognitive Assessment.

Blood biomarkers for cognitive impairment in patients with heart failure at 1-year follow-up. Plasma p-tau181 (A) and NfL (B) levels in each group. Changes in plasma p-tau181 (C) and NfL (D) levels from baseline in each group. Dead patients were excluded from the analysis. Data are presented as mean±SEM. P values were calculated using unpaired t-tests. ARNI, angiotensin-receptor-neprilysin inhibitors; MMSE-J, Mini-Mental State Examination Japanese; MoCA-J, Japanese version of Montreal Cognitive Assessment; NfL, neurofilament light chain; p-tau 181, tau phosphorylated at threonine 181.
This is the first study to examine the association between the use of ARNI and cognitive impairment in Japanese patients with CHF using blood biomarkers. We observed no difference in cognitive function assessed by MMSE-J and MoCA-J scores and blood biomarkers for cognitive impairment, including plasma p-tau181 and NfL, between the non-ARNI and ARNI groups at baseline and 1-year follow-up.
Neprilysin is an enzyme that breaks down Aβ in the central nervous system. Its expression and activity in the brain decrease with normal aging and in patients with AD, and this decline inversely correlates with Aβ accumulation.18,19 Such findings raise concerns about the long-term neurocognitive safety associated with ARNI use. However, data from primates and humans have yielded inconclusive findings regarding the association between ARNI use and Aβ clearance in the central nervous system.20,21 In addition, clinical studies have not found an association between ARNI use and cognitive dysfunction or increased AD risk in patients with CHF.12,13 However, given the relatively short duration of the clinical studies and the potentially long time required to assess changes in cognitive function, some concerns remain. Furthermore, there is limited data on the effect of ARNI on cognitive function in Japanese populations.
In our study, we assessed cognitive function in Japanese patients with CHF using the Japanese versions of MMSE and MoCA (MMSE-J and MoCA-J), which are widely used cognitive testing methods. Based on the traditional cutoff score (<28 for MMSE-J; 26 for MoCA-J), indicating mild cognitive impairment, both the MoCA-J and MMSE-J scores revealed the presence of mild cognitive impairment in the patients with CHF in our study. The MoCA-J demonstrated a more substantial decline from its cutoff score, whereas the MMSE-J exhibited only a slight decline, which is consistent with previous studies reporting that the MoCA test is more sensitive in detecting mild cognitive impairment in patients with CHF.22 Notably, we did not find any significant difference in cognitive function between the non-ARNI and ARNI groups of Japanese patients with CHF at either baseline or 1-year follow-up, supporting the safety of ARNI for cognitive function. However, assessing the decline in cognitive function using cognitive tests is highly challenging within the limited duration of clinical studies.
Blood biomarkers offer a great advantage for AD diagnosis compared with conventional approaches such as clinical assessment or neural imaging, thus emerging as a promising approach for the early detection of AD.23 Previously, we and other groups have shown that the plasma level of p-tau181 is higher in AD patients than in healthy controls.24,25 A recent study reported that plasma p-tau181 levels can differentiate pathology-confirmed AD from normal controls, using a cutoff value of 3.44 pg/mL.25 Additionally, a plasma NfL concentration cutoff ranging from 35.02 to 50.00 pg/mL has been suggested to identify neurodegeneration.26 However, despite these proposed thresholds, no widely accepted standardized reference ranges are currently available for plasma p-tau181 or NfL levels. Therefore, in our study, we included participants from the PADNI cohort as control samples for biomarker evaluation to demonstrate the magnitude of differences in biomarker levels between patients with and without cerebral Aβ deposition. In our current study, we found that the Aβ-positive control group showed significantly higher plasma p-tau181 levels than the Aβ-negative control group, supporting the reliability of using plasma p-tau181 levels to assess AD-related brain changes, even in studies with a small sample size. In contrast, there was no significant difference in plasma p-tau181 levels between the non-ARNI and ARNI groups at either baseline or 1-year follow-up. We also evaluated plasma NfL as a general neurodegeneration marker, and found no difference between the non-ARNI and ARNI groups at either time point. Our results are consistent with a recently published study in which a secondary analysis of a clinical trial investigating the effect of ARNI on cardiac remodeling in patients after myocardial infarction demonstrated no correlation between ARNI use and changes in AD blood biomarkers, including plasma p-tau181 and NfL.13,27 Furthermore, although detailed results are not yet available, the PERSPECTIVE trial (NCT02884206) reported that ARNI use did not affect brain Aβ deposition, as observed by PET, in patients with CHF with mildly reduced and preserved EF, further supporting the safety of ARNI for cognitive function.28
Study LimitationsThere are several that should be acknowledged. First, this was an observational study conducted in a single center, and the number of patients was small, leading to a selection bias of patients. To assess the effect of sample size, we performed a hypothetical calculation using G*Power version 3.1,29 which estimated that 64 participants per group would be needed for sufficient statistical power (80% power, effect size 0.5, α=0.05). Given the small sample size in our study, we recognize that drawing definitive conclusions remains challenging. Additionally, another potential source of selection bias arises from the timing of our study: patients were recruited between 2020 and 2021, shortly after ARNI (sacubitril/valsartan) was approved for clinical use in Japan. During this period, ARNI had not yet been widely adopted, and clinicians may have unconsciously refrained from prescribing it to relatively high-risk patients, although we have no direct evidence to support this assumption. This potential selection bias could have resulted in differences in the baseline characteristics of the ARNI and non-ARNI groups, potentially contributing to the higher mortality rate observed in the non-ARNI group. Second, cerebrospinal fluid analysis and PET images in CHF patients were not evaluated, and these techniques might be more sensitive in detecting neurological damage.30 Finally, the 1-year follow-up period might be too short to fully assess any neurologically deleterious effects of neprilysin inhibition. Although clinical examinations over a 1-year period may be insufficient to evaluate changes in cognitive function, previous studies suggest that biomarkers could still capture cognitive alterations within this timeframe in the natural history of AD.31,32 However, it remains uncertain whether biomarkers can adequately assess drug-induced cognitive changes over 1 year.
Despite these limitations, our findings suggest that neprilysin inhibition-induced impairment of Aβ degradation in the brain, if present, may not be substantial enough to cause clinically significant cognitive impairment. However, other mechanisms may also compensate for this impairment, warranting further consideration. Importantly, bradykinin, whose degradation is also mediated by neprilysin, is implicated in both neuroprotective and neurotoxic processes in AD pathophysiology. Although bradykinin is involved in promoting neuroinflammation and tau phosphorylation, processes contributing to AD progression, it also exerts neuroprotective effects through the promotion of Aβ clearance and attenuation of proinflammatory responses.33 Additionally, the potential improvement in cerebral blood flow regulation resulting from the alleviation of HF with ARNI use might have had a positive effect on cognitive function. Given these complex and multifaceted interactions, neprilysin inhibition alone may not necessarily lead to cognitive impairment in patients receiving ARNI therapy.
In summary, our pilot-scale study with short-term follow-up suggests that ARNI treatment in Japanese patients with CHF is not associated with significant cognitive decline or an increase in blood biomarkers for cognitive dysfunction. Although these preliminary findings support the neurological short-term safety of neprilysin inhibitors, further large-scale, long-term studies are needed to draw definitive conclusions about their cognitive effects.
This work was supported by the Japan Heart Foundation Research Grant (F.K.-M.).
The authors declare no conflicts of interest associated with this manuscript. This work was supported by the Japan Heart Foundation Research Grant (F.K.-M.).
This study was approved by the Institutional Review Board of the Kyoto Prefectural University of Medicine (ERB-C-2027-1).
The de-identified participant data will not be shared.
Please find supplementary file(s);
https://doi.org/10.1253/circrep.CR-24-0175