Abstract
Background: Heart failure (HF) is an increasing public health concern in Japan, largely related to the aging population. This protocol describes the rationale, objectives, and methods of the Hokuriku-plus Heart Failure Registry (HpHFR), designed to establish a comprehensive clinical and digital database to assess novel prognostic indicators in patients with HF.
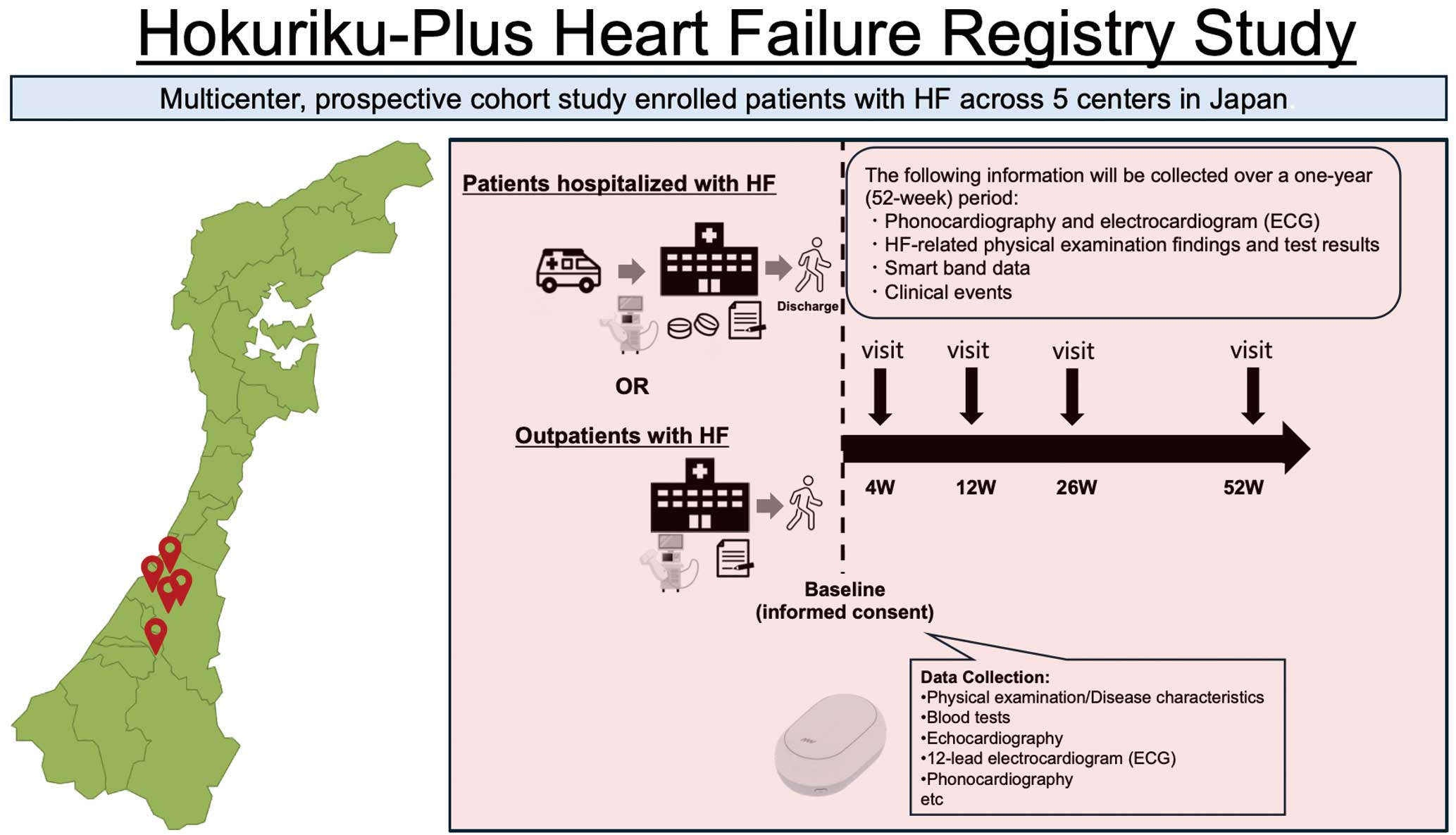
Methods and Results: HpHFR is a prospective, multicenter observational cohort study that has enrolled patients with acute or chronic HF from 5 Japanese centers. Eligible patients provided informed consent and underwent baseline clinical, laboratory, and biometric assessment, including digital phonocardiography and wearable device data. Follow-up assessment will be conducted at 4, 12, 26, and 52 weeks. Primary outcomes include all-cause death, HF-related hospitalization, and worsening HF. Secondary outcomes are the changes in clinical parameters over time. A digital substudy will investigate continuous biometric monitoring, and a genomic substudy explores the loss of chromosome Y as a prognostic biomarker.
Conclusions: In conclusion, this study protocol summarizes the development of a valuable prospective cohort resource. This registry will provide a unique dataset enabling multifaceted investigations to improve HF prediction and management by systematically integrating comprehensive clinical and laboratory data with biometric indicators derived from digital devices and genomic markers.
Central Figure
Cardiovascular diseases remain the second leading cause of death in Japan, with heart failure (HF) as a major contributor.1–3 The HF burden, driven by the aging population, is increasing by approximately 10,000 hospitalizations annually, and projections estimate up to 1.3 million HF patients by 2030 in Japan, signaling an “HF pandemic.”4–12 This rise and the economic impact strain the healthcare system.13 Addressing these challenges emphasizes the need for strategies to optimize HF management, prevent readmission, mitigate cardiovascular events, and improve survival.
Current HF management has advanced with combination therapies, including angiotensin receptor-neprilysin inhibitors, β-blockers, mineralocorticoid-receptor antagonists, and sodium-glucose cotransporter 2 inhibitors, which significantly reduce cardiovascular events.14–19 The availability of robust clinical markers for accurately predicting acute decompensation and subsequent rehospitalization among patients with chronic HF remains limited despite the therapeutic advances. Although several risk models have been developed and validated, including those for Japanese patients, their integration into routine clinical practice within Japan has been suboptimal.20–23 Therefore, a pressing unmet clinical need exists for validated prognostic indicators that are capable of guiding therapeutic decision-making through accurate disease progression identification, clinical decompensation prediction, and mortality risk assessment.
Advanced hemodynamic monitoring paradigms that extend beyond conventional diagnostic approaches may provide novel information for this pressing clinical challenge. Digitizing phonocardiographic and electrocardiographic signals into biomarkers can capture subtle signs imperceptible to the human ear or eye, enabling new approaches to cardiovascular assessment.24,25 The combination of traditional clinical parameters with genomic profiling and real-time digital biometric data represents a promising strategy for developing innovative prognostic indicators in HF management.26 Notably, wearable technology has appeared as a valuable digital solution, enabling continuous biometric data collection during daily activities. This approach facilitates real-time monitoring of physiological parameters, providing opportunities to detect early clinical deterioration.
In this study we aim to build a clinical HF database integrating biometric data from digital devices with conventional variables to identify and validate novel prognostic indicators, including a digital biomarker-based risk stratification tool.
Methods
Study Design and Overview
The Figure is a schematic of the study. This multicenter, prospective cohort study enrolled patients who presented with acute or chronic HF and met the predefined inclusion criteria at 5 participating centers in Japan. Enrollment commenced in February 2024. After obtaining written informed consent from eligible outpatients or inpatients, we collected baseline data from electronic medical records, including demographics, clinical findings, disease characteristics, and laboratory results. The study protocol stipulates the acquisition of electrocardiogram (ECG), echocardiography, and digital phonocardiogram (PCG) recordings at specified time points. A validated electric data capture (EDC) system captures and manages all study data.

The cohort study adheres to the principles outlined in the Declaration of Helsinki and complies with the Ethical Guidelines for Medical and Biological Research Involving Human Subjects, alongside other relevant guidelines in Japan. The Medical Ethics Committee of Kanazawa University approved the study protocol, which was registered in the University Medical Information Network Clinical Trial Registry (UMIN-CTR ID: UMIN000053521).
Participants
Patients with a clinical diagnosis of either acute or chronic HF were enrolled from February 2024. Eligibility was determined by fulfilling all inclusion criteria and none of the exclusion criteria (Supplementary Appendixes 1,2). All participants signed written informed consent using forms approved by the Medical Ethics Committee of Kanazawa University before enrollment.
Outcomes
The primary outcome included a composite of cardiac events, defined as all-cause death, HF-related hospitalization, and worsening HF. Event definitions adhered to the American Heart Association criteria.27 Supplementary Appendix 3 lists detailed operational definitions for death, HF-related hospitalization, and worsening HF. Secondary outcomes were changes from baseline in clinical parameters, laboratory findings, physiological test results, and imaging modalities at the 4-, 12-, 26-, and 52-week follow-up points.
Data Collection and Management
REDCap (Research Electronic Data Capture; Vanderbilt University, Nashville, TN, USA), a secure, web-based, metadata-driven EDC platform, was used to collect study data. The Table presents the assessment schedule and data collection time points after enrollment. Supplementary Appendix 4 provides a comprehensive list of collected variables and measurement parameters.
Table.
Data Collection and Follow-up Schedule
| Follow-up period |
0 week |
4 weeks† |
12 weeks† |
6 months |
1 year |
At events |
| Baseline |
Follow-up |
Clinical
outcomes |
| Informed consent |
● |
|
|
|
|
|
| Basic information |
● |
|
|
|
|
|
| Disease characteristics |
● |
|
|
|
|
|
| Adverse events |
 |
● |
| Clinical findings |
| Physical examination |
● |
● |
● |
● |
● |
● |
| HF severity |
● |
● |
● |
● |
● |
● |
| Frailty/sarcopenia |
○‡ |
|
|
|
○‡ |
|
| SPPB |
○‡ |
|
|
|
|
|
| Blood tests |
● |
● |
● |
● |
● |
● |
| Chest X-ray |
● |
○ |
○ |
● |
● |
● |
| Electrocardiogram* |
● |
● |
● |
● |
● |
● |
| Phonocardiogram* |
● |
● |
● |
● |
● |
● |
| Echocardiography* |
● |
○ |
○ |
● |
● |
● |
| Depression Scale |
● |
|
|
|
● |
|
| Body composition analysis |
○ |
|
|
|
|
|
| Cardiopulmonary exercise test |
○ |
|
|
|
|
|
| Sympathetic nervous activity |
○‡ |
|
|
|
|
|
| Smart Band§ |
○  |
|
|
|
|
| Confirmation of cardiac event information |
 |
● |
●: Mandatory measurement item; ○: Optional measurement item. *Data will be used from tests conducted on the same day, in principle, for these examination items. †The responsible researcher or co-investigator may allow the 4-week and 12-week follow-up visits and examinations to be skipped if a primary care physician conducted regular outpatient visits as part of a hospital-clinic collaboration. ‡Conducted only at Kanazawa University Hospital. §We used either the Fitbit Inspire 3 device (Google/Fitbit, San Francisco, CA, USA) or the Axivity AX3 (Axivity Ltd., Newcastle, UK) for participants who provided informed consent. HF, heart failure.
Digital PCG
The digital PCG system (AMI-SSS01 series, developed by AMI, Inc. [Kagoshima, Japan]) used for this study integrates bipolar ECG acquisition with PCG, which was classified into 4 frequency bands. It utilizes a previously described artificial intelligence/machine learning (ML) model to automatically predict cardiac stress levels and classify valvular heart disease severity based on the combined ECG and PCG data.28 In the current study protocol, a trained examiner acquired 8-s waveform recordings from 4 standard precordial locations (2RSB, 2LSB, 4LSB, and 5LMCL) at baseline and subsequently at 4, 12, 26, and 52 weeks post-enrollment.
Wearable Device Substudy
The wearable device substudy was exclusively conducted at Kanazawa University Hospital. It aimed to recruit 100 participants from the main cohort who provided additional consent to wear a Fitbit Inspire 3 device (Google/Fitbit, San Francisco, CA, USA) for continuous biometric data collection. Details of the wearable device substudy are described in Supplementary Appendix 5.
Flow Cytometry, Somatic Variant Investigation, and Gene Expression Analysis
At Kanazawa University, peripheral blood samples were collected into specific anticoagulated tubes for distinct downstream analyses. Specifically, 10 mL of blood was collected into heparin tubes for flow cytometry, 7 mL samples into ethylenediaminetetraacetic acid tubes for somatic variant analysis, and 2 separate 2.5-mL samples into PAXgene Blood RNA Tubes (Qiagen, Hilden, Germany), designated for gene expression analysis (RNA-Seq) and loss of chromosome (e.g., LoX or LoY) assessment, respectively.
Follow-up Schedule
The Table outlines the schedule of assessments and follow-up visits. The follow-up schedule is described in Supplementary Appendix 6.
An independent clinical research associate performed systematic monitoring. Initial monitoring visits were scheduled after enrolling the first participants at each site, with subsequent reviews occurring at regular intervals (e.g., monthly or bi-monthly, depending on enrollment pace) until completing the verification of the final participant’s case report form. An independent data manager, who issues data queries to site personnel as required to ensure data accuracy, consistency, and completeness, regularly monitors and validates the study database.
Statistical Analysis
Baseline characteristics and outcome data will be summarized using descriptive statistics. Continuous variables will be reported as mean±standard deviation or median (interquartile range), depending on distribution. Categorical variables will be presented as frequencies and percentages. Student’s t-test or Mann-Whitney U test will be conducted for continuous variables, and chi-square tests or Fisher’s exact tests will be used for categorical variables. Multivariable analyses, including logistic regression (for binary outcomes) and Cox proportional hazards regression (for time-to-event outcomes), will be conducted to determine independent predictors of the primary and secondary outcomes, adjusting for relevant baseline covariates. Model performance and predictive accuracy will be assessed utilizing appropriate metrics (e.g., C-statistic/area under the curve).
To develop novel prognostic models, both conventional and ML approaches will be applied. ML will be used to extract features from ECG waveforms, digital PCG, and physiological data (e.g., accelerometry, photoplethysmography) obtained from devices. Physical activity patterns and derived indices from temporal clustering of acoustic, ECG, and echocardiographic features will be explored for their association with clinical outcomes. Model validation will use techniques such as k-fold cross-validation and C-index calculation. Advanced ML methods, including deep learning models (e.g., DeepSurv), may be considered. The prognostic value of digital biomarkers, particularly acoustic features, will be evaluated using regression or Cox models adjusted for established clinical risk factors.
Discussion
The aim of this multicenter, prospective study is to establish a comprehensive clinical database tailored for prognostic assessment in patients with HF through systematic enrollment across multiple Japanese centers. A principal strength of this initiative is the planned integration of multimodal biometric data, acquired via contemporary digital devices, with conventional clinical parameters. This integrated approach can help develop and validate novel prognostic indicators for HF.
The escalating prevalence of HF, popularly known as the “HF pandemic” and largely driven by aging populations globally, poses a substantial public health challenge in Japan.10–12 Recent pharmacological advancements have expanded the therapeutic armamentarium for HF beyond traditional approaches; however, a critical unmet need persists for reliable clinical indicators and biomarkers.14–17 Such tools are crucial for accurately predicting acute decompensation events, optimizing therapeutic interventions, and ultimately preventing recurrent hospitalizations among individuals with chronic HF.
Our study aims to address a notable gap in Japanese cardiovascular research by establishing, prospectively from inception, a registry that incorporates digital health parameters specifically for developing innovative prognostic tools in HF. The centralized management structure designed for this multilayered, multidimensional dataset is anticipated to not only facilitate the identification of novel pathophysiological information and inform future treatment strategies, but also to contribute to standardized data infrastructure establishment. Such an infrastructure could be a foundation element for next-generation, community-based integrated care systems in Japan. Implementing such systems, informed by data, such as those collected in our study, can improve cardiovascular disease management at both regional and national levels, thereby helping reduce disparities in health expectancy and overall public health promotion.
A significant aspect of this study is the planned digital PCG data collection at both baseline and follow-up assessments. Concurrent advancements in ML-enhanced digital stethoscopes demonstrate an increasing potential, including applications for non-invasively estimating cardiac stress biomarkers such as plasma B-type natriuretic peptide. Moreover, the use of quantitative acoustic cardiography features in predicting adverse cardiac events is an active area of investigation to which this study is well positioned to contribute. Another strength is collecting continuous, biometric data with wearable devices in a dedicated sub-cohort. The analysis of these data streams provides a unique opportunity to assess the potential use of passively collected physiological metrics in predicting or detecting early signs of HF exacerbation outside of traditional clinical settings.
Furthermore, using the collected biological samples, we plan to assess the association between the loss of chromosomes in peripheral blood cells and HF outcomes within our cohort. Previous seminal work has revealed the LoY as a potential contributor to the development and progression of cardiovascular diseases, including HF.29 Exploring chromosome loss as a biomarker for risk stratification, combined with digital biomarkers, may yield novel insights into HF prediction and personalized management.
In conclusion, this study protocol summarizes the development of a valuable prospective cohort resource. This registry will provide a unique dataset enabling multifaceted investigations aimed at improving HF prediction and management by systematically integrating comprehensive clinical and laboratory data with biometric indicators derived from digital devices and genomic markers.
Acknowledgments
We appreciate the support and collaboration of the co-investigators participating in the Hokuriku-plus Heart Failure Registry study.
IRB Information
The present study was approved by the Institutional Review Board at Kanazawa University. Reference number: 2023-181, 714391.
Funding
This study was supported by research and development funds jointly provided by the New Energy and Industrial Technology Development Organization (NEDO, Project No. JPNP23019, NEDO Deep Tech Startup Support Fund/Support Program) and AMI Inc. (Kagoshima, Japan).
Competing Interest Statement
Takeshi Kato received lecture fees from AstraZeneca, Ono Pharmaceutical, Novartis Pharma, Abbott Medical Japan, Boston Scientific and Medtronic Japan.
Supplementary Files
Please find supplementary file(s);
https://doi.org/10.1253/circrep.CR-25-0160
References
- 1.
Vital Statistics 2023. Ministry of Health, Labour and Welfare, Japan. https://www.mhlw.go.jp/toukei/saikin/hw/jinkou/kakutei22/ (accessed June 1, 2025).
- 2.
Hirata A, Hirata T. Clinical practice for acute heart failure in Japan from the nationwide registry. Circ J 2024; 88: 1274–1275.
- 3.
Kanaoka K, Iwanaga Y, Sumita Y, Nakai M, Miyamoto Y. Management and outcomes of acute heart failure hospitalizations in Japan. Circ J 2024; 88: 1265–1273.
- 4.
Yasuda S, Miyamoto Y, Ogawa H. Current Status of Cardiovascular Medicine in the Aging Society of Japan. Circulation 2018; 138: 965–967.
- 5.
Shiba N, Nochioka K, Miura M, Kohno H, Shimokawa H. Trend of westernization of etiology and clinical characteristics of heart failure patients in Japan. Circ J 2011; 75: 823–833.
- 6.
Conrad N, Judge A, Tran J, Mohseni H, Hedgecott D, Crespillo AP, et al. Temporal trends and patterns in heart failure incidence: A population-based study of 4 million individuals. Lancet 2018; 391: 572–580.
- 7.
Shimokawa H, Miura M, Nochioka K, Sakata Y. Heart failure as a general pandemic in Asia. Eur J Heart Fail 2015; 17: 884–892.
- 8.
Tsuji K, Sakata Y, Nochioka K, Miura M, Yamauchi T, Onose T, et al. Characterization of heart failure patients with mid-range left ventricular ejection fraction: A report from the CHART-2 Study. Eur J Heart Fail 2017; 19: 1258–1269.
- 9.
Sidney S, Go AS, Jaffe MG, Solomon MD, Ambrosy AP, Rana JS. Association between aging of the US population and heart disease mortality from 2011 to 2017. JAMA Cardiol 2019; 4: 1280.
- 10.
Okura Y, Ramadan MM, Ohno Y, Mitsuma W, Tanaka K, Ito M, et al. Impending epidemic future projection of heart failure in Japan to the year 2055. Circ J 2008; 72: 489–491.
- 11.
Konishi M, Ishida J, Springer J, von Haehling S, Akashi YJ, Shimokawa H, et al. Heart failure epidemiology and novel treatments in Japan: Facts and numbers. ESC Hear Fail 2016; 3: 145–151.
- 12.
Isobe M. The heart failure “pandemic” in Japan: Reconstruction of health care system in the highly aged society. JMA J 2019; 2: 103–112.
- 13.
Shiba N, Nochioka K, Miura M, Kohno H, Shimokawa H. Trend of westernization of etiology and clinical characteristics of heart failure patients in Japan: First report from the CHART-2 study. Circ J 2011; 75: 823–833.
- 14.
McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. 2023 Focused Update of the 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2023; 44: 3627–3639.
- 15.
McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021; 42: 3599–3726.
- 16.
Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, et al. 2022 AHA/ACC/HFSA Guideline for the management of heart failure: Executive summary. J Am Coll Cardiol 2022; 79: 1757–1780.
- 17.
Tsutsui H, Ide T, Ito H, Kihara Y, Kinugawa K, Kinugawa S, et al. JCS/JHFS 2021 Guideline focused update on diagnosis and treatment of acute and chronic heart failure. J Card Fail 2021; 27: 1404–1444.
- 18.
Tsutsui H, Momomura SI, Saito Y, Ito H, Yamamoto K, Sakata Y, et al. Long-term treatment with sacubitril/valsartan in Japanese patients with chronic heart failure and reduced ejection fraction: Open-label extension of the PARALLEL-HF Study. Circ J 2023; 88: 43–52.
- 19.
Kitai T, Kohsaka S, Kato T, Kato E, Sato K, Teramoto K, et al. JCS/JHFS 2025 Guideline on diagnosis and treatment of heart failure. Circ J 2025; 89: 1278–1444.
- 20.
Lee DS, Stitt A, Austin PC, Stukel TA, Schull MJ, Chong A, et al. Prediction of heart failure mortality in emergent care: A cohort study. Ann Intern Med 2012; 156: 767–775, W-261, W-262.
- 21.
Sartipy U, Dahlström U, Edner M, Lund LH. Predicting survival in heart failure: Validation of the MAGGIC heart failure risk score in 51,043 patients from the Swedish heart failure registry. Eur J Heart Fail 2014; 16: 173–179.
- 22.
Shiraishi Y, Kohsaka S, Nagai T, Goda A, Mizuno A, Nagatomo Y, et al. Validation and recalibration of Seattle Heart Failure Model in Japanese acute heart failure patients. J Card Fail 2019; 25: 561–567.
- 23.
Levy WC, Mozaffarian D, Linker DT, Sutradhar SC, Anker SD, Cropp AB, et al. The Seattle Heart Failure Model: Prediction of survival in heart failure. Circulation 2006; 113: 1424–1433.
- 24.
Ogawa S, Namino F, Mori T, Sato G, Yamakawa T, Saito S. AI diagnosis of heart sounds differentiated with super StethoScope. J Cardiol 2024; 83: 265–271.
- 25.
Ogawa S, Ishii M, Saito S, Seki H, Ikeda K, Yasui Y, et al. Deep learning for cardiac overload estimation: Predicting B-type natriuretic peptide (BNP) levels from heart sounds and electrocardiogram. Circ J 2025; 89: 1684–1692.
- 26.
Nomura A. Digital health, digital medicine, and digital therapeutics in cardiology: Current evidence and future perspective in Japan. Hypertens Res 2023; 46: 2126–2134.
- 27.
Stout KK, Daniels CJ, Aboulhosn JA, Bozkurt B, Broberg CS, Colman JM, et al. 2018 AHA/ACC Guideline for the management of adults with congenital heart disease: Executive summary. J Am Coll Cardiol 2019; 73: 1494–1563.
- 28.
Nomura A, Takeji Y, Shimojima M, Takamura M. Digitalomics: Towards artificial intelligence/machine learning-based precision cardiovascular medicine. Circ J 2025, doi:10.1253/circj.CJ-24-0865.
- 29.
Sano S, Horitani K, Ogawa H, Halvardson J, Chavkin NW, Wang Y, et al. Hematopoietic loss of Y chromosome leads to cardiac fibrosis and heart failure mortality. Science 2022; 377: 292–297.




