2012 Volume 37 Issue 2 Pages 81-87
2012 Volume 37 Issue 2 Pages 81-87
Directing the axis of cell division toward extrinsic and intrinsic cues plays a fundamental role in morphogenesis, asymmetric cell division, and stem cell self-renewal. Recent studies highlight the misorientation of the cell division axis as a cause of mammalian diseases, including polycystic kidney disease. Although the core regulators for oriented cell division have been identified in invertebrate model systems, we still have an imprecise picture of the relevant signaling networks in the mammalian system. The reasons for this include the lack of established approaches in mammalian cells to survey the molecules required for the spindle orientation. Here we summarize our recent study on a genome-scale RNA-mediated interference screen of human kinases to identify a new player for the oriented cell division in both culture cells and developing mammalian tissues.
One major strategy of multicellular organisms to maintain their homeostatic balance is to gradually replace old cells with new ones, or other types of cells, without altering the basic configuration of their bodies. Many types of stem cells contribute to the tissue metabolism by providing the tissues with fresh cells at the right time and the right place (Hsu and Fuchs, 2012). This strategy, however, runs the risk of stochastical errors, whose accumulation eventually leads to a critical exhaustion of regenerative potential on the one hand, or loss of control of proliferation on the other, and ultimately the death of the organism (Conboy and Rando, 2005; Pekovic and Hutchison, 2008). The crisis-management strategies include precise control of cell proliferation, cell differentiation, and cell death. Failures of the control mechanisms are often associated with oncogenesis and cancer (Holland and Cleveland, 2009; Thompson et al., 2010; Janssen and Medema, 2011; McCaffrey and Macara, 2011).
Recent studies have shed light on the orientation of cell division as another management system to regulate tissue homeostasis. The oriented cell division allows stem cells to divide symmetrically or asymmetrically by directing the spindle axis parallel or perpendicularly, respectively, to their attached niche or the extracellular matrix. The balance between symmetric and asymmetric cell division defines the number of undifferentiated stem cells as well as the bulk of differentiated cells in a given tissue. The deregulation of spindle orientation in proliferative cells has been shown to cause abnormal stem cell differentiation, aberrant organ structures and functions, and to lead to the onset of disorders that include tumorigenesis and polycystic kidneys (Fischer et al., 2006; Morrison and Kimble, 2006; Cicalese et al., 2009; Fleming et al., 2009; Neumuller and Knoblich, 2009; Quyn et al., 2010; Scaffidi and Misteli, 2011).
The core regulators of spindle orientation were discovered through forward genetics and mutant analysis, using invertebrate model organisms, such as Drosophila and C. elegans (Kemphues et al., 1988; Suzuki and Ohno, 2006; Knoblich, 2008; Siller and Doe, 2009; Morin and Bellaiche, 2011). The mechanism, at least in part, has been shown to be evolutionarily conserved in mammals (Knoblich, 2008; Siller and Doe, 2009; Morin and Bellaiche, 2011). However, the nature of the extrinsic and/or intrinsic cortical cues that determine the spindle orientation, as well as the signaling pathways between cortical cues and the spindle in mammalian cells, remains largely unknown. In this review, we introduce our recent study on novel spindle orientation control in both mammalian cultured cells and skin epithelium by ABL1 tyrosine kinase, which was identified in a newly established high-throughput screening method (Matsumura et al., 2012).
The evolutionarily conserved regulators for spindle orientationIn asymmetrically dividing Drosophila neuroblasts, the asymmetrically distributed cortical polarity proteins align the spindle with the polarity axis to ensure an unequal segregation of the cell-fate determinants. Bazooka (Baz; Par3 in C. elegans and mammals), Par6, and atypical protein kinase C (aPKC) function as polarity determinants, whereas Inscuteable (Insc), Partner of Inscuteable (Pins; LGN/AGS3 in mammals), Gαi, mushroom body defective (Mud; NuMA in mammals), and Discs large (Dlg) regulate spindle orientation (Fig. 1) (Knoblich, 2008; Siller and Doe, 2009; Morin and Bellaiche, 2011). Pins interacts with Gαi through its C-terminal GoLoco domain and is thought to link the astral microtubules with cortical cues through motor proteins dynein and kinesin Khc-73 (Siegrist and Doe, 2005; Bowman et al., 2006; Izumi et al., 2006; Siller et al., 2006). A recent report using induced cell polarity in Drosophila S2 cells has elucidated the role of Pins-Dlg-Khc73 and Pins-Mud-dynein pathways for the control of microtubule attachment to the cortex and microtubule shortening (force generation), respectively, during spindle orientation (Johnston et al., 2009).
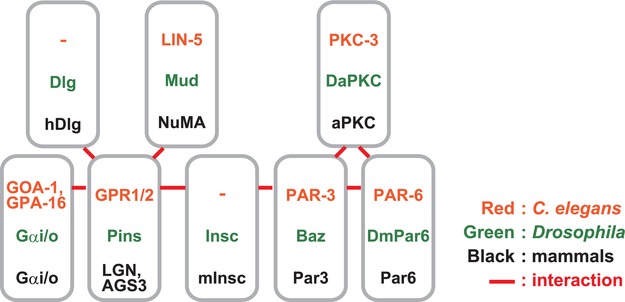
Summary of nomenclature of the core regulators of spindle orientation and their protein interactions.
The Pins-Gα pathway is evolutionarily conserved both in Caenorhabditis elegans and in mammals (Fig. 1) (Knoblich, 2008; Siller and Doe, 2009; Morin and Bellaiche, 2011). In mammalian culture cells, NuMA, a microtubule binding protein that associates with a microtubule minus-end directed motor protein, dynein (Merdes et al., 1996), has been shown to bind to LGN during M phase. This association induces the conformational switch of LGN to an open state, allowing it to associate with Gα at the cortex (Du and Macara, 2004). It is believed that NuMA associates with astral microtubules at the cortex and regulates their attachment to dynein or to a cortical attachment factor, which provides the cortical pulling force on the astral microtubules to orient the spindle (Du and Macara, 2004).
A genome-wide screening method to identify novel regulators for spindle orientation in mammalian cellsWe have previously shown that in non-polarized mammalian adherent cells, such as HeLa cells, spindles are aligned along the cell-substrate adhesion plane, which ensures both daughter cells are attached to the substrate after cell division. In this spindle orientation mechanism, integrin-mediated cell-substrate adhesion provides vertical signals along the z-axis, which results in the accumulation of PtdIns(3,4,5)P3 at the midsection of the cortex during metaphase. PtdIns(3,4,5)P3 recruits the dynactin/dynein complex to the midcortex to orient the spindle parallel to the substratum (Fig. 2A) (Toyoshima et al., 2007b). This mechanism also requires the actin cytoskeleton, astral microtubules, the microtubule plus-end-tracking protein EB1 and myosin X (Toyoshima and Nishida, 2007a). In addition, Cdc42, a Rho family small GTPase, has been shown to regulate the spindle orientation through two distinct pathways: the Cdc42-PAK2-βPix-actin pathway and the Cdc42-PI3K-PtdIns(3,4,5)P3 pathway (Fig. 2A) (Mitsushima et al., 2009).
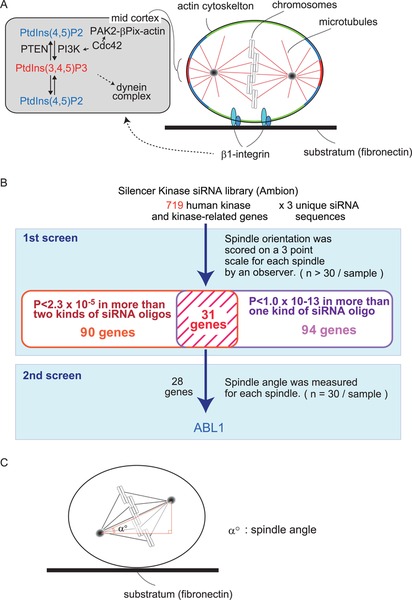
Spindle orientation control in HeLa cells. (A) PtdIns(3, 4, 5)P3 is accumulated in the mid sections at the cell cortex (mid cortex), which is generated by PI3K at the mid cortex via the β1-integrin signaling pathway. PtdIns(3, 4, 5)P3 is required for the localization of the dynein/dynactin complex at the mid-cortex. The boundary between PtdIns(3, 4, 5)P3 at the mid-cortex is defined by the activity of the negative regulator, PTEN, which dephosphorylates PtdIns(3, 4, 5)P3 to PtdIns(4, 5)P2. At the mid-cortex, a small GTPase, Cdc42, upregulates PI3K as well as the PAK2-βPix pathway to regulate actin reorganization. (B) A schematic figure of an RNA-mediated interference screening of human kinases for spindle orientation. (C) The analysis of spindle orientation along the Z-axis relative to the substratum surface. The spindle angle (α°) between the axis of the spindle and that of the substratum is measured from a series of Z-stack images, 0.5 mm-thick sections of metaphase cells stained with anti γ-tubulin antibody, anti α-tubulin antibody and Hoechst.
This HeLa cell system is suitable for a high-throughput screen to identify new regulators of spindle orientation, with the following three advantages: 1) HeLa cells can be efficiently synchronized in M phase, which allows us to obtain enough numbers of metaphase cells for quantitative analyses. 2) Gene expression can be efficiently downregulated by siRNA. 3) The spindle orientation can be easily quantified by directly measuring the angle between the axis of the spindle and that of the substratum. In addition, recent studies have identified several molecules that regulate spindle orientation not only in HeLa cells but also in mouse embryonic tissues (Lechler and Fuchs, 2005; Taddei et al., 2008; Godin et al., 2010). This prompted us to perform an siRNA-based genome-wide screen to identify additional molecules required for proper spindle orientation in HeLa, and to further analyze their functions in mouse tissues in vivo.
We used a commercially available siRNA library, which targets 719 genes of human kinases and kinase-related molecules, and performed a two-step screening procedure. In the first step, the spindle orientation in each metaphase cell was blindly scored on a three-point scale by an observer (n>30/sample) (Fig. 2B). Three individual siRNAs per gene were tested, and p-values were obtained for each siRNA by performing the Mann-Whitney U-test. We judged 31 genes to be hits in the first screening, which met the following two criteria: 1) the P-values were less than 2.3×10−5 in more than two kinds of siRNA; 2) the P-values were less than 1.0×10−13 in more than one kind of siRNA (Fig. 2B). In the second step, we quantified the spindle orientation phenotypes by measuring the spindle angle between the axis of the spindle and that of the substratum (Fig. 2C). Among the 28 candidate genes, ABL1 displayed the highest score (Fig. 2B).
ABL1 is a new player in the core machinery of spindle orientationKnockdown of ABL1 elicits spindle misorientation, which can be rescued by a siRNA-resistant form wild-type ABL1, confirming a definitive role of ABL1 in the control of spindle orientation in HeLa cells. However, both the Cdc42-PAK2-βPix-actin pathway and the PI3K-PtdIns(3,4,5)P3 pathway function normally in the ABL1-depleted cells. It appears that LGN and NuMA, the core machinery of spindle orientation in many systems, play minor roles in the HeLa cell system, since the spindles are normally oriented parallel to the substratum in the LGN or NuMA-depleted cells. In the ABL1-depleted cells, however, we found that an excessive amount of LGN protein accumulated at the cortex in metaphase cells. Indeed, this accumulation of LGN at the cortex has been shown to induce the abnormal rotational motion of the spindle about the z-axis, which leads to spindle misorientation. Thus, ABL1 regulates the core machinery of spindle orientation by preventing the excessive accumulation of LGN protein at the cortex in metaphase.
Another important role of ABL1 in the core machinery is anchoring NuMA at the cortex by directly phosphorylating NuMA at tyrosine 1774. In HeLa cells, NuMA is co-localized with LGN at the cell cortex (Fig. 3A) (Du and Macara, 2004; Matsumura et al., 2012). Downregulation of ABL1 causes the disappearance of NuMA from the cortex in metaphase HeLa cells, even though LGN accumulates at the cortex (Fig. 3B). The phosphorylation of Y1774 by ABL1 is required for anchoring NuMA at the cortex during metaphase, but dispensable for targeting NuMA to the cortex during prometaphase or binding NuMA with LGN, which is required for targeting the NuMA/LGN complex to the cortex (Fig. 3A) (Du and Macara, 2004; Matsumura et al., 2012). That is to say, NuMA is localized to the cortex by two steps: 1) it is recruited to the cortex via binding to LGN; 2) it is anchored to the cortex via the ABL1-catalyzed Y1774 phosphorylation of NuMA. This second step has been further shown to be required for directing the spindle axis toward the center of the LGN crescent which is localized at the mid-cortex (Fig. 3B). Thus, ABL1 has a dual role in the core spindle orientation machinery; regulation of the cortical localization of LGN, and anchoring NuMA at the cortex to align the spindle with the LGN polarity axis (Fig. 3B).
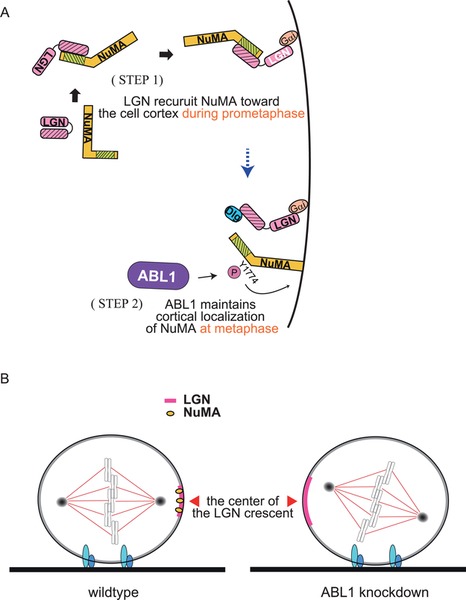
ABL1-mediated spindle orientation in HeLa cells. (A) The two step mechanism for the cortical localization of NuMA during M phase. In the 1st step, in prometaphase, NuMA binds to LGN, which induces the conformational switch of LGN to an open state, following association of NuMA/LGN complex with cortical-anchored Gα. In the 2nd step, in metaphase, NuMA dissociates from LGN and is anchored to the cortex via the ABL1-catalyzed Y1774 phosphorylation of NuMA. (B) Dual role of ABL1 in the control of spindle orientation: 1) ABL1 focuses the LGN crescent in the mid-cortex; 2) ABL1 phosphorylates NuMA at Y1774 and anchors it to the cortex, which is necessary for the alignment of spindles toward the center of the cortical LGN crescent.
Skin is a multilayered epithelium consisting of a basal layer of proliferative cells that adhere to the basement membrane, and upper layers of differentiated cells (Blanpain and Fuchs, 2009). It is believed that a small number of stem cells and progenitor cells are localized in the basal layer. Basal cells divide parallel to or perpendicularly to the basement membrane, undergoing symmetric or asymmetric cell division, respectively (Lechler and Fuchs, 2005). In parallel (symmetric) cell division, both daughter cells remain attached to the basement membrane and thus retain their proliferative potential. In perpendicular (asymmetric) cell division, one daughter cell remains attached to the basement membrane, whereas the other daughter cell is detached from the basement membrane, stops dividing, and undergoes differentiation. Therefore, the asymmetric cell divisions promote the multilayered structure of skin (Lechler and Fuchs, 2005). The asymmetric cell division in murine skin tissues requires Par-aPKC, LGN, NuMA and mInsc. In LGN/NuMA-knockdown epidermis, asymmetric cell division is significantly reduced, and the majority of the basal cells undergo symmetric cell division, which results in skin barrier dysfunction, leading to death by dehydration (Williams et al., 2011).
We have shown that in ABL1 knockout mice, or in mice treated with the ABL1 inhibitor Glivec, spindles were mis-oriented in the basal skin cells. In the wild-type epidermis, LGN is localized at the apical cortex in basal cells, whereas in the ABL1 knockout epidermis, LGN is randomly positioned at the cortex. Consequently, spindles are aligned with these randomly positioned LGN crescents at the cortex in the ABL1 knockout basal cells (Matsumura et al., 2012). A key to this dysregulation is that NuMA loses its cortical association in the ABL1 knockout epidermis. These phenotypes are highly similar to those observed in the ABL1-depleted HeLa cells. We speculate that HeLa cells and skin basal cells share common mechanisms for spindle orientation. It is interesting to note that the spindle orientation control in both HeLa cells and mouse epidermal basal cells depends on β1-integrin-mediated cell-substratum adhesion (Lechler and Fuchs, 2005; Toyoshima and Nishida, 2007a). In addition, in the β1-integrin knockout epidermis, similar to the ABL1 knockout epidermis, LGN is randomly positioned at the cortex (Lechler and Fuchs, 2005). Thus, ABL1 might have a role in the β1-integrin signaling pathway both in mouse skin and HeLa cells.
The switching machinery between symmetric and asymmetric cell division in the epidermisIn both symmetrically and asymmetrically dividing epidermal basal cells, LGN is localized to the apical cortex (Lechler and Fuchs, 2005; Matsumura et al., 2012). It has recently been reported that exogenously expressed mInsc, another binding partner of LGN that is co-localized with LGN at the apical cortex, induces a temporary increase in the number of asymmetric cell divisions (Poulson and Lechler, 2010). However, this effect is not sustained over a long time. The proportion of the asymmetric cell divisions returns to the normal level beyond 3 days post-induction of mInsc. Thus, the mInsc/LGN polarity is uncoupled from the spindle axis in a significant population of symmetrically dividing cells. Therefore, apical localization of LGN is necessary but not sufficient for asymmetric (perpendicular) cell division. Based on the observation that NuMA is co-localized with mInsc/LGN at the apical cortex in asymmetrically dividing cells, but has lost its cortical association in symmetrically dividing cells, the dynamic localization of NuMA might constitute a switching control for symmetric and asymmetric cell division (Fig. 4) (Poulson and Lechler, 2010). A recent report showing that in the NuMA knockdown epidermis, the majority of basal cells divide symmetrically, even though LGN is still localized at the apical cortex (Williams et al., 2011), supports this hypothesis.

A hypothetical switching mechanism of spindle orientation in asymmetric and symmetric cell division in skin basal cells. (A) During prometaphase, LGN/NuMA complex associates with Gα at the cortex. LGN dissociates from NuMA at the cortex and binds to mInsc (dotted line), which is localized at the apical cortex via Par3/Par6/aPKC complex. (B) In asymmetric cell division, ABL1 phosphorylates NuMA, which may induce association of NuMA with an unknown cortical factor at the apical cortex (designated by “X”). The cortically anchored NuMA binds to dynein and drives the microtubule-pulling force at the apical cortex, which aligns the spindle along the apical-basal axis of the cell. LGN-Dlg-Kif13B complex may partially contribute to the apical-basal spindle orientation by capturing microtubules at the apical cortex. (C) In symmetric cell division, NuMA is not anchored to the cortex, which unable the cells to produce the strong microtubule-pulling force at the apical cortex. As a result, other mechanism(s) for spindle orientation may function dominantly to align the spindle parallel to the basement membrane.
Structural biological approaches have shown that mInsc and NuMA bind competitively to LGN (Culurgioni et al., 2011; Yuzawa et al., 2011; Mauser and Prehoda, 2012). Thus, LGN cannot bind mInsc and NuMA at the same time. Therefore, in asymmetric cell division, which requires mInsc, LGN, and NuMA, LGN and NuMA should be anchored to the cortex independently. We have found that ABL1-mediated phosphorylation of NuMA at Y1774 anchors NuMA to the cortex. It would be interesting to examine the possibility that the kinase activity of ABL1 during mitosis together with a cortical binding partner of Y1774-phosphorylated NuMA could be a switching control for symmetric and asymmetric cell division.
In the asymmetric cell division of Drosophila neuroblasts, a Pins/Dlg/Khc73 pathway, in addition to a Pins/Mud/dynein pathway, provides a link between astral microtubules and the cortex (Siegrist and Doe, 2005; Johnston et al., 2009). In ABL1-depleted HeLa cells, spindles display a rotational motion in an LGN/Dlg-dependent manner, even in the absence of cortical NuMA. In addition, in the ABL1 knockout epidermis, spindles are aligned with the randomly positioned cortical LGN, even in the absence of cortical NuMA (Matsumura et al., 2012). Therefore, the LGN/Dlg pathway might regulate spindle orientation in the epidermis, which would partially contribute to asymmetric cell division. Our hypothetical model for the switching mechanism for spindle orientation in asymmetric/symmetric cell division is shown in Fig. 4.
The switching mechanism between symmetric and asymmetric cell division plays an essential role in stem cell self-renewal, cell differentiation, and tissue morphogenesis. The spatiotemporal analysis of the regulators using both mammalian culture systems and mouse tissues in vivo should reveal the signaling networks for this mechanism in future studies.