2013 Volume 38 Issue 2 Pages 207-223
2013 Volume 38 Issue 2 Pages 207-223
Activations of mitochondrial calpains cause apoptosis-inducing factor-dependent apoptosis of retinal photoreceptor cells in the Royal College of Surgeons (RCS) rat, an animal model of retinitis pigmentosa. In the present study, we attempted to develop specific inhibitors of mitochondrial calpains that would prevent the retinal degeneration. We examined the inhibitory potency of 20-mer peptides of the m-calpain for mitochondrial calpains activity, determined the inhibitory regions, and conjugated the cell-penetrating peptides (CPP). The cytotoxicity and delivery of the peptide was evaluated using mouse photoreceptor-derived 661W cells. After intravitreal injection of the peptide in RCS rats, we examined the peptide delivery to the retina, photoreceptor cell death numbers, responses of the electroretinogram (ERG), concentrations of intracellular ATP, and changes of retinal morphology. Results showed that one of the peptides inhibited the activity of the mitochondrial m-calpain. The HIV-1 tat-conjugated m-calpain peptide, HIV-Nm, could preserve the inhibitory potency of the mitochondrial m-calpain, and penetrate into the 661W cells. While intravitreal injection of HIV-Nm made it possible to deliver to the retina, it did not prevent photoreceptor cell death. Furthermore, it caused the ERG attenuation and the decrease in the intracellular ATP only a day after the injection. Although HIV-Nm did not cause histological change of the retina after 1 or 2 days of the administration, the morphological abnormality of the retina was observed after 3–14 days. Our results demonstrated that HIV-Nm failed to prevent the photoreceptor cell death, but rather caused the attenuation of ERG response and the decrease of ATP.
There are three types of calpains (EC 3.4.22.17) found in the mitochondria, μ-calpain (Badugu et al., 2008; Garcia et al., 2005; Ozaki et al., 2007, 2008; Polster et al., 2005), m-calpain (Ozaki et al., 2009) and calpain-10 (Arrington et al., 2006). All three of these have been shown to play significant roles in pathophysiological conditions, including in apoptotic and necrotic cell deaths (Kar et al., 2010). Our previous study shown that mitochondrial μ-calpain initiates apoptotic signals by limited cleavage of the apoptosis-inducing factor [AIF (Norberg et al., 2010)] in the rat model of retinal photoreceptor degeneration (Mizukoshi et al., 2010). We also demonstrated that mitochondrial m-calpain releases truncated AIF from the mitochondrial intermembrane space (IMS) to the cytoplasm by developing VDAC-Bax complex in isolated rat liver mitochondria (Ozaki et al., 2009). In cytoplasm, calpains have been shown to modulate a variety of cellular functions under physiological conditions, including survival signaling, gene expression, cell motility, differentiation, and proliferation (Goll et al., 2003; Ono and Sorimachi, 2012). However, the physiological role of mitochondrial calpains has yet to be elucidated.
Calpains- or AIF-dependent cell death pathways contribute to the apoptosis of photoreceptors (Doonan et al., 2005; Kaur et al., 2011; Mizukoshi et al., 2010; Murakami et al., 2008; Nguyen et al., 2012; Paquet-Durand et al., 2006, 2010) and ganglion cells (Huang et al., 2010; Qu et al., 2010; Ryu et al., 2012) in animal models of retinitis pigmentosa (RP) and glaucoma. RP and glaucoma are a major cause of blindness in humans and thus, specific inhibition of the mitochondrial calpains- and AIF-dependent cell death pathways could potentially be an effective method for treating these retinal diseases.
Recently, we identified the HIV-1 tat-conjugated mitochondrial μ-calpain inhibitory peptide, Tat-μCL (Ozaki et al., 2012). In addition, we found that intravitreal injections or eyedrops containing Tat-μCL prevented retinal degeneration in the Royal College of Surgeons (RCS) rat, which is an animal model of RP (D’Cruz et al., 2000). However, while the intravitreal injection protected retinal photoreceptor apoptosis in ~90% of the animals, the Tat-μCL eyedrops only prevented ~50% of the photoreceptor apoptosis. Our previous study also demonstrated that intravitreal injection of the calpain inhibitors, ALLN or PD150606, prevented AIF-dependent photoreceptor apoptosis by ~50% in these RCS rats (Mizukoshi et al., 2010). Based on these findings, the current study attempted to identify stronger peptide inhibitors of RP by targeting the mitochondrial m-calpain, which stimulates AIF-dependent cell death. We hypothesized that co-application of Tat-μCL and a novel candidate, mitochondrial m-calpain inhibitory peptide, should result in a greater protective effect against retinal degeneration.
In the present study, we decided to focus on around the m-calpain C2L domain, as while the specific functions of the C2L domain have yet to be determined (Goll et al., 2003). Our previous studies suggested that the sequence of the mitochondrial m-calpain C2L domain differed from that of cytosolic m-calpain, and that one of the mitochondrial chaperones, Grp75, could bind to the C2L domain (Ozaki et al., 2009). Therefore, we speculated that the m-calpain peptide derived around the C2L domain would be able to competitively inhibit the mitochondrial m-calpain-Grp75 interaction, inducing a loss of its stability and protease activity.
Based on all of the previous findings and in accordance with our hypotheses, the initial aim of our present study was to identify the peptide inhibitor of mitochondrial m-calpain. Once identified, we then planned to examine whether this peptide inhibitor could protect against retinal degeneration in the RCS rat. Although we were able to successfully identify a peptide inhibitor of the mitochondrial m-calpain, intravitreal injection of this inhibitor in the RCS rats prevented the electroretinogram (ERG) responses. As a result, we altered our study aim and tried to determine why inhibition of mitochondrial m-calpain led to an attenuation of the ERG responses.
All experimental procedures were designed to conform to the Association for Research in Vision and Ophthalmology (ARVO) Statement for Use of Animals in Ophthalmic Vision Research and the Hirosaki University Guidelines for animal experiments. Sprague-Dawley (SD) rats were used to prepare liver cytosolic and mitochondrial fractions, and for partial purification of cytosolic and mitochondrial calpains. RCS rats were used to determine the effects of calpain peptide inhibitors on retinal photoreceptor apoptosis and retinal function. SD rats, dystrophic RCS (rdy–/–) rats, and congenic RCS (rdy+/+) rats were all purchased from Clea Japan, and were housed at the Hirosaki University Graduate School of Medicine Animal Care Service Facility under a 12-h light (50 lux illumination) and 12-h dark (<10 lux illumination) cycle. Care was taken not to cause photoreceptor light damage to the rats.
AntibodiesThe following antibodies were purchased: rabbit polyclonal anti-bodies against anti-AIF (Abcam, Cambridge, MA; ab1998), anti-adenylate kinase 2 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA; sc-28786), anti-glyceraldehyde-3-phosphate dehydrogenase (anti-GAPDH; Santa Cruz Biotechnology, Inc.; sc-25778), anti-voltage-dependent anion-selective channel (anti-VDAC; Calbiochem, La Jolla, CA; PC548), anti-pyruvate dehydrogenase (Molecular Probes, Eugene, OR; A-21323), mouse monoclonal anti-trans-Golgi network-46 (Abcam; ab2809), anti-HIV1 tat (Abcam; ab63957), rat monoclonal anti-zonula occludens-1 (anti-ZO-1; Chemicon, Temecula, CA; MAB1520), and normal rabbit IgG (Santa Cruz Biotechnology, Inc.).
Synthesis of m-calpain peptidesAs seen in Table I, Table II, and Table III, we separately synthesized m-calpain peptides. Each peptide was synthesized by the fluorenylmethyloxycarbonyl method using an automated peptide synthesizer (PSSM-8; Shimazdu, Kyoto, Japan). The resulting peptides were purified by reverse-phase HPLC using a C18 column (Jupiter 250 mm×10 mm; Phenomenex, Torrance, CA). The molecular weight and purity of each peptide was confirmed by MALDI-TOF mass spectrometry with a Voyager RP-DE (Applied Biosystems, Foster City, CA), as previously described (Ozaki et al., 2008). No minor peaks were observed other than the correctly synthesized peptide.
Abbreviations: AA, numbers of amino acids; M, molecular mass.
The indicated CPP were conjugated to the N- or C-terminal regions of the m-calpain peptide N2-10-1. Abbreviations: HIV, HIV-1 tat peptide; OR, oligo-arginine peptide; MTS, membrane transporting sequence peptide; AA, numbers of amino acids; M, molecular mass.
Abbreviations: HIV-Nm, HIV-1 tat-conjugated m-calpain peptide N2-10-1; AA, numbers of amino acids; M, molecular mass.
To purify cytosolic μ- and m-calpains, the liver cytosolic fraction was prepared from 8-week-old SD rats, as previously described (Ozaki et al., 2007). All chromatographic procedures were performed at 4°C with DEAE-Sepharose CL-6B, Sephacryl S-300 and Sepharose 6B columns (Amersham Pharmacia Biotech, Buckinghamshire, UK).
Partial purification of mitochondrial μ- and m-calpainsRat liver mitochondria and mitochondrial compartments were prepared from 8-week-old SD rats, as previously described (Ozaki et al., 2009). The purity of the mitochondrial compartments was determined by immunoblotting with anti-VDAC antibody for the mitochondrial outer membrane, anti-adenylate kinase 2 for the IMS, anti-AIF antibody for the inner membrane, anti-pyruvate dehydrogenase antibody for the matrix, anti-GAPDH antibody for the cytosolic fraction, anti-ZO-1 for the plasma membrane, anti-calnexin for the ER, and anti-trans-Golgi network-38 for the Golgi apparatus. Mitochondrial μ- and m-calpains were purified from rat liver mitochondrial IMS. All chromatographic procedures were performed at 4°C with DEAE-Sepharose CL-6B, Sephacryl S-300 and Sepharose 6B columns.
Calpain activity assayVarious concentrations of m-calpain peptides were incubated with 25 μg of rat liver IMS proteins or partially purified calpains at 4°C for 4 h. Calpain activity was then assayed using succinyl-Leu-Tyr-7-amino-4-methylcoumarin (Suc-Leu-Tyr-AMC; Bachem, Bubendorf, Switzerland) as previously described (Ozaki et al., 2007).
SDS-PAGE, native-PAGE and western blottingIMS proteins incubated with each m-calpain peptide were subjected to SDS-PAGE on 10% SDS-polyacrylamide gels (40 μg protein per lane) as described (Ozaki et al., 2007). Nondenaturing (native) PAGE was carried out to evaluate the protein–protein interactions using a modified zymography method as described (Ozaki et al., 2008). After SDS-PAGE or native-PAGE, western blotting was performed. The immunoreactive signals were developed with an enhanced chemiluminescence western blotting detection kit (Amersham Biosciences) and quantified with a luminescent image analyzer (LAS-3000; Fujifilm Co., Tokyo, Japan).
Cathepsin, papain, and proteasome activity assaysTo determine the specificity of m-calpain peptides, we examined the effects of m-calpain peptides on the enzymatic activities of cathepsin, papain and proteasome. Cathepsin activity was assayed using a cathepsin fluorogenic substrate (Z-Phe-Arg-AMC; Biomol, Farmingdale, NY) in accordance with the method reported by Tavares et al (Tavares et al., 2004). Papain activity was assayed by using Bz-Arg-AMC (Bachem) as the fluorescent substrate (Alvarez-Fernandez et al., 1999). For the proteasome inhibition assay, we used the fluorogenic substrate Suc-Leu-Leu-Val-Tyr-AMC (Suc-LLVY-AMC; A. G. Scientific Inc., San Diego, CA), as previously described (Meng et al., 1999).
Intravitreal injection of m-calpain peptide inhibitor in RCS ratsThe effects of m-calpain peptide inhibitors on retinal photoreceptor cell death in RCS rats were determined. RCS rats at postnatal day (PND) 25 were anesthetized by intramuscular injection of ketamine (80–125 mg/kg) and xylazine (9–12 mg/kg). After topical application of 0.02% oxybuprocaine hydrochloride to the cornea, each animal received an intravitreal injection of 2 μl of 20 mM HIV-Nm (GRKKRRQRRRPPQ-HYSRLEICNL) in PBS (0.14 M NaCl and 10 mM phosphate buffer, pH 7.4). The same volume of 20 mM HIV-Nm scrambled peptide (GRKKRRQRRRPPQ-NCLRISYHEL), 20 mM HIV-Nm C21A mutant (GRKKRRQRRRPPQ-HYSRLEIANL), 20 mM HIV-1 tat (GRKKRRQRRRPPQ), 10 μM rotenone, or PBS were injected as controls of HIV-Nm. Details of peptides used were described in Table III. Using a Hamilton syringe with a 32-gauge needle, the solutions were injected through the area of the ciliary body (1 mm posterior to the corneal limbus) into the vitreous cavity.
ImmunohistochemistryImmunohistochemistry of the peptide was performed as previously described (Ozaki et al., 2012). The cryosections were blocked with 1% skim milk in PBS plus 0.05% Tween 20 (PBS-T) for 2 h at room temperature. Sections were incubated overnight at 4°C with mouse monoclonal anti-HIV1 tat antibody (Abcam, ab63957, 1:200) diluted in 1% skim milk in PBS-T. Sections were then incubated with tetramethylrhodamine isothiocyanate isomer R (TRITC)-conjugated rabbit anti-mouse IgG (DAKO, Glostrup, Denmark; R0270, 1:200) overnight at 4°C. The nuclei were counterstained with DAPI. Immunofluorescent images were acquired by laser scanning confocal microscopy (FV1000-D; Olympus, Tokyo, Japan).
TUNEL assayTo determine retinal photoreceptor cell death, cleavage of DNA was visualized in situ by TUNEL assay as described (Ozaki et al., 2012). We performed TUNEL assay using In Situ Apoptosis Detection Kits (Takara Bio, Shiga, Japan) in accordance with the manufacturer’s instructions. The nuclei were counterstained with DAPI. Immunofluorescent images were acquired by laser scanning confocal microscopy (FV-1000D, Olympus, Tokyo, Japan). The numbers of TUNEL-positive cells were counted in 20 sections per 9 eyes from each group.
ElectroretinographyThe preparation, recording technique and measurement of the ERG responses have been previously described (Ozaki et al., 2012). Responses to a 200-ms duration white flash (3, 30, 300, 1000 lux) were recorded using Neuropack (MES-3102 and 9102; Nihon Kohden, Tokyo, Japan). The a-wave amplitude was determined as the baseline to the bottom of the a-wave. The b-wave amplitude was determined as the bottom of the a-wave to the top of the b-wave.
Assay of ATP concentrations in RCS rat retinaTo determine the concentration of ATP in RCS rat retina, we used an ATP measurement kit (Bio Assay Systems, Hayward, CA), as previously described (Ozaki et al., 2011). This kit provides a rapid method to measure intracellular cytosolic or organelle ATP. In the presence of luciferase, ATP immediately reacts with the substrate D-luciferin to produce light. The light intensity is a direct measurement of the ATP concentration. After enucleation of the eyes of HIV-Nm-, HIV-Nm scrambled peptide-, HIV-Nm C21A mutant-, rotenone-, or PBS-treated RCS rats, the retinas were isolated. Subsequently, retinas were homogenized with 200 μl of cold PBS, with the homogenates further centrifuged at 12,000×g for 5 min to pellet any debris. We then transferred 1–10 μl of the supernatants to each well and brought the volume to 10 μl with PBS. The ATP concentrations were assayed using the ATP measurement kit in accordance with the manufacturer’s instructions.
Hematoxylin and eosin stainingHematoxylin and eosin staining was performed as described (Ozaki et al., 2013a). Hematoxylin staining was performed with New Hematoxylin Type M solution (Muto Pure Chemicals, Tokyo, Japan) for 10 min. After a wash with running water, eosin staining was performed with 1% eosin Y solution (Muto Pure Chemicals) for 30 sec. The sections were dehydrated with 70%, 80%, 90%, 95%, 100% ethanol and xylene, and then enclosed with MGK-S (Matsunami Glass, Osaka, Japan).
Cell cultureMouse photoreceptor-derived 661W cells were obtained from Dr. Muayyad R. Al-Ubaidi (University of Oklahoma, Oklahoma City, OK). The cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM; Nissui Seiyaku Co., Tokyo, Japan) containing 10% heat-inactivated fetal bovine serum (FBS; Biocell, Carson, CA), 2 mM glutamine, 100 U/mL penicillin and 100 μg/mL streptomycin. Cells were grown in a humidified incubator containing 5% CO2 and 95% air at 37°C.
ImmunocytochemistryThe 661W cells were seeded on coverslips, treated with 40 μM HIV-Nm and 250 nM MitoTracker in serum free DMEM for 45 min at 37°C. Cells were washed with PBS and fixed with acetone for 15 min at −20°C. Cells were washed with PBS and incubated with 1% skim milk in PBS-T for 2 h at room temperature. Cells were incubated overnight at 4°C with mouse monoclonal anti-HIV1 tat antibody (Abcam, ab63957, 1:200) diluted in 1% skim milk in PBS-T. Cells were then washed with PBS-T and incubated with rabbit anti-mouse IgG-Biotinylated (DAKO, E0354, 1:200) diluted in 1% skim milk in PBS-T overnight at 4°C. Subsequently, cells were washed with PBS-T and incubated with Streptavidin-FITC (DAKO, F0422, 1:200) diluted in 1% skim milk in PBS-T for 2 h at room temperature. Stained slides were washed with PBS-T and mounted using Vectashield H-1200 with DAPI (Vector Laboratories Inc., Burlingame, CA). Immunofluorescent images were acquired by laser scanning confocal microscopy (FV1000-D; Olympus).
Cell viability assay and morphological analysisCell viability was measured by the MTS assay (Promega, Madison, WI). MTS [3-(4,5-dimethylthiazol)2H-tetrazolium] was utilized in accordance with the manufacturer’s instructions. The 661W cells were plated in a 96-well plate at 1×104 cells/100 μL/well followed by seeding for 8 hours. After the seeding, 661W cells were treated with 400 nM-4 mM HIV-Nm, HIV-Nm scrambled peptide, or HIV-Nm C21A mutant for 12 hours in a humidified incubator containing 5% CO2 and 95% air at 37°C. Then 20 μL of the MTS solution was added to each well and incubated for 4 hours in the 5% CO2 incubator at 37°C. After incubation, the intensity of absorbance at 490 nm was measured with a 96-well plate reader. Results were expressed as the percentage of the MTS reduction as compared to the controls. For the morphological analysis, 661W cells were plated in a 24-well plate at 1×104 cells/1 mL/well and seeding for 8 hours. After the seeding, 661W cells were treated with 4–400 μM HIV-Nm, HIV-Nm scrambled peptide, or HIV-Nm C21A mutant for 12 hours in a 5% CO2 incubator at 37°C. HIV-Nm-treated 661W cells were fixed and stained using the Diff-Quick™ Staining System (Dade Behringer, Newark, DE).
Statistical analysisStatistical analysis was performed using GraphPad Prism 6 (GraphPad Software, San Diego, CA). A one-way ANOVA was used to statistically compare results between multiple groups. An unpaired t-test was used to determine significance between the two groups. A p-value <0.05 indicated statistical significance.
We separately synthesized peptides derived from the m-calpain as 20 amino acid residues around the C2L domain (Fig. 1A). Three amino acids overlapped and were synthesized in each 20-mer peptide. The inhibitory potency of the peptides against mitochondrial calpains was tested using the rat liver mitochondrial IMS, with each of the samples assayed for calpain activity (Fig. 1B). One m-calpain peptide, the peptide 2 from the C-terminal region of the CysPc domain (N2), was found to significantly inhibit mitochondrial calpain activity at the final concentration of 50 μM. Therefore, we examined the specificity of this peptide against several proteases, including rat liver cytosolic μ- and m-calpain, mitochondrial μ- and m-calpain, human recombinant cathepsin L, proteasome purified from human erythrocytes, and papain. The m-calpain peptide potently inhibited mitochondrial m-calpain (Fig. 1C). None of these peptides affected the activities of cathepsin L, proteasome or papain.

(A) Sequences of the synthetic peptides of rat m-calpain large subunit (CAPN2). The peptides were synthesized for every 20 residues around the C2L domains. The shaded sequences represent three amino acids that overlapped in each synthesized peptide. The accession number for CAPN2 is NP_058812. The CAPN2 contains the autolytic N-terminal sequence (1–19 aa), protease (CysPC) domain (24–342 aa), C2L domain (356–514 aa), and the PEF(L) domain (531–700 aa) as shown in previous report (Sorimachi et al., 2011). (B) Inhibition of mitochondrial calpain activity by m-calpain 20-mer peptides. Each peptide (final concentration, 50 μM) was pretreated with 25 μg of mitochondrial IMS protein, as described in the Methods section. **P<0.01. (C) Specificity of the m-calpain peptide N2. Peptide specificity was tested by incubating each peptide with partially purified rat liver cytosolic μ- and m-calpain, mitochondrial μ- and m-calpain, human recombinant cathepsin L, proteasome, and papain. All data are expressed as means±SD (n=3).
We further examined whether the m-calpain peptide N2 could induce degradation of mitochondrial m-calpain and dissociation of calpain–chaperone binding. SDS-PAGE and western blotting showed that the m-calpain peptide N2 did not influence the mitochondrial m-calpain large subunit (data not shown). Additionally, native-PAGE and western blotting found no shifts or decreases of the mitochondrial m-calpain complex by m-calpain peptide N2 (data not shown). Thus, these results demonstrate that m-calpain peptide N2 does not cause degradation of the mitochondrial m-calpain or dissociation of the m-calpain–chaperone binding.
Determination of the inhibitory regions of m-calpain peptide N2To determine the inhibitory regions of the m-calpain peptide N2, we spliced the 20-mer m-calpain peptide N2 into 7–12 mer (as shown in Table I), and then determined the inhibitory potencies (Fig. 2A). One of the 10-mer peptides (N2-10-1) strongly inhibited the activity of the mitochondrial calpains at the final concentration of 50 μM, whereas the 12- and the 7-mer peptides lost all of their inhibitory effects. Subsequently, we tested the specificity of N2-10-1 against seven proteases (rat liver cytosolic μ- and m-calpain, mitochondrial μ- and m-calpain, human recombinant cathepsin L, proteasome purified from human erythrocytes and papain). The m-calpain peptide N2-10-1 strongly inhibited both mitochondrial μ- and m-calpains, but did not have any effect on the other proteases (Fig. 2B).

Identification of the inhibitory regions of the m-calpain peptide N2 and conjugation of the CPP. Specific segments of each peptide (final concentration, 50 μM) were incubated with 25 μg of rat liver mitochondrial IMS protein (A) or the indicated proteases (B). (A) Inhibitory effects of the m-calpain peptide N2 segments are listed in Table I. The m-calpain peptide N2-20 inhibited enzyme activity by ~50% and N2-10-1 inhibited activity by ~60%. (B) The m-calpain peptide N2-10-1 inhibited both mitochondrial μ- and m-calpains. The CPP-conjugated m-calpain peptides listed in Table II were pretreated with 25 μg of rat liver mitochondrial IMS protein (C) or the indicated proteases (D). (C) The m-calpain peptide N2-10-1 inhibited enzyme activity by ~60%. HIV-conjugated N2-10-1 at the N-terminal region of N2-10-1 (HIV-N) inhibited enzyme activity by ~50%. No inhibitory activity was noted for any of the other CPP-conjugated peptides. (D) HIV-conjugated m-calpain peptide N2-10-1 inhibited mitochondrial μ-calpain activity by ~55% and mitochondrial m-calpain activity by ~70%. Data are expressed as means±SD (n=3).
To transport the peptides across the plasma and mitochondrial outer membranes, we conjugated the m-calpain peptide N2-10-1 to the HIV-1 tat peptide (HIV) (Fawell et al., 1994), the oligo-arginine peptide (OR) (Suzuki et al., 2002), or the membrane transporting sequence peptide (MTS) (Lin et al., 1995) as a CPP at the N- or C-terminal region of N2-10-1 (Table II). We then evaluated the inhibitory effects of the resulting m-calpain peptide–CPP complexes. As shown in Fig. 2C, only the m-calpain peptide N2-10-1 conjugated with the HIV peptide at the N-terminal region (HIV-N) retained its inhibitory potency at the final concentration of 50 μM. In contrast, conjugation with a CPP interfered with the calpain inhibitory abilities of the other peptides tested. Subsequently, we examined the specificity of HIV-conjugated m-calpain N2-10-1, HIV-Nm. The peptide inhibited mitochondrial m-calpain activity by ~70% and mitochondrial μ-calpain activity by ~55% (Fig. 2D).
Effects of HIV-Nm on cell viability and morphology of 661W cellsWe examined the cytotoxicity of HIV-Nm in the mousederived photoreceptor 661W cells. We determined the effects of HIV-Nm on the viability of the 661W cells using the MTS assay at the final concentration of 400 nM-4 mM. We also used the HIV-Nm scrambled peptide or HIV-Nm C21A mutant, which do not have the calpain inhibitory potency, as controls of the HIV-Nm. Cell viability was not inhibited by HIV-Nm at the concentrations of ≤40 μM in the culture media (Fig. 3A). The HIV-Nm scrambled peptide and HIV-Nm C21A mutant did not prevent the cell viability at the concentrations of ≤400 μM and ≤40 μM, respectively. We subsequently examined the effects of HIV-Nm on the morphology of the 661W cells. After treating the 661W cells with various concentrations of HIV-Nm, the cells were stained by the Diff-Quick™ Staining System. The results showed that HIV-Nm did not affect the morphology of the 661W cells at the concentrations of 4 and 40 μM (Fig. 3B).

Cell viability assay and morphological analysis for HIV-Nm-treated 661W cells. Details are described in the Methods section. (A) Cell viability assay for 400 nM-4 mM HIV-Nm-, scrambled peptide-, or C21A mutant-treated 661W cells. Cell viability was measured by the MTS assay. Data are expressed as means±SD (n=3). *P<0.05, ***P<0.001. (B) Morphological analysis of the 4–400 μM HIV-Nm-treated 661W cells. HIV-Nm-treated 661W cells were stained with Diff-Quick. Original magnification: 100×.
In the present study, we intravitreally injected 2 μl of 20 mM HIV-Nm in the rats. Assuming the volume of the rat vitreous was about 30 μl on PND 21–30 (Sha and Kwong, 2006), the final concentration of the peptide in the vitreous would be about 1.25 mM immediately after the injection. However, we expected that the concentration of the peptide in the mitochondria of photoreceptor would be around the range of fiftieth to one-hundredth of 1.25 mM, i.e., 12.5–25 μM. Because the peptides would be diluted by ocular circulation, degraded by many proteases, and/or blocked by the several barriers including cell-to-cell adhesion, plasma membrane, and mitochondrial outer membrane. The concentrations of 12.5–25 μM exert no cytotoxic effects on the photoreceptors as shown in Fig. 3A. In addition, we reported that the intravitreous injection of 2 μl of 20 mM Tat-μCL, a peptide inhibitor of mitochondrial μ-calpain, in the rat vitreous had no cytotoxicity on the retinal cells (Ozaki et al., 2012, 2013b). Therefore, we speculated that the injection of 2 μl of 20 mM HIV-Nm would have no cytotoxic effects on the photoreceptors.
Localization of the HIV-Nm peptideTo explore the intracellular and mitochondrial translocation of HIV-Nm, we performed immunocytochemistry using anti-HIV1 tat antibody in 661W cells. Results showed that HIV-Nm was translocated to the cell, with localization observed at least in the mitochondria and the cytoplasm (data not shown).
Delivery of HIV-Nm into retinal cellsWe further determined whether HIV-Nm was efficiently delivered into the retinal cells following intravitreal injection (Fig. 4). In this part of the study, 2 μL of 20 mM HIV-Nm was injected on PND 25, after which the eyes were enucleated after 1, 2, and 3 days. HIV-Nm was detected on the retinal sections by immunofluorescence with anti-HIV1 tat antibody. At a day after the injection, HIV-Nm was fully present in the retina, ranging from the outer limiting membrane (OLM) to the inner limiting membrane (ILM). In addition, the immunopositivity of the HIV-Nm was observed in choroid and sclera. The results suggested that the relatively low molecular mass of the peptide (~2.9 kDa) and the conjugated cell penetrating-peptide, HIV-1 tat sequence, made it possible to penetrate from vitreous to sclera. Sanharawi et al also reported that protein permeability was ≤150 kDa through the sclera, ≤200 kDa through the choroid-Bruch’s layer, ≤30 kDa through the RPE, and ≤76 kDa through the neuroretina (Sanharawi et al., 2010). At 2 days after the injection, we found that the HIV-Nm was decreased in the retinal cells. At 3 days after the injection, a small amount of HIV-Nm remained in the inner nuclear layer (INL).

Delivery of HIV-Nm into the RCS rat retinal cells following intravitreal injection. RCS (rdy–/–) rats received an intravitreal injection of 2 μL of 20 mM HIV-Nm at PND 25. The eyes were enucleated after 1, 2, or 3 days. The retinal sections were stained with anti-HIV1 tat antibody (red) and DAPI (blue). On the day after the injection, the HIV-Nm was fully present in the retina ranging from the OLM to ILM. After 2 days, the HIV-Nm was decreased in the retinal cells. After 3 days, only a small amount of HIN-Nm remained in the INL. Data are representative of three independent experiments [n=6 eyes (3 rats) per group]. Abbreviations: GCL, ganglion cell layer; INL, inner nuclear layer; ONL, outer nuclear layer; RPE, retinal pigment epithelium; OLM, outer limiting membrane; ILM, inner limiting membrane.
We determined the inhibitory effects of HIV-Nm on apoptosis of the retinal photoreceptors in dystrophic RCS (rdy–/–) rats. These results are shown in Fig. 5. On PND 25, rats received an intravitreal injection of 2 μL of vehicle (PBS), 20 mM HIV-Nm, with the eyes then enucleated on PND 28. HIV-Nm did not inhibit the photoreceptor apoptosis. Although we used the HIV-Nm scrambled and C21A mutant peptides as controls of HIV-Nm, these peptides also did not affect the number of TUNEL-positive cells (data not shown).
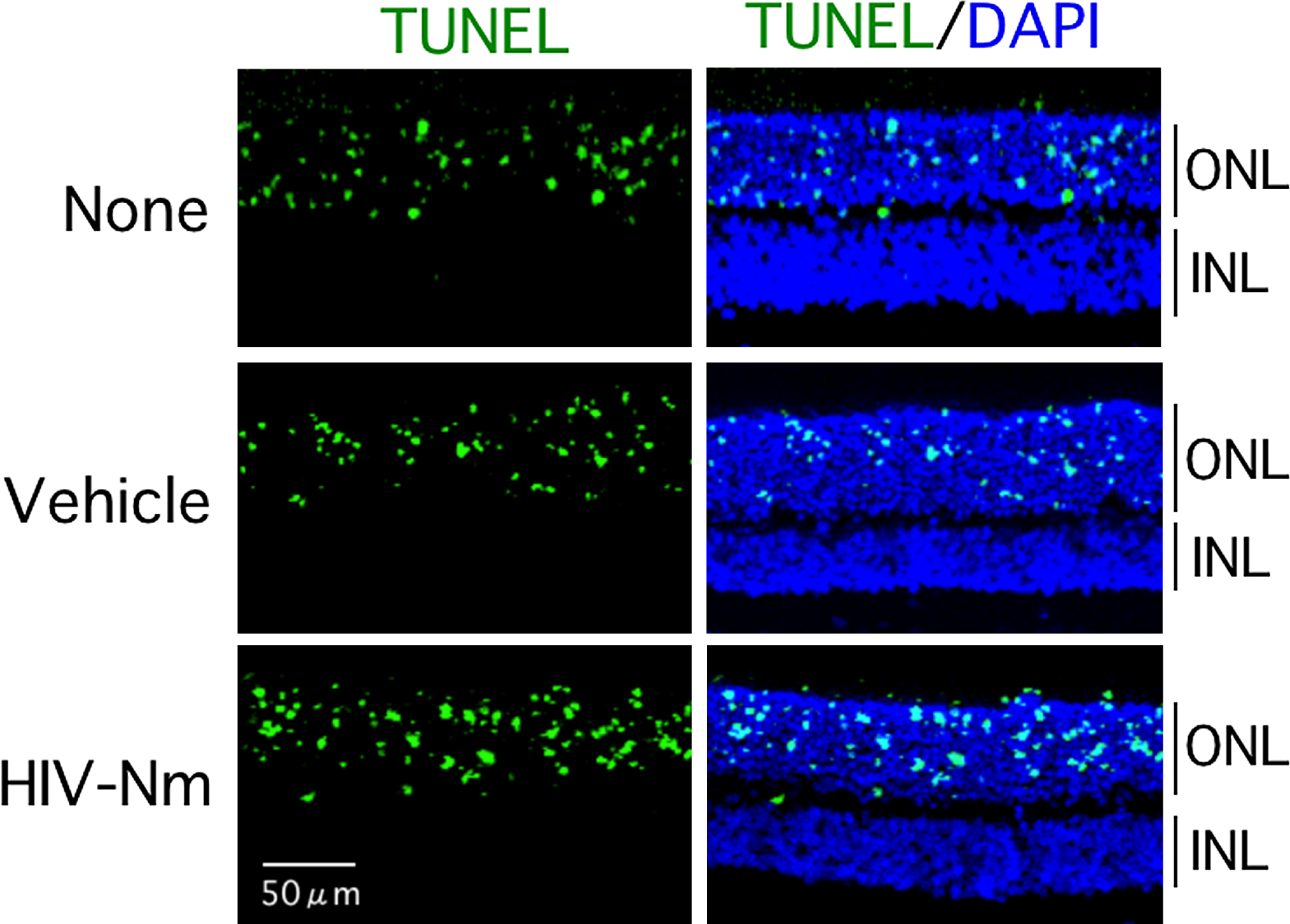
TUNEL assay of the retinal sections of the RCS rats treated with HIV-Nm. After dystrophic RCS (rdy–/–) rats received an intravitreal injection of 2 μL of vehicle alone (PBS) or 20 mM HIV-Nm on PND 25, the eyes were enucleated on PND 28. Retinal sections were then stained with TUNEL (green) and DAPI (blue). HIV-Nm did not cause any decrease in the number of apoptotic photoreceptor nuclei in the ONL. Abbreviations: ONL, outer nuclear layer; INL, inner nuclear layer.
We analyzed the ERG responses in order to determine the effect of HIV-Nm on the preservation of the retinal function in RCS rats. Intravitreal injection of 2 μL of vehicle, 20 mM HIV-Nm, or 20 mM HIV-1 tat was performed in dystrophic RCS (rdy–/–) rats on PND 25, with the ERG responses then determined on PND 28 (Fig. 6A). As compared to the vehicle- or HIV-1 tat-treated retina, HIV-Nm-treated retina showed attenuation of the a- and b-wave amplitudes. This indicated that HIV-Nm administration prevented the retinal function in dystrophic RCS (rdy–/–) rats. Based on this finding, we decided to examine the effect of HIV-Nm on ERG responses over a longer time scale. Intravitreal injections of 2 μL of vehicle or 20 mM HIV-Nm were administered in dystrophic RCS (rdy–/–) or congenic RCS (rdy+/+, wild type) rats on PND 18. The ERG responses were then determined on PND 21, 24, 28, 35, and 42 (Fig. 6B and 6C). In both dystrophic RCS (rdy–/–) and congenic RCS (rdy+/+) rats, HIV-Nm irreversibly induced disappearance of the a- and b-waves. The HIV-Nm scrambled peptide or HIVNm C21A mutant did not have any affect on the ERG responses of the dystrophic RCS (rdy–/–) and congenic RCS (rdy+/+) rats (data not shown).

Effects of HIV-Nm on scotopic ERG in the RCS rats. (A) Representative ERG traces. Dystrophic RCS (rdy–/–) rats were treated with vehicle alone (PBS), 20 mM HIV-Nm, or 20 mM HIV-1 tat on PND 25. Scotopic ERGs were recorded using 3, 30, 300, and 1000 lux on PND 28. The arrowheads indicate the time at which the eyes were exposed to light. After HIV-Nm treatment, the a- and b-waves were absent. This indicates that administration of the HIV-Nm prevents retinal function in the dystrophic RCS rat. (B and C) The effects of HIV-Nm on the a- and b-waves during a longer time period. Dystrophic RCS (rdy–/–) or congenic RCS (rdy+/+) rats were treated with HIV-Nm, or vehicle (PBS) on PND 18, with the scotopic ERGs then recorded on PND 21, 24, 28, 35, and 42. (B) Changes of the a-wave. (C) Changes of the b-wave. After the intravitreal injections, there were gradual irreversible decreases of the ERG responses in the dystrophic and congenic RCS rats. Data are expressed as means±SD [n=12 eyes (6 rats) per group].
We speculated that the loss of the ERG responses in the HIVNm-treated rats was caused by a reduction of ATP levels in the retina. Therefore, we decided to compare the effects of HIV-Nm and rotenone on the ERG responses and the ATP levels in the RCS rat retinas (Fig. 7). Rotenone is a potent inhibitor of mitochondrial complex I (NADH-ubiquinone oxidoreductase), which is responsible for bioenergetics defects and mitochondrial dysfunction. After performing intravitreal injections of 2 μL of 20 mM HIV-Nm or 10 μM rotenone in dystrophic RCS (rdy–/–) or congenic RCS (rdy+/+) rats, ERG recordings and ATP levels were determined at 12, 24, 48, and 72 hours after the injection. As shown in Fig. 7A, dystrophic RCS (rdy–/–) rats treated with HIV-Nm or rotenone exhibited a time-dependent decrease in the amplitudes of the a- and b-waves. At 72 hours after the injection, the ERG responses completely disappeared in both groups. Similarly, the ERG responses disappeared in the HIV-Nm- and rotenone-treated congenic RCS (rdy+/+) rats (Fig. 7B). For the ATP levels, a gradual decrease was noted in the dystrophic and congenic RCS rat retinas after the intravitreal injection of HIV-Nm or rotenone (Fig. 7C and 7D). The HIV-Nm scrambled peptide or HIV-Nm C21A mutant did not cause the decrease of the intracellular ATP concentrations of either the dystrophic RCS (rdy–/–) or the congenic RCS (rdy+/+) rat retinas (data not shown).

Effects of HIV-Nm on scotopic ERG and the retinal ATP levels in dystrophic or congenic RCS rats. Intravitreous injections of 2 μl of 20 mM HIV-Nm or 10 μM rotenone (inhibitor of mitochondrial complex I NADH-ubiquinone oxidoreductase) were performed in dystrophic RCS (rdy–/–) or congenic RCS (rdy+/+) rats on PND 25. Details are described in the Methods section. (A) Representative ERG traces for the HIV-Nm- or rotenone-treated dystrophic RCS (rdy–/–) rats. Both HIV-Nm and rotenone caused a decrease in the amplitude of the a- and b-waves in a time-dependent manner. (B) Representative ERG traces of the HIV-Nm- or rotenone-treated congenic RCS (rdy+/+) rats. ERG responses gradually decreased after the intravitreal injection. (C) ATP levels in the dystrophic RCS rat retinas. ATP concentrations in the HIV-Nm- or rotenone-treated rat retinas drastically decreased in conjunction with the decreases of the ERG responses. (D) ATP levels of congenic RCS rat retinas. When compared to vehicle-treated rats, the retinas of the HIV-Nm- or rotenone-treated rats lost more than half of the intracellular ATP. Data are expressed as means±SD [n=12 eyes (6 rats) per group].
We investigated the morphological basis for the attenuations of the ERG responses in HIV-Nm-treated congenic RCS (rdy+/+) rat retina (Fig. 8). After intravitreal injections of 2 μL of 20 mM HIV-Nm, HIV-Nm scramble peptide, or HIV-Nm C21A mutant in congenic RCS rats at PND 25, the eyes were enucleated after 1, 2, 3, 4, 5, 7, and 14 days. The retinal sections were stained with hematoxylin and eosin. No morphological changes were observed at 1 and 2 days after the administration of HIN-Nm. After 3 days, vacuoles were observed in the inner segments (IS). At 5 days after the injection, retinal layer was completely disrupted and the rosettes were formed. After 7–14 days, the retinal layer became thinner with the rosette formation. The HIV-Nm scrambled peptide- or HIV-Nm C21A mutant-injection had no affect on the retinal morphology at 14 days.

Histologic analysis of congenic RCS rat retina treated with HIV-Nm. After congenic RCS (rdy+/+) rats received an intravitreal injection of 2 μL of 20 mM HIV-Nm, HIV-Nm scrambled peptide, or HIV-Nm C21A mutant on PND 25, the eyes were enucleated after 1, 2, 3, 4, 5, 7, or 14 days. Retinal sections were stained with hematoxylin and eosin. Abbreviations: RPE, retinal pigment epithelium; IS/OS, inner segment/outer segment; ONL, outer nuclear layer; INL, inner nuclear layer; GCL, ganglion cell layer.
The aim of the present study was to determine the specific peptide inhibitor of the mitochondrial calpains that were involved in the degeneration of the retinal photoreceptor. Although we were able to identify the specific peptide inhibitor of the mitochondrial μ- and m-calpains, we also showed that the HIV-Nm peptide did not have any inhibitory potency in the RCS rat retinal photoreceptor degeneration. Additionally, we also determined that HIV-Nm caused a loss of the scotopic ERG response and a reduction of the intracellular ATP without histological abnormality. These results suggested that HIV-Nm can cause drug toxicity and that mitochondrial m-calpain plays a critical role in both the retinal and visual function.
We performed sequence alignment of the human m-calpain large subunit (CAPN2) with other isoforms of the calpain superfamily in order to observe the homology of the m-calpain peptide N2 (Fig. 9A). Although the PC2 domain was highly conserved, there was much heterogeneity in the C2L domain. This analysis revealed that m-calpain peptide N2 corresponds to the link between the PC2 and C2L domains. The homology of the N2 peptide region was relatively low between the CAPN2 and other calpain isoforms.

(A) Conservation and plasticity of 15 human calpains. The amino acid sequences of 15 human calpains were aligned using the L-INS-i procedure provided in the MAFFT software. The accession numbers for the isoforms are: NP005177 (CAPN1), NP001739 (CAPN2), NP000061 (CAPN3), NP004046 (CAPN5), NP055104 (CAPN6), NP055111 (CAPN7), NP001137434 (CAPN8), NP006606 (CAPN9), NP004046 (CAPN10), NP008989 (CAPN11), NP653292 (CAPN12), NP653176 (CAPN13), NP001138594 (CAPN14), NP005623 (CAPN15) and EAW47827 (CAPN16). The identity between isoforms is indicated in black (100%) or pink (>50%). The Ca2+-binding site (CBS) is present within the PC2 domain. The C2L domain is also indicated. The m-calpain peptide N2 (m-N-2) is indicated by the green box. (B) Three-dimensional (3D) ribbon structure of active human m-calpain and the regions targeted by the m-calpain peptide N2 generated using the MolFeat software version 4.5. The large subunit is comprised of the CysPc (green), C2L (blue), PEF(L) (ocher), and PEF(S) (brown) domains. The side chains of the catalytic triad residues are circled in black. Red spheres indicate the Ca ions. The region corresponding to the m-calpain peptide N2 is colored pink. The side chains of D346 and D351 in this region form salt bridges with K234 and K230, respectively. The distances of D346–K234 and D351–K230 are 5.35 Å and 2.78 Å, respectively.
To understand the mechanism how the m-calpain peptides inhibit m-calpain, we sought to identify the regions targeted by the inhibitory peptides in the 3D structure of m-calpain (Fig. 9B). The large catalytic subunit contains the CysPC (green), C2L (blue), Ca2+-binding PEF(L) (ocher), and PEF(S) (brown) domains. The m-calpain peptide N2 is colored pink. The side chains corresponding to the peptide N2, D346 and D351 in the PC2-C2L linker region, formed salt bridges with K234 and K230 in the PC2 domain, respectively. The distances of D346–K234 and D351–K230 are 5.35 Å and 2.78 Å, respectively. As we identified that a molecular chaperone, Grp75, interacts with mitochondrial m-calpain (Ozaki et al., 2009), the two salt bridges may be important for the interaction. Thus, it is possible that the peptide N2 competitively prevents the interaction or the affinity between Grp75 and mitochondrial m-calpain, causing the inhibition of the enzymatic activity.
Furthermore, calpastatin, an endogenous inhibitor of calpains, is known to modulate the activity of calpains by reversibly binding to CysPc and Ca2+-bound PEF(L)/(S) domains of a calpain molecule (Goll et al., 2003). Although we have not tested whether calpastatin also modulates the activity of mitochondrial m-calpain, the peptide N2 would not affect the interactions of calpastatin to calpains, because their binding regions do not overlap in the 3D structure.
One of the major molecular differences between cytosolic and mitochondrial calpains involves the binding of chaperone to calpain. It has been previously reported that in cytosol, calpastatin mainly controls the calpain activity (Goll et al., 2003), while in mitochondria, chaperones such as ERp57 or Grp75, can respectively stabilize mitochondrial μ- and m-calpains (Kar et al., 2010). Based on these findings, we tried to inhibit the mitochondrial m-calpain-Grp75 interaction and inactivate the calpain activity. However, our results indicated that the peptide inhibitor did not affect the mitochondrial m-calpain and its complex. This demonstrated that the peptide inhibitor can neither cause the release of chaperone from calpain nor the destabilization of the mitochondrial calpains. Based on these results, we hypothesized that the peptide inhibitor may have been able to prevent the conformational change of the mitochondrial m-calpain from the preactive to the active one. Another possibility is that this peptide could have influenced the conformation of the mitochondrial m-calpain-Grp75 complex.
We also found that the 20-mer m-calpain N2 peptide inhibited m-calpains as shown in Fig. 1C, while the 10-mer m-calpain N2-10-1 peptide lost the specificity and inhibited both mitochondrial μ- and m-calpains as shown in Fig. 2B. This is probably because the regions targeted by the 10-mer m-calpain N2-10-1 peptide appear to be similar in the 3D structure between mitochondrial μ- and m-calpains. And also, the aa sequence of the N2-10-1 peptide is highly conserved in the corresponding sequence of μ-calpain (among 10 aas, seven or eight are identical or similar between μ- and m-calpains, respectively; the rest of the N2 peptide only have six identical aas out of 10). In any case, a further study that specifically clarifies the molecular mechanisms of calpain inhibition will need to be undertaken. Conversely, we cannot exclude the possibility that HIV-Nm has inhibitory effects on other molecules than mitochondrial m-calpain. We should illustrate the possibility with a biochemical technique in the near future.
The present study also demonstrated that HIV-Nm could not protect a photoreceptor cell death in RCS rats (Fig. 5), and caused a loss of the ERG response and a reduction of the intracellular ATP concentration of the retina without histological abnormality (Fig. 6, Fig. 7, Fig. 8). In contrast, the calpain inhibitor, PD150606, protected the photoreceptor cell death and preserved the a- and b-wave amplitudes of ERG, as shown in our previous study (Ozaki et al., 2012). A possible reason for the different results between HIV-Nm and PD150606 is that 50 μM HIV-Nm inhibits mitochondrial m-calpain better than that of μ-calpain (see Fig. 2D), whereas PD150606 may inhibit mitochondrial μ-calpain better than mitochondrial m-calpain in retinal cells. In fact, Ki values of PD150606 are 210 nM and 370 nM for cytosolic μ- and m-calpains, respectively. Alternatively, since interaction sites of PD150606 and HIV-Nm are in the PEF domain and the PC2-C2L linker region, respectively, this difference may cause distinct results.
The present study revealed that the inhibition of the mitochondrial m-calpain by HIV-Nm could have been responsible for the defects in the electron transfer chain in the mitochondria. If so, these defects could induce ATP reductions, bioenergetics defects, mitochondrial dysfunction, and the generation of oxidative stress. This hypothesis is supported by our results that showed administration of rotenone, which is a potent inhibitor of the mitochondrial complex I (NADH-ubiquinone oxidoreductase), led to a depression of the ERG response (Esteve-Rudd et al., 2011) and a decrease of the retinal ATP concentration, similar to that observed after the HIV-Nm intravitreal injection. In ischemia and reperfusion injury, a rapid exhaustion of intracellular ATP occurs due to insufficient oxygen and the consumption of glucose, thereby leading to a reduction in the aand b-wave amplitudes of the ERG (Ettaiche et al., 2001). ATP-liposome has also been shown to protect against ischemia- and reperfusion-induced reduction of the intracellular ATP and retinal ganglion cell death (Dvoriantchikova et al., 2010). These previous reports along with our current findings indicate that exhaustion of intracellular ATP is partially responsible for the reduction of the ERG response. In addition, the decrease of intracellular ATP would damage the cellular functions, following by the retinal cell degeneration and the morphological abnormality.
Mitochondrial calpains play significant roles in pathological conditions such as apoptotic and necrotic cell deaths (Kar et al., 2010). However, their physiological roles have yet to be elucidated. The present study showed that inhibition of the mitochondrial calpains by HIV-Nm caused a severe decrease of the intracellular ATP of the retina (Fig. 7). This suggested that mitochondrial calpains were involved in the ATP production in the mitochondria. Furthermore, it also has been reported that after Ca2+-induced mitochondrial dysfunction, mitochondrial calpain-10 cleaves ND6 and NDUFV2, which are components of the electron transport chain complex I (Arrington et al., 2006). Additionally, another study has demonstrated that diabetes-induced renal mitochondrial dysfunction and renal injury may result after the loss of mitochondrial calpain-10 (Smith et al., 2012). Therefore, we expected that during physiological states, mitochondrial m-calpain acts in conjunction with other substrates in order to target the components of electron transport chain, ATP synthase, or ATP-transporter such as adenine nucleotide translocator (ANT). To definitively clarify the speculations, further studies are necessary to determine how mitochondrial m-calpain modulates the ATP production or transport.
In summary, the present study demonstrated that the inhibition of mitochondrial m-calpain caused a decrease of retinal intracellular ATP and a loss of the ERG response.
We thank Dr. Muayyad R. Al-Ubaidi (Department of Cell Biology, University of Oklahoma) for the providing the 661W cell line. This study was supported by several grants including Exploratory Research by Young Scientists from Hirosaki University (to T.O.), AS242Z02574Q from the Japan Science and Technology Agency (JST) (to T.O.), a Grant-in-Aid for Young Scientists B-25861611 from the Japan Society for the Promotion of Science (JSPS) (to T.O.), a Grant-in-Aid C-24592616 from JSPS (to M.N.), and a grant from the North-Tohoku Three National Universities (to S.I.). This study was done in part at the Gene Research Center, Hirosaki University.