2016 Volume 41 Issue 2 Pages 137-143
2016 Volume 41 Issue 2 Pages 137-143
Over the past decade, many studies have been conducted on extracellular vesicles (EVs) in the fields of basic and clinical research. EVs are small sized membranous vesicles generated from many type of cells upon activation by environmental stresses such as heat, hypoxia, and irradiation. EVs theoretically consist of microparticles/microvesicles, exosomes, and apoptotic bodies by different productive mechanisms. Clinically, EVs are observed in the blood stream of patients suffering from acute and chronic inflammation evoked by various diseases, and number of EVs in blood flow is often dependent on the inflammatory status and severity of the diseases. To date, it has been reported that small molecules such as RNAs and proteins are encapsulated in EVs; however, the functions of EVs are still unclear in the biological, pathological, and clinical aspects. In this review, we summarize and discuss the biogenesis-based classification, expected function, and pathobiological activities of EVs.
Extracellular vesicles (EVs) are small membranous globules that are released from many cells including endothelial cells, leukocytes, and cancer cells (Kawamoto et al., 2012; Leroyer et al., 2007; Yamamoto et al., 2015). Clinically, the number of EVs has been observed to increase in the bloodstream of patients suffering from acute and chronic inflammation evoked by diseases such as sepsis, stroke, preeclampsia, atherosclerosis, diabetes mellitus, metabolic syndrome, and cancer (Agouni et al., 2008; Boulanger et al., 2006; Koga et al., 2005; Mostefai et al., 2008; Petrozella et al., 2012; Simak et al., 2006). Several studies have reported that EVs mediate intercellular communications by releasing encapsulated materials such as messenger RNAs (mRNAs), microRNAs (miRNAs), and proteins, to target cells (Al-Nedawi et al., 2009, 2008; Fujita et al., 2014; Kawamoto et al., 2012; Tominaga et al., 2015; Waldenstrom et al., 2012; Yamamoto et al., 2015). This review summarizes and discusses the current understanding of the pathophysiological role of EVs in various diseases.
EVs including microparticles/microvesicles, exosomes, and others (Beyer and Pisetsky, 2010; Gould and Raposo, 2013; Kosaka et al., 2010; Raposo and Stoorvogel, 2013) are released from different cell types by different productive mechanisms (Fig. 1). EVs are commonly classified into three major groups based on their theoretical biogenesis pathways and sizes (Table I) (Gyorgy et al., 2011; Kalra et al., 2012).
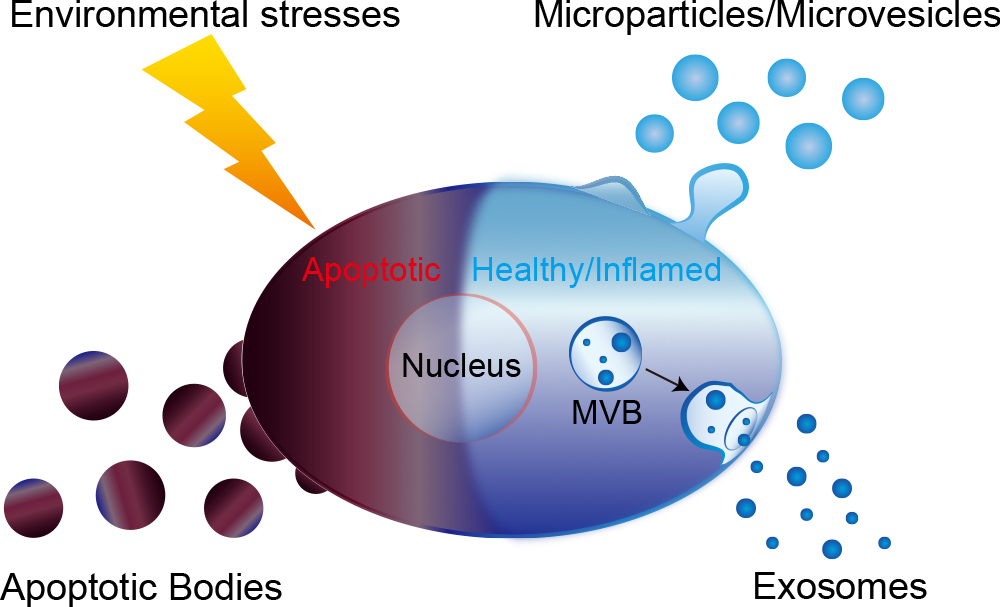
Mechanisms of extracellular vesicle (EV) production. A major population of EVs is comprised of microparticles/microvesicles, exosomes, and apoptotic bodies. These are produced from different cell types (e.g. vascular endothelial cells and tumor cells) and by different mechanisms of production in response to various stimulating factors. Microparticles/microvesicles are shed and then released from the plasma membranes of cells. Exosomes are internalized in early endosomes called as multivesicular bodies (MVB). When MVBs merge with the plasma membrane and split open, exosomes are released to the extracellular space. Apoptotic bodies are broken off from cells undergoing apoptosis. These EVs can carry bioactive molecules to recipient cells.
| Classification | Biogenesis | Size | Contents | Functions | Related diseases |
|---|---|---|---|---|---|
| Membrane shedding type EVs | Budding from plasma membrane and released to the circulating blood or interstitial fluid. | 50–3,000 nm | mRNA, miRNA, genomic DNA, mitocondrial DNA, protein, lipid, etc. | Inflammation1, educating recipient cell1,2, promoting hypercoagulability3, thrombotic activity4, production of inframmatory cytokines5,6, pulmonary oedema7, enhance permeabilization8, pulmonary injury9, pulmonary hypertension10. | Stroke11, myocardial infarction12, Kawasaki disease13,14, sepsis15, preeclampsia16, atherosclerosis17, diabetes mellitus18, metabolic syndrome19, cancer20, infectious disease21. |
| Multivesicular body derived EVs | Produced in the cellular endosome called multivesiclur body. Released to the blood flow or interstitial fluid as EVs when multivesiclur body fuses with plasma membrane. | 40–100 nm | mRNA, miRNA, genomic DNA, mitocondrial DNA, protein, lipid, etc. | Leukotrien biogenesis22, prostagrandin biogenesis23, phosphatase activity24, educating recipient cell25, angiogenesis26,27, chemotaxis22, immunosuppression23, tumor suppressor28, immune evasion29. | Cancer30,31, infectious disease21, heart failure after acute myocardial infarction32, acute kidney injury33, acute podocyte injury33, Alzheimer’s disease34. |
| Apoptosis derived EVs | Broken off from cells that are undergoing programmed cell death. | 800–5,000 nm | Oncoviral DNA, genomic DNA, protein, lipid, etc. | Transferring oncoviral DNA35, tumorigenic conversion36, inappropriate clearance of apoptotic derived EVs37. | Autoimmune disease37, viral infection35. |
References are described in Supplementary Information.
Platelet dust has been identified in human plasma (Wolf, 1967). This was the first report to describe microparticles/microvesicles. When the plasma membrane is activated by extrinsic stimuli such as inflammatory cytokines and lipopolysaccharide (LPS), membranous globular objects of submicron to micron sizes (0.05–3 μm) are shed from the plasma membrane surface, and subsequently released (Morhayim et al., 2014; Raposo and Stoorvogel, 2013; Yamamoto et al., 2015). These globular objects comprised of the plasma membrane are categorized as microparticles/microvesicles among EVs (Fig. 1, Fig. 2 and Table I) (Beyer and Pisetsky, 2010; Yamamoto et al., 2015).
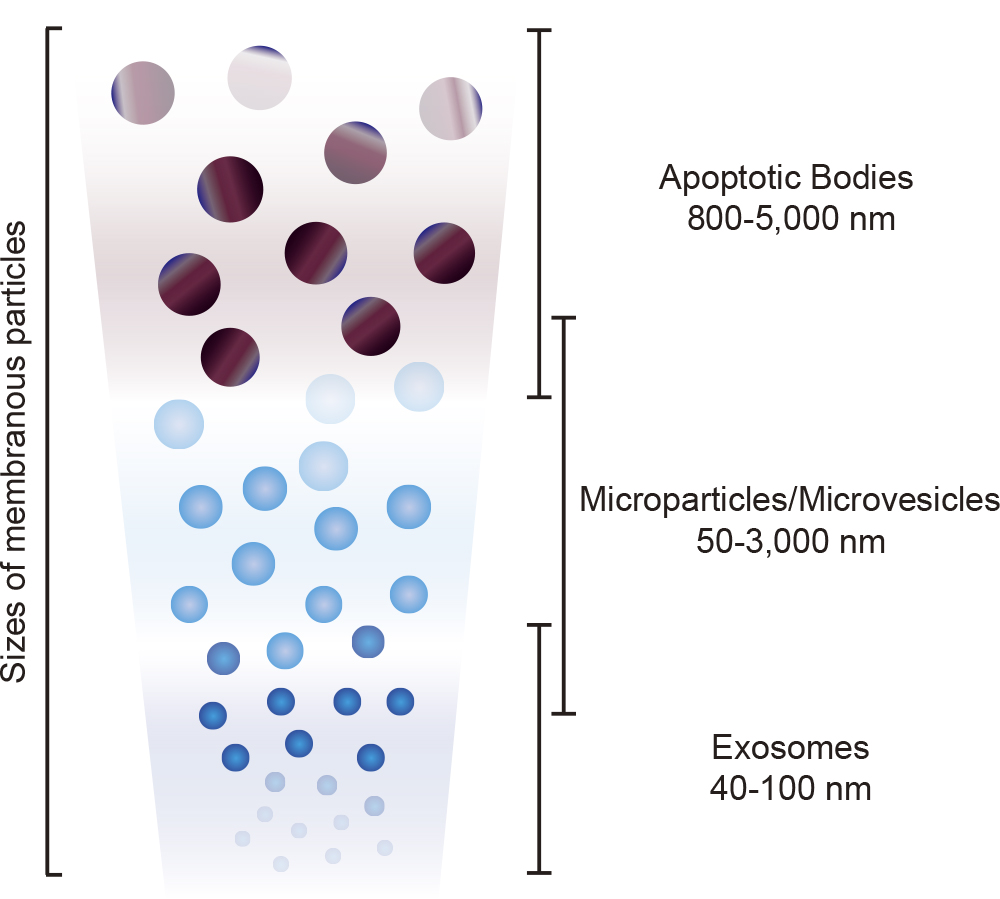
Correlation between productive manner and diameter range of EVs. Exosomes are relatively small sized membranous particles (40–100 nm), whereas apoptotic bodies are large vesicles (800–5,000 nm). Microparticles/microvesicles are reported as medium sized membranous globules (50–3,000 nm) among EVs. The distribution of diameters might overlap with each other.
Exosomes were firstly discovered in maturing mammalian reticulocytes (Johnstone et al., 1987). In endosomes, a part of the membranous organelles from cells can be internalized into nanometer-sized vesicles (40–100 nm), in which case the vesicle-filled endosomes are called as multivesicular bodies (MVBs) (Fujita et al., 2014). When MVBs merge with a plasma membrane, and release the nanometer-sized vesicles into the extracellular space, these small vesicles become exosomes and are classified as a subclass of EVs (Fig. 1, Fig. 2 and Table I) (Beyer and Pisetsky, 2010; Gruenberg and van der Goot, 2006).
Apoptosis derived EVs (apoptotic bodies)Cells suffering from many kinds of environmental stresses undergo apoptosis (Steller, 1995). Apoptotic bodies (ABs), found as membranous vesicles of relatively large diameter (800–5,000 nm), are broken off from cells that are undergoing programmed cell death, and are also classified as a subclass of EVs (Fig. 1, Fig. 2 and Table I) (Beyer and Pisetsky, 2010; Kosaka et al., 2010; Morhayim et al., 2014).
A number of studies have demonstrated that RNA, DNA, and proteins are encapsulated in EVs as bioactive molecules (Table I) (Al-Nedawi et al., 2009; Cai et al., 2013; Waldenstrom et al., 2012; Yamamoto et al., 2015). Some reports have suggested that the bioactive molecules encapsulated in EVs play important roles in intercellular communication, and in specific signal transductions in the pathophysiological states of cells both in vitro and in vivo (Kawamoto et al., 2012; Tominaga et al., 2015; Yamamoto et al., 2015).
NucleotidesSpecific coding and non-coding RNAs such as mRNAs, miRNAs, retrotransposon RNAs, Alu transposable elements, and chromosomal DNA and mitochondrial DNA have been reported to be enriched in tumor cell-derived EVs (Balaj et al., 2011; Cai et al., 2013; Guescini et al., 2010; Rak and Guha, 2012; Ronquist et al., 2012; Waldenstrom et al., 2012). Recent studies have demonstrated that specific miRNAs encapsulated in EVs can educate the recipient cells through intercellular communication. We recently revealed that inflammation-related miRNAs are specifically encapsulated in endothelial-derived EVs (E-EVs) in response to inflammatory stimuli (Yamamoto et al., 2015). We also demonstrated that E-EVs act as a tool for intercellular communication between endothelial cells and pericytes/vascular smooth muscle cells (vSMCs), in which inflammation-related miRNAs modulate mRNA expression of vascular endothelial growth factor, and mediate protein biogenesis in pericytes/vSMCs (Fig. 3). Along this line, miR-143/145 clusters contained in E-EVs were shown to control the vSMC phenotypes (Hergenreider et al., 2012). This study also suggested that reciprocal communication between endothelial cells and vSMCs through miRNAs contained in E-EVs plays an important role in protection from pathogenesis of atherosclerosis. These data suggest that cells can reciprocally communicate with other cells through small molecules encapsulated in EVs.
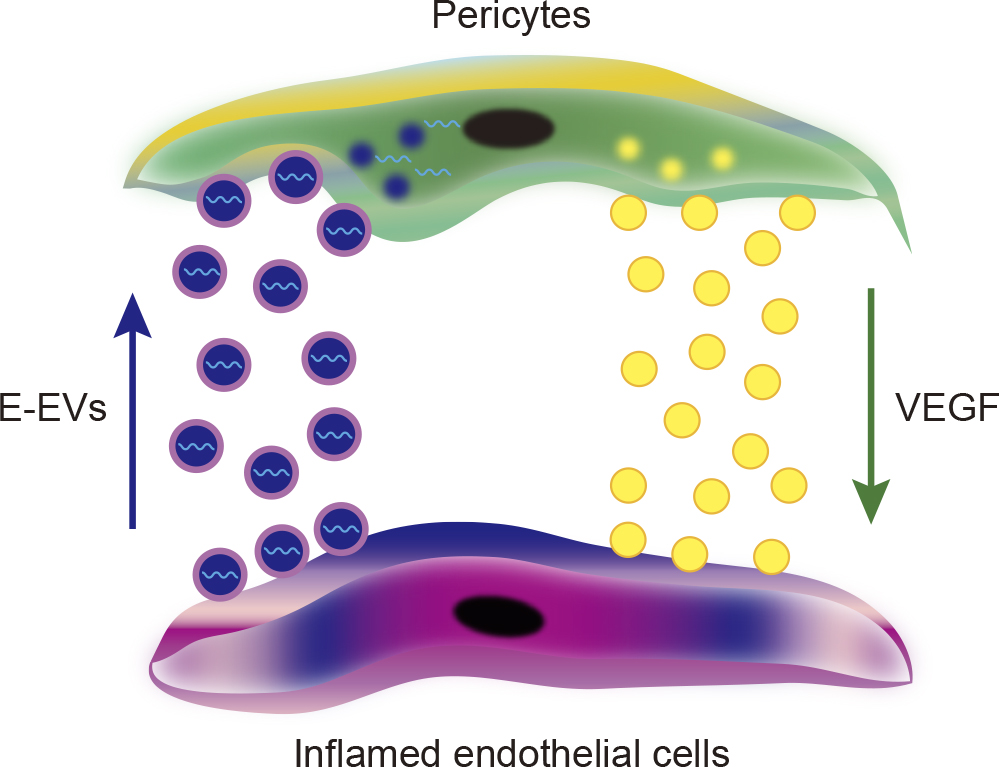
Reciprocal intercellular communication. Under inflammatory conditions in endothelial cells, inflammation-related miRNAs are specifically encapsulated in E-EVs in response to inflammatory stimuli. The composition of inflammation-related miRNAs encapsulated in E-EVs might depend on the strength of inflammation (Yamamoto et al., 2015). When E-EVs are incorporated into pericytes/vSMCs, the inflammation-related miRNAs modulate the mRNA expression levels of vascular endothelial growth factor, and mediate protein biogenesis in pericytes/vSMCs. These reciprocal intercellular communications involving EVs might be common a biological intercellular response in pathophysiological conditions.
Other studies suggest that specific proteins such as receptor type tyrosine kinases contained in EVs can transduce intracellular signals to the recipient cells (Al-Nedawi et al., 2009; Al-Nedawi et al., 2008). The blood vessels in tumors can be educated by EVs derived from tumor cells. Incorporation of EGF receptor-containing EVs into endothelial cells leads to the activation of MAPK and Akt pathways and triggers the endogenous expression of VEGF, followed by the activation of VEGFR2 (Al-Nedawi et al., 2009). EVs may contribute to the secretion of morphogens, and be responsible for their tissue gradient formation. Several lines of studies have shown that morphogens such as Dll4, Wnt, and Hedgehog (Beckett et al., 2013; Gross et al., 2012; Luga et al., 2012; Sheldon et al., 2010; Tanaka et al., 2005) tightly associate with the EV membrane surface. EV-associated Dll4 was incorporated to the recipient endothelial cells, and could inhibit Notch signaling in vitro. Such recipient endothelial cells reduced expression of the stalk cell markers, and appeared to switch the phenotype toward tip cells. (Sheldon et al., 2010). Regarding Wnt signaling, Wnt ligands bind to the EV surface through the cargo receptor, Evi/WIS, during Drosophila melanogaster development and in human cells (Gross et al., 2012). Wnts associated with EVs are implicated in signal transduction; however, EVs were unlikely to contribute essentially to the Wnt gradient formation in D. melanogaster (Beckett et al., 2013). In addition, EVs derived from fibroblasts can mediate autocrine Wnt signaling, and promote the cellular dynamics of tumor cells (Luga et al., 2012). In the pathological status of the central nervous system, pathogenic proteins involved in central nervous system diseases, such as β-amyloid peptide, superoxide dismutase, α-synuclein, and prions (Emmanouilidou et al., 2010; Fevrier et al., 2004; Gomes et al., 2007; Rajendran et al., 2006), are also released in association with EVs.
In patients suffering from inflammation evoked by various diseases, EVs are often observed in the plasma and in other body fluids (Antonyak and Cerione, 2014; Koga et al., 2005; Nozaki et al., 2009; Simak et al., 2006), and the number of EVs are increased in response to the disease severity. These evidences may suggest that EVs are functionally active in the intercellular communication between EV-producing cells and other cells (Kawamoto et al., 2012; Yamamoto et al., 2015), and can be a potential biomarker for diagnosis, for response to medical treatment by therapeutic agents, and for prognosis (Table I) (Amabile et al., 2012; Fujita et al., 2014; Nozaki et al., 2009).
Common diseases related to inflammationClinically, EVs are often found in circulating blood, and the number of EVs is elevated in response to acute and chronic inflammation associating with common diseases such as stroke, myocardial infarction, Kawasaki disease, sepsis, preeclampsia, atherosclerosis, diabetes mellitus, and metabolic syndrome (Agouni et al., 2008; Boulanger et al., 2006; Ding et al., 2014; Koga et al., 2005; Mostefai et al., 2008; Petrozella et al., 2012; Simak et al., 2006; Tan et al., 2013). Nonetheless, EVs are produced by many types of cells in response to inflammation, vascular endothelial cells are thought to be one of the major cell types that release EVs into the bloodstream (Martinez et al., 2005). The number of E-EVs circulating in the bloodstream correlates with the disease severity (Boulanger et al., 2006). Several lines of study have suggested that regionally derived EVs are incorporated into remotely located cells (Ono et al., 2014; Peinado et al., 2012). Furthermore, we and others have demonstrated that cells under inflammatory conditions actively communicate with their adjacent cells through EVs (Hergenreider et al., 2012; Yamamoto et al., 2015). Accordingly, E-EVs may be functionally involved in the cellular communication in number of diseases; however, more evidence needs to be accumulated in in vivo models. Further clinical and experimental approaches are needed to elucidate the significance of EVs in inflammatory diseases.
Infectious diseasesIt is necessary to pay careful attention to the crosstalk between host cells and pathogenic organisms in infectious diseases. EVs from the pathogenic organisms such as Trypanosoma spp., Cryptococcus spp., and Leishmania spp. may carry virulent factors and facilitate their delivery to host cells (Goncalves et al., 1991; Rodrigues et al., 2008; Silverman et al., 2010; Torrecilhas et al., 2012), subsequently promoting the dissemination of pathogens. In fact, proteomic analyses of parasite-produced EVs have identified virulent factors in EV proteomes (Bayer-Santos et al., 2013; Geiger et al., 2010; Silverman et al., 2010). Virulent factors in EVs such as proteases, toxins, and adhesins (Amano et al., 2010; Torrecilhas et al., 2012) are involved in the regulation of gene expression. Moreover, parasites have developed many strategies to survive and reproduce in host animals. One of the ingenious strategies employed by parasites is immune evasion (Lambertz et al., 2012) by establishment of self-tolerance, alteration of host antigens, immunosuppression and functional immune inactivation, and molecular mimicry between host antigens and parasite polypeptides by acquisition of sialic acid motifs from host cells and incorporation of sialoglycoconjugates from the host serum, which leads to modulation of host NETosis (Brinkmann et al., 2004; Hahn et al., 2013). Production of EVs by pathogenic organisms appears to be involved in many of these self-protection mechanisms.
CancerCancer cells also secrete EVs in addition to the stromal cells from the tumor microenvironment. These secreted EVs contribute to tumor progression by promoting angiogenesis and tumor cell migration towards metastases (Hood et al., 2011; Rak, 2010). Some reports have a provided fascinating notion of tumor derived EVs. Tumor derived EVs can encapsulate and enrich coding and non-coding RNAs, retrotransposon RNAs, Alu transposable elements, and chromosomal and mitochondrial DNA (Balaj et al., 2011; Guescini et al., 2010; Rak and Guha, 2012; Ronquist et al., 2012; Waldenstrom et al., 2012). EVs from tumor cells can also crosstalk, reprogram, and permanently educate bone marrow progenitor cells to mobilize out from their niche (Peinado et al., 2012). Consistent with these contexts, vascular endothelial cells in tumors can be educated by EVs derived from tumor cells (Al-Nedawi et al., 2009; Kawamoto et al., 2012). Such educated vascular endothelial cells exhibited a highly proliferative phenotype and in some cases show endothelial karyotype abnormalities implying cellular immortality. Tumor-derived EVs also encapsulate immunosuppressive molecules that can inactivate natural killer cells or T-lymphocytes, or promote the differentiation of myeloid cells or regulatory T-lymphocytes for suppressing immune responses (Zhang and Grizzle, 2011). These studies strongly suggest that EV-based cellular education is a universal phenomenon in pathophysiological conditions in vivo. These findings suggest that such “education” is mediated by specific miRNAs or proteins encapsulated in the EVs.
As a therapeutic biomarker, EVs may have the potential for prediction and prognosis of various diseases, particularly in cardiovascular diseases. Detectable levels of EVs from different cellular origins are commonly found to be circulating in the plasma of healthy humans. In cardiovascular functional disorders, although E-EVs represent a relatively small population of the circulating EVs in healthy humans, increase in their plasma levels may reflect important clinical information in patients (Chironi et al., 2009; Dignat-George and Boulanger, 2011). Several studies have suggested that measurement of E-EVs from the plasma of patients with cardiovascular diseases has prognostic potential. In fact, a large number of E-EVs that express CD31 but not CD41 (platelet marker) might be a diagnostic biomarker for cardiovascular mortality, whereas such potential was not observed in EVs derived from other cell types (Amabile et al., 2012). In pulmonary hypertension, the number of circulating E-EVs expressing CD62E (E-selectin) can be used for predicting a one year outcome (Amabile et al., 2009). In case of acute ischemic stroke, E-EV levels were associated with lesion volume and clinical outcome (Simak et al., 2004). In a high risk of coronary heart disease, the basal level of E-EVs that expressed CD144 (VE-cadherin) could predict the outcome independently of the Framingham score derived from C-reactive protein (CRP) and brain natriuretic peptide levels (Nozaki et al., 2009). Similar findings were observed in chronic renal failure. In the patient with Kawasaki disease, a significantly large number of E-EVs could be observed in the blood of patients, suggesting that E-EVs could be used as a potential biomarker for endothelial damage (Ding et al., 2014; Tan et al., 2013). These data suggest that E-EVs in plasma might be used in the future as a remarkable biomarker for the prediction of clinical stage in high-risk patients who may have cardiovascular complications.
Over the past decade, many studies have revealed abundant EVs that encapsulate many bioactive molecules in body fluids and show potent regulatory functions in target cells. These encapsulated molecules including RNAs and proteins are deeply correlated with the severity and malignancy of various diseases. It is obvious that EVs are a fascinating target for molecular-targeting therapeutic strategies to overcome various diseases. Thus, many scientists and physicians are focusing on EVs as a target for medical treatment, and as a prognostic and therapeutic biomarker. At the present time, however, technical difficulties hinder the progress of the field of EVs. Simple and accurate methods have not been established to discriminate among these EVs from clinical samples. Therefore, EVs need to be collected by ultracentrifugation, and column and solid phased methods, during research regarding the pathobiology of EVs. More simple, specific, and reproducible methods need to be established for the analysis of specific EVs in both clinical and basic research. Conducting of wider and deeper studies to develop methods for the specific detection and analysis of circulating EVs may help us to precisely understand the onset and progression of diseases. Further technical advances in this research field will open new avenues to clarify their respective functions.
SY involved in designing, coordinate and writing the manuscript, EA and MM involved in writing and improving the appearance of the manuscript, TH, YI and MS involved in revising the manuscript critically for important intellectual content. All authors read and approved the final manuscript.
We thank members of the Department of Pathology at the University of Toyama for helpful discussions. We also thank N. Yamamoto, A. Yamamoto, R. Yamamoto for broad-based support. This work was supported by a Grant-in-Aid for Scientific Research and Scientific Research on Innovative Areas (JP21590589/JP24659112 and JP23122506; to S.Y.) from the Ministry of Education, Culture, Sports, Science and Technology.