2018 Volume 43 Issue 1 Pages 85-94
2018 Volume 43 Issue 1 Pages 85-94
It takes several months to form the 3-dimensional morphology of the human embryonic brain. Therefore, establishing a long-term culture method for neuronal tissues derived from human induced pluripotent stem (iPS) cells is very important for studying human brain development. However, it is difficult to keep primary neurons alive for more than 3 weeks in culture. Moreover, long-term adherent culture to maintain the morphology of telencephalic neuron aggregates induced from human iPS cells is also difficult. Although collagen gel has been widely used to support long-term culture of cells, it is not clear whether human iPS cell-derived neuron aggregates can be cultured for long periods on this substrate. In the present study, we differentiated human iPS cells to telencephalic neuron aggregates and examined long-term culture of these aggregates on collagen gel. The results indicated that these aggregates could be cultured for over 3 months by adhering tightly onto collagen gel. Furthermore, telencephalic neuronal precursors within these aggregates matured over time and formed layered structures. Thus, long-term culture of telencephalic neuron aggregates derived from human iPS cells on collagen gel would be useful for studying human cerebral cortex development.
Key words: Induced pluripotent stem cell, forebrain neuron, collagen gel, long-term culture
Adult somatic cells can be reprogrammed into induced pluripotent stem (iPS) cells simply by forcing expression of a defined set of transcription factors, i.e., OCT4 (octamer-binding transcription factor 4), SOX2 (sex determining region Y-box 2), KLF4 (Kruppel-like factor 4) and c-MYC (Takahashi et al., 2007; Nakagawa et al., 2008). iPS cells have been shown to be functionally equivalent to embryonic stem (ES) cells, as they can maintain an undifferentiated state indefinitely and differentiate into derivatives of all three germ layers: the ectoderm, mesoderm and endoderm. Furthermore, adult chimaeric mice with germ line transmission ability were generated from iPS cells (Maherali et al., 2007; Okita et al., 2007; Wernig et al., 2007). In addition, when using human iPS cells, there are no ethical difficulties concerning the use of human embryos. It is also possible to make gene-manipulated iPS cells (Hockemeyer et al., 2009, 2011; Zou et al., 2009). Therefore, we can perform human developmental research by analysing the developmental stages of organs derived from human iPS cells.
The molecular mechanisms underlying human brain development are not well understood, which is mainly due to the ethical difficulties of using human embryonic brains and the lack of a culture method for long-term neuronal tissue culture in vitro. It takes several months to form the 3-dimentional morphology of the human brain (Kim et al., 2008). Therefore, it is important for developmental research regarding the human brain to develop a long-term culture system using human iPS cell-derived neuronal tissue.
The cerebral cortex is the outer layer of the cerebrum, which consists of six layers. The cerebral cortex functions in memory, attention, perception, awareness, thought, language and consciousness (Shipp, 2007). An efficient culture method for telencephalic selective neural differentiation of human ES/iPS cells has been established (Eiraku et al., 2008; Vaccarino et al., 2011). It has also been reported that a layered structure was observed inside these neuron aggregates at 46 days (Eiraku et al., 2008). Thus, the establishment of a long-term culture method is useful for analysing human cerebral cortex development. However, it is difficult to maintain the morphology of these neural aggregates for more than a month because these aggregates attach to each other or to the bottom of the plate.
Collagen gel is an extracellular matrix that is known to be useful for long-term culture and maturation of neuronal cells (Bellamkonda et al., 1995; Krewson et al., 1994; O’Connor et al., 2001). Collagen is the main component of connective tissues in vertebrates and is the most abundant mammalian protein, accounting for about 20–30% of total body proteins (Lee et al., 2001). It has been suggested that neuronal cells adhere strongly and receive important signals from collagen gel, which play critical roles in cell development, function and survival (O’Connor et al., 2001). The use of collagen gel is expected to keep the morphology of these aggregates for a long time because it is less stiff than collagen-coated culture dishes.
In the present study, we showed that human iPS cell-derived telencephalic neuron aggregates could be cultured for more than 3 months on collagen gel. Furthermore, telencephalic neuronal precursors within the aggregates matured over time and formed layered structures.
The human iPS line 253G1 was obtained from CiRA (Center for iPS Cell Research and Application). These human iPS cells were maintained on a feeder layer of mitomycin-C-treated SNL cells (a mouse fibroblast STO cell line transformed with neomycin resistance and LIF genes; ReproCELL, Yokohama, Japan) in primate ES medium supplemented with 4 ng/mL basic fibroblast growth factor (ReproCELL) in a humidified atmosphere of 5% CO2 and 95% air at 37°C. These iPS cells were passaged with Dissociation Solution for human ES/iPS cells (ReproCELL) every 4–5 days.
Human iPS cell-derived cortical neural differentiationTo obtain telencephalic aggregates derived from human iPS cells (passages 32–45), we partially modified the method described previously (Eiraku et al., 2008; Watanabe et al., 2005). The human iPS cells were cultured for 1 hour in the presence of 50 μM Y27632 (Wako Pure Chemical, Osaka, Japan) in Neuronal Differentiation Medium consisting of DMEM/F12 (Sigma, St. Louis, MO, USA) supplemented with 20% Knockout Serum Replacement (Life Technologies, Carlsbad, CA, USA), 5 μM SB431542 (Wako Pure Chemical), 10 μM CKI-7 (Sigma), 1.5 mg/mL soluble human Bone Morphogenetic Protein Receptor IA (BMPRIA)-Fc (R&D Systems, Minneapolis, MN, USA), 2 mM glutamine (Life Technologies), 1 mM pyruvate, 0.1 mM nonessential amino acids (Life Technologies) and 0.1 mM 2- mercaptoethanol (Life Technologies). After washing with PBS, the human iPS cells were dissociated to single cells with Accutase (Innovative Cell Technologies, San Diego, CA, USA) and quickly aggregated in 50 μM Y27632 in Neuronal Differentiation Medium (9000 cells/well, 150 μL) using 96-well low cell-adhesion plates (Sumitomo Bakelite Co., Ltd., Tokyo, Japan). Medium was changed to fresh Neuronal Differentiation Medium with 50 μM Y27632 every day. On day 8, medium was changed to Neuronal Differentiation Medium without Y27632. On day 18, medium was changed to DMEM/F12/Glutamax-1 (Sigma) supplemented with N2 (Sigma). On day 25, cell aggregates were replaced onto poly-d-lysine/laminin/fibronectin (BD Biosciences, Bedford, MA, USA)-coated 6-well culture plates and cultured in Neurobasal medium (Sigma) supplemented with B27 (Sigma) and 2 mM l-glutamine. After replating, medium was changed every 2–3 days. Percentage of number of human iPS cell-derived telencephalic neuron aggregates adhering to poly-d-lysine/laminin/fibronectin coated plates was calculated from 25 aggregates.
Preparation of collagen-coated platesOne volume of type I collagen solution (Cell-matrix, Type I-C; Nitta Gelatin Inc., Osaka, Japan) was mixed with nine volumes of 1 N HCl (pH 3.0) and kept on ice. This Type I-C collagen solution is used for collagen coating (Nagai et al., 2013; Iino et al., 2014). Aliquots (1.0 mL) of this collagen solution were placed in the wells of 6-well culture plates (Corning, Corning, NY, USA), and warmed to 37°C overnight. The coated plates were rinsed twice with sterile PBS and an appropriate volume of pre-warmed Neurobasal Medium was added. On day 25, cell aggregates were replaced onto collagen-coated 6-well culture plates. Percentage of number of human iPS cell-derived telencephalic neuron aggregates adhering to collagen-coated plates was calculated from 23 aggregates.
Preparation of collagen gelEight volumes of type I collagen solution (Cell-matrix, Type I-P; Nitta Gelatin Inc.) were mixed with one volume each of 10× concentrated DMEM (Invitrogen), reconstitution buffer (260 mM NaHCO3 in 100 mL of 50 mM NaOH and 200 mM HEPES; Nitta Gelatin Inc.) and kept on ice. This Type I-P collagen solution is used for making collagen gel (Haga et al., 2005; Nagai et al., 2007). Aliquots (1.5 mL) of this reconstituted collagen solution were placed in the wells of 6-well culture plates (Corning), and immediately warmed to 37°C to allow gel formation. The gel-formed plates were rinsed twice with sterile PBS and an appropriate volume of pre-warmed Neurobasal Medium was added. On day 25, cell aggregates were replaced onto collagen gel in 6-well culture plates. Percentage of number of human iPS cell-derived telencephalic neuron aggregates adhering to collagen gel was calculated from 25 aggregates.
Immunocytochemical stainingCell aggregates were fixed with 4% paraformaldehyde at 4°C overnight, immersed in 30% sucrose, embedded in OCT Compound (Sakura Finetek, Torrance, CA, USA) and cut into section 10 μm thick on a cryostat (HM 550; Thermo Fisher Scientific Inc., Runcorn, UK). Staining was visualised using secondary antibodies conjugated with Alexa Fluor® 488 (green) or Alexa Fluor® 555 (red). The nuclei were stained with Hoechst 33342 (Thermo Fisher Scientific Inc., #H3570, 1:2000). Commercial antibodies used for immunostaining were as follows: FoxG1 (Abcam, #ab18259, 1:500), Nestin (Covance, # PRB-570C, 1:500), neural class III β-tubulin (Covance, #MRB-435P, 1:500, #MMS-435P, 1:500), Oct3/4 (R&D systems, #AF1759, 1:500) and MAP2 (Sigma, #M2320, 1:500). Staining was observed by confocal microscopy (BZ-9000; Keyence, Tokyo, Japan).
Real-time quantitative polymerase chain reaction (qPCR)Total RNA was prepared from each 3–5 neuron aggregates derived from human iPS cells cultured at various conditions using TRIzol reagent (Life Technologies) according to the manufacturer’s instructions. Reverse transcription was carried out using 1 μg of total RNA in a final volume of 1000 μL using SuperScript III First-Strand Synthesis SuperMix (Life Technologies) according to the manufacturer’s instructions. Human fetal cerebral cortex cDNA was purchased from BioChain (Hayward, CA, USA). Real-time qPCR was performed with the 7500 Real-Time PCR System using Power SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA, USA). The following primers were purchased from Takara Bio Inc. (Shiga, Japan): TUJ1 (forward: 5'-AAGTACGTGCCTCGAGCCATTC-3', reverse: 5'-GACCCTTGGCCCAGTTGTTG-3'); OCT4 (forward: 5'-TGAAGCTGGAGAAGGAGAAGCTG-3', reverse: 5'-TCTTTCTGCAGAGCTTTGATGTCCT-3'); NANOG (forward: 5'-TCCAACATCCTGAACCTCAGCTA-3', reverse: 5'-AGTCGGGTTCACCAGGCATC-3'); SOX17 (forward: 5'-TGCAGGCCAGAAGCAGTGTTAC-3', reverse: 5'-CCCAAACTGTTCAAGTGGCAGA-3'); BRACHYURY (forward: 5'-GACAGGTACCCAACCCTGAGGA-3', reverse: 5'-AGCATGGATAAACATGCAGGTGAG-3'); CK17 (forward: 5'-AAGGATGCCGAGGATTGGTTC-3', reverse: 5'-CGAGATCTCACTCTTGCCACTCTG-3'); FOXG1 (forward: 5'-TTAACATCCCTGGGACCAGACTGTA-3', reverse: 5'-ACATTGCACCTCGCTGACACTC-3'); MAP2 (forward: 5'-TGCAACCAGAAATTGGATACCTCA-3', reverse: 5'-CCATCACGCTGCTGGAACTC-3'); GLUR1 (forward: 5'-TTTGTTGGAAGTGAGAATCCCTGAC-3', reverse: 5'-GACACATGGCACGCACTGAA-3'); VGLUT1 (forward: 5'-CAAGGCAGCTCCCAGGTTTAGA-3', reverse: 5'-CAAACACAGCTGCTACTGCCTGA-3'); NESTIN (forward: 5'-CTCCAAGAATGGAGGCTGTAGGAA-3', reverse: 5'-CCTATGAGATGGAGCAGGCAAGA-3'); SYNAPSIN1 (forward: 5'-CCATGCCAATGGTGGATTCTC-3', reverse: 5'-CAATGACCAAACTGCGGTAGTCTC-3') and GAPDH (forward: 5'-GCACCGTCAAGGCTGAGAAC-3', reverse: 5'-TGGTGAAGACGCCAGTGGA-3').
Ethics statementAll experiments were approved by the Ethics Committee of Shionogi & Co., Ltd and conducted in accordance with the Declaration of Helsinki.
An efficient culture method for telencephalic selective neural differentiation of human ES/iPS cells has been established using suspension culture in serum-free medium on low cell-adhesion 96-well plates (serum-free culture of embryoid body-like aggregates quickly [SFEBq]) (Eiraku et al., 2008; Vaccarino et al., 2011). To investigate whether telencephalic neurons were differentiated from human iPS 253G1 cells, we used a partially modified SFEBq culture method with small molecules (TGF-β inhibitor SB431542 and Wnt pathway inhibitor CKI-7) instead of recombinant Lefty-A protein and Dkk1 protein respectively as shown in Fig. 1A. When neural stem cells were induced from 253G1 cells, 25 days cultured embryoid body-like aggregates became larger than 18 days those (Fig. 1B) and immunocytochemical staining showed that cell aggregates contained large numbers of TUJ1 (class-III β-tubulin)-positive neurons and NESTIN-positive neural precursors (Wada et al., 2009) (Fig. 1C). qPCR analysis also confirmed induction of TUJ1 expression (Fig. 1D). In contrast, there were few cells positive for the undifferentiated state marker OCT4 (Scholer et al., 1990) on day 18, and they disappeared completely on day 25 (Fig. 1E). The results of qPCR analysis indicated that the expression levels of OCT4 and another undifferentiated state marker, NANOG (Chambers et al., 2003), were decreased during this culture period (Fig. 1F, G). Furthermore, expression levels of the definitive endoderm marker SOX17 (Kanai-Azuma et al., 2002), the early mesoderm marker BRACHYURY (Wilkinson et al., 1990) and the basal epithelial marker cytokeratin 17 (CK17) (Troyanovsky et al., 1992) were also decreased (Fig. 1H, I, J).

Differentiation of telencephalic neurons from human iPS cells. (A) Experimental scheme of neural differentiation from human iPS cells, 253G1. (B) SFEBq-cultured human iPS cell aggregates on days 18 and 25. Scale bar, 500 μm. (C) TUJ1 (green)-positive neurons and NESTIN (purple)-positive neural precursors were observed in cryosections of SFEBq-cultured human iPS cell aggregates on days 18 and 25. Nuclei were counterstained with Hoechst 33342 (blue). Scale bar, 100 μm. (D) Expression levels of TUJ1. (E) Immunocytochemical staining of OCT4 (green)-positive cells on days 18 and 25. Scale bar, 100 μm. Expression levels of (F) OCT4, (G) NANOG, (H) SOX17, (I) BRACHYURY, (J) CK17 and (K) FOXG1 on days 0, 18 and 25. Expression levels were determined by qPCR analysis and normalised relative to that of GAPDH. “Fold expression” is shown as the ratio of day 18/day 0 or day 25/day 0. “Cortex” indicates human fetal cerebral cortex cDNA. (L) Cryosections of SFEBq-cultured human iPS cell aggregates on days 18 and 25 were stained for FOXG1 (purple). Scale bar, 100 μm.
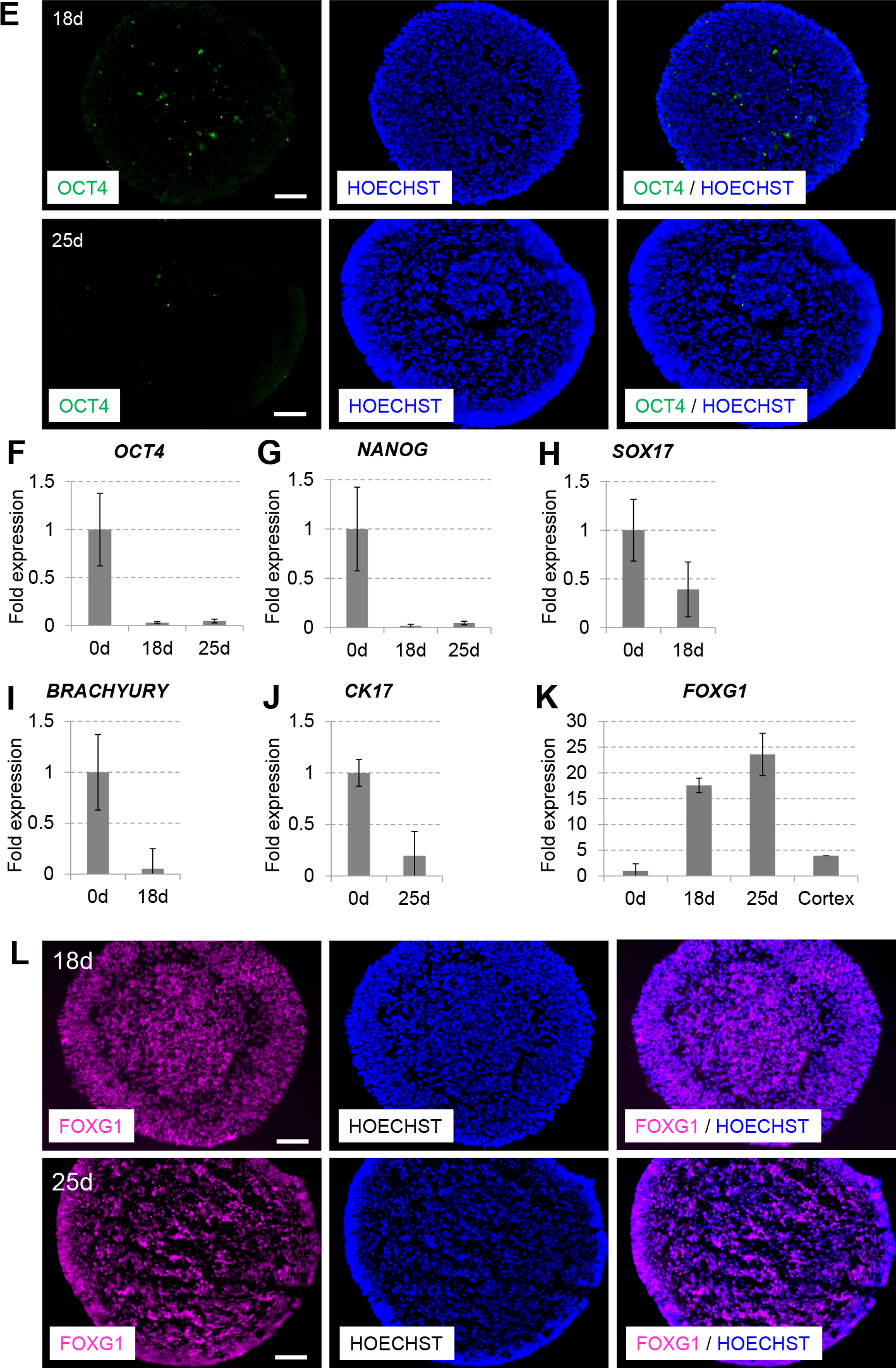
Differentiation of telencephalic neurons from human iPS cells. (A) Experimental scheme of neural differentiation from human iPS cells, 253G1. (B) SFEBq-cultured human iPS cell aggregates on days 18 and 25. Scale bar, 500 μm. (C) TUJ1 (green)-positive neurons and NESTIN (purple)-positive neural precursors were observed in cryosections of SFEBq-cultured human iPS cell aggregates on days 18 and 25. Nuclei were counterstained with Hoechst 33342 (blue). Scale bar, 100 μm. (D) Expression levels of TUJ1. (E) Immunocytochemical staining of OCT4 (green)-positive cells on days 18 and 25. Scale bar, 100 μm. Expression levels of (F) OCT4, (G) NANOG, (H) SOX17, (I) BRACHYURY, (J) CK17 and (K) FOXG1 on days 0, 18 and 25. Expression levels were determined by qPCR analysis and normalised relative to that of GAPDH. “Fold expression” is shown as the ratio of day 18/day 0 or day 25/day 0. “Cortex” indicates human fetal cerebral cortex cDNA. (L) Cryosections of SFEBq-cultured human iPS cell aggregates on days 18 and 25 were stained for FOXG1 (purple). Scale bar, 100 μm.
After culturing the cells for 18–25 days, the expression level of the telencephalic marker Forkhead box g1 (FOXG1) (Shimamura and Rubenstein, 1997) was increased and the strong FOXG1-positive cells were also increased in spite of the decrease of the cell density in cell aggregates (Fig. 1K, L). qPCR analysis indicated that the level of FOXG1 expression was increased by approximately sixfold compared to the human fetal cerebral cortex (Fig. 1K). Therefore, human iPS 253G1 cells could be differentiated efficiently into telencephalic neural cells when cultured under the partially modified SFEBq culture conditions.
Long-term culture of human iPS cell-derived telencephalic neuron aggregates on collagen gelNext, we examined whether human iPS cell-derived telencephalic neuron aggregates could be cultured on the extracellular matrix for a long time. We seeded these aggregates on plates coated with type I collagen (collagen-coated plates), type I collagen gel (collagen gel) or poly-d-lysine/laminin/fibronectin (PDL/laminin/fibronectin-coated plates), and then counted the number of aggregates adhering on each plate. Human iPS cell-derived telencephalic neuron aggregates that had been seeded on PDL/laminin/fibronectin-coated plates or collagen-coated plates initially adhered to the plates, but they gradually came off the plates over time (Fig. 2A). Aggregates adhering to these plates on day 35 were collapsing with distorted neurites as demonstrated by expression of the neuronal marker, TUJ1 (Fig. 2B), and all of the aggregates came off the plate by day 45 (Fig. 2A). On the other hand, human iPS cell-derived telencephalic neuron aggregates that had been seeded on collagen gel could be cultured for more than 35 days and significantly extended neurites into the gel (Fig. 2C, D). In addition, these aggregates became larger in a time-dependent manner and could be cultured for more than 95 days (Fig. 2E, F). These results demonstrated that human iPS cell-derived telencephalic neuron aggregates could be cultured on the collagen gel for more than 3 months.

Long-term culture of telencephalic neuron aggregates derived from human iPS cells on collagen gel. (A) Percentage of human iPS cell-derived telencephalic neuron aggregates adhering to plates [● collagen gel, ■ collagen-coated plates and ▲ PDL/laminin/fibronectin-coated plates]. (B-(a)) SFEBq-cultured human iPS cell-derived telencephalic neuron aggregates on collagen-coated plates on day 35 were seen to be collapsing with distorted neurites. Scale bar, 500 μm. (B-(b)) These neurites were labelled with anti-TUJ1 antibody and stained using goat anti-mouse Alexa Fluor® 488 (green) secondary antibody. Scale bar, 250 μm. (C-(a)) On the other hand, human iPS cell-derived telencephalic neuron aggregates cultured on collagen gel significantly extended neurites into the gel on days 35. Scale bar, 500 μm. (C-(b)) Neurites extended from human iPS cell-derived telencephalic neuron aggregates cultured on collagen gel were labelled with anti-TUJ1 antibody and stained using goat anti-mouse Alexa Fluor® 555 (red) secondary antibody. Scale bar, 250 μm. Human iPS cell-derived telencephalic neuron aggregates cultured on collagen gel increased in size in a time-dependent manner on days (D) 53 and (E) 95. Scale bar, 500 μm. (F) Diameter of SFEBq-cultured human iPS cell aggregates on collagen gel. Diameter at each time point was calculated as the average diameter of 2-4 aggregates. Error bars shows standard deviations
The cerebral cortex layer contains approximately 80% glutamatergic neurons, and this layered structure is formed by the precise migration and differentiation of neuronal progenitor cells (Wonders and Anderson, 2006, Molyneaux et al., 2007). As described above, human iPS cell-derived telencephalic neuron aggregates on the collagen gel became larger in a time-dependent manner (Fig. 2F) and extended neurites into the gel (Fig. 2C, D). These results suggested that human iPS cell-derived telencephalic neuron aggregates matured over time. To examine the maturation of human iPS cell-derived telencephalic neuron aggregates on the collagen gel, we cultured these aggregates for 3 months and analysed the expression of mature neuron markers. The results of qPCR analysis indicated that the expression levels of the mature neuron marker MAP2 (microtubule-associated protein 2), glutamate receptor marker GLUR1 and vesicular glutamate transporter marker VGLUT1 were elevated until day 95. A gradual increase in expression of the presynaptic marker SYNAPSIN1 mRNA was also observed during the differentiation process. On the other hand, the expression level of the neural stem cell marker NESTIN was decreased over this schedule (Fig. 3A). These results indicated that the differentiation process proceeded from telencephalic precursors to differentiated glutamatergic neurons in human iPS cell-derived telencephalic neuron aggregates by culture on collagen gel over a long period.

Maturation of telencephalic neuron aggregates derived from human iPS cells on collagen gel. (A) Expression levels of MAP2, NESTIN, SYNAPSIN1, GLUR1 and VGLUT1 on days 0, 18, 25, 35 and 95. Expression levels of 3–5 human iPS cell-derived telencephalic neuron aggregates were measured by qPCR analysis and normalised relative to that of GAPDH. Cryosections of SFEBq-cultured human iPS cell aggregates on days (B) 35 and (C) 95 were stained for the neural precursor marker NESTIN (red) and mature neuron marker MAP2 (green). Nuclei were counterstained with Hoechst 33342 (blue). A mushroom-shaped structure was enclosed by white lines, and the inner region was delineated by white-dotted lines. Scale bar, 100 μm.
Previous studies have shown that a cerebral cortex-like layered structure is formed in human ES cell-derived telencephalic neuron aggregates on day 46 in culture on PDL/laminin/fibronectin-coated plates (Eiraku et al., 2008). As described above, human iPS cell-derived telencephalic neuron aggregates that had been seeded on collagen gel could be cultured for more than 35 days (Fig. 2C, D, E, F). We examined whether the cerebral cortex-like layered structure was formed in human iPS cell-derived telencephalic neuron aggregates cultured on collagen gel. Immunocytochemical analysis of these aggregates on day 35 revealed a mushroom-shaped structure formed by NESTIN-positive neural precursors (Fig. 3B). After culturing these aggregates on collagen gel, there were many NESTIN-positive layer-like structures and increased numbers of MAP2-positive mature neurons around these layer-like structures on day 95 (Fig. 3C). These results indicated that layer-like structures were formed inside the human iPS cell-derived telencephalic neuron aggregates in long-term culture on collagen gel.
Our data demonstrated that human iPS 253G1 cells could be differentiated efficiently into telencephalic neuron aggregates when cultured under partially modified SFEBq culture conditions using the low-cost and stable-activity chemical inhibitors instead of recombinant proteins. These aggregates could be cultured for more than 3 months on collagen gel. Furthermore, telencephalic neuronal precursors within the aggregates matured over time and formed layered structures. A previous study indicated that a cerebral cortex-like layered structure is formed in hES cell-derived telencephalic neuron aggregates cultured on PDL/laminin/fibronectin-coated plates (Eiraku et al., 2008). However, we found that human iPS cell-derived telencephalic neuron aggregates adhering to PDL/laminin/fibronectin-coated plates or collagen-coated plates gradually came off the substrate. This might be caused by the difference in a favored extra-matrix for attachment between human iPS cells and ES cells (Lam and Lonqaker, 2012), but human iPS cell-derived telencephalic neuron aggregates that had been seeded on collagen gel could be cultured for more than 45 days (Fig. 2E, F). We suggest two possible reasons for these results. First, the human iPS cell-derived telencephalic neuron aggregates are fragile and are thus suitable for culture on collagen gel, which is softer than the collagen or PDL/laminin/fibronectin coating (Yunoki et al., 2011, Mizutani et al., 2007). Alternatively, the cellular crawling into the 3D-pore structure in the collagen gel contributed to the tight adhesiveness between these aggregates and the collagen gel. To test which hypothesis is correct, the use of collagen-coated acrylamide gel (soft and poreless) is preferable. In conclusion, our data indicated that collagen gel are more suitable for long-term culture of telencephalic neuron aggregates derived from human iPS cells than collagen-coated plates or PDL/laminin/fibronectin-coated plates.
In addition, the spherical morphology of human iPS cell-derived telencephalic neuron aggregates was maintained when cultured on collagen gel. However, it was difficult to keep the spherical morphology of human iPS cell-derived telencephalic neuron aggregate adhering to PDL/laminin/fibronectin-coated or collagen-coated plates (Fig. 2A, B). This is probably because human iPS cell-derived telencephalic neuron aggregates are suitable for culture on softer collagen gel. In addition, the stiffness of collagen gel is close to that of the brain (0.1–1.0 kPa) (Engler et al., 2006; Janmey and Miller, 2011, Byfield et al., 2009). Thus, it was suggested that human iPS cell-derived telencephalic neuron aggregates on collagen gel could be cultured under conditions of stiffness close to that of the brain.
Furthermore, qPCR analysis indicated that the expression levels of the mature neuron marker MAP2, glutamate receptor marker GLUR1 and vesicular glutamate transporter marker VGLUT1 still increased in human iPS cell-derived telencephalic neuron aggregates cultured on collagen gel over a long period. In addition, this study revealed that telencephalic neuron aggregates derived from human iPS cells increased in size in a time-dependent manner (Fig. 2F), and significantly extended neurites into the collagen gel as observed by monitoring expression of the neuronal marker TUJ1 (Fig. 2C, D). It has been reported that soft fibronectin surfaces cause differentiation and increased neurite extension of mouse hippocampal neurons (Kostic et al., 2007). As described above, collagen gel is soft (0.1–1.0 kPa), similar to the brain. Cell function and differentiation have been reported to be regulated by substrate stiffness corresponding to the mechanical properties of the tissue in which the cells are normally located (Engler et al., 2004). It has also been reported that the majority of human mesenchymal stem cells exhibit a branched, filopodia-rich morphology and high levels of neurogenic transcript expression on soft collagen-coated gel (0.1–1.0 kPa) (Engler et al., 2006). Therefore, it was suggested that NESTIN-positive neural precursors were promoted to differentiate and increase neurite extension by adhering to collagen gel.
In addition, cells that were positive for TUJ1, a neuronal marker, were present not only at the periphery of cellular aggregates but also at the inner aggregates on the collagen gel as shown in Fig. 2C(b). On the other hand, TUJ1-positive cells were present only at the periphery of cellular aggregates on the collagen-coated plate condition as shown in Fig. 2B(b). These results suggested that the cells at the inner aggregates differentiated more on the collagen gel than the collagen-coated plate. As described above, soft fibronectin surfaces induced differentiation of mouse hippocampal neurons (Kostic et al., 2007). It was also reported that a cell stiffening response was induced due to substrate properties (Vichare et al., 2014). From these reports, we hypothesized that not only do the outer cells adhere to the collagen gel, but also the inner cells in the cellular aggregates might sense a soft extracellular environment and thus differentiate more efficiently.
In this study, we established defined conditions for the long-term culture of human iPS cell-derived telencephalic neuron aggregates with layered structures using collagen gel. The formation of the layered structures inside the human iPS cell-derived telencephalic neuron aggregates is expected to be applicable to research on human brain development and the pathogenesis of neuronal developmental disorders, such as autism, Asperger’s disorder and attention deficit hyperactivity disorder. Thus, our method for culturing human iPS cell-derived telencephalic neuron aggregates on collagen gel will be useful for a variety of neurodevelopmental research.