2024 年 89 巻 1 号 p. 3-5
2024 年 89 巻 1 号 p. 3-5
Regeneration is the process of reconstituting or developing new cells, tissues, and organs. Recent research has revealed that the priming mechanism plays a crucial role in regeneration. This mechanism involves the cell entering a primed state after the first stimulus, which could be a change in differentiation state, metabolic alteration, inflammatory response due to pathogen stimulation, or environmental stimuli. In this primed state after the initial stimulus, histone modifications and chromatin open/closed states remain in the primed gene loci. Upon arrival of the second stimulus, whether the same or different, these epigenetically primed genes are transcribed more quickly and potently than in response to the first stimulus. This is because RNA polymerase II can bind to the promoters of the primed genes immediately. This mechanism of priming enables quick shoot regeneration from callus in plants and prompt wound repair in mammals. This review focuses on recent reports regarding the primed state of callus and mammalian skin stem cells. As Professor Louis Pasteur famously stated, ‘Chance favors the prepared mind.’ Similarly, regeneration is more likely to occur in the epigenetically primed state.
Regeneration is crucial for restoring or developing new cells, tissues or organs in both plants and animals (Birnbaum and Alvarado 2008). Plants have a high potential for regeneration due to their continuous growth throughout their life, known as postembryonic development (Birnbaum and Alvarado 2008). Plant tissue culture systems have been established to regenerate through a pluripotent cell mass called callus (Morinaka et al. 2023). After induction by changing the phytohormone balance, organs can be regenerated from stem cells that emerge from the callus (Sugimoto et al. 2019, Hirano and Tanaka 2020). In contrast, the regenerative capacity of animals varies among species. While most animals complete their body axis development during the embryonic stage, some animal species, such as planaria, hydra, and amphibian limbs, have a high potential for organ regeneration (Poss 2010). Stem cells are undifferentiated cells with self-renewal properties found in the organs that can differentiate into different cell types with specialized functions. Animals possess various stem cells in each tissue and organ. In mammals, limited organs such as the liver (Michalopoulos and Bhushan 2021) exhibit large-scale organ regeneration in the natural state, while small-scale regeneration, such as skin wound healing (Naik and Fuchs 2022), is a common phenomenon. Medical engineering for organ and tissue regeneration using stem cells has also been actively pursued (Ajmal et al. 2023). This review summarizes recent reports on the mechanism of primed states of callus in plants and skin stem cells in mammals that contribute to the rapid response to second stimuli.
Epigenetics can explain phenomena that cannot be explained by conventional genetics, such as differences in personality and health status between monozygotic twins who are genomically similar. Conventional studies have compared gene expression before and after a significant phenotypic change caused by environmental stimuli or induction of differentiation. Recently, epigenetic analyses have allowed to explain the mechanism of preparation for future growth, differentiation, and regeneration. During the preparation, the phenotype may not be apparent but is likely to progress without the alternation of gene expression in the organism. This process, known as epigenetic priming, involves the preparation of the organism for rapid and coordinated transcription in response to environmental stimuli or differentiational induction (Dillon 2012, Bonifer and Cockerill 2017).
In the dicot model plant Arabidopsis thaliana, the epigenetic modification factor LYSINE-SPECIFIC DEMETHYLASE 1-LIKE 3 (LDL3) specifically removes demethylated lysine 4 of histone H3 (H3K4me2) in the callus derived from root cells. This facilitates the subsequent activation of genes involved in shoot formation (Ishihara et al. 2019). LDL3 is an ortholog of human LYSINE-SPECIFIC DEMETHYLASE 1 (LSD1), which removes mono- and dimethyl groups from H3K4 (Shi et al. 2004, Toyoda and Matsunaga 2019). Ishihara et al. (2019) identified the ldl3 mutant, which is incapable of regenerating shoots from callus derived from roots. Callus is induced from explants on a callus induction medium (CIM) containing a high auxin ratio. The resulting callus is then cultured on a shoot induction medium (SIM) with a high cytokinin ratio to induce the regeneration of shoots (Valvekens et al. 1988). Genome-wide chromatin immunoprecipitation followed by sequencing (ChIP-seq) analysis showed that LDL3 eliminated H3K4me2 of genes required for shoot regeneration from the callus. However, this demethylation occurred during callus formation did not affect gene expression (Ishihara et al. 2019). The histone modifications of H3K4me2 did not change when plants were transferred from the CIM to the SIM. LDL3 removes H3K4me2 during callus formation before transfer to SIM, indicating that LDL3 is an epigenetic priming factor that recognizes genes required for shoot regeneration, removes H3K4me2 during callus formation, and facilitates the rapid activation of genes to form shoot regeneration (Fig. 1A) (Ishihara et al. 2019). Our discovery of epigenetic priming based on the removal of H3K4me2 by LDL3 was the first report in both plants and animals to implicate a histone modification eraser in priming.
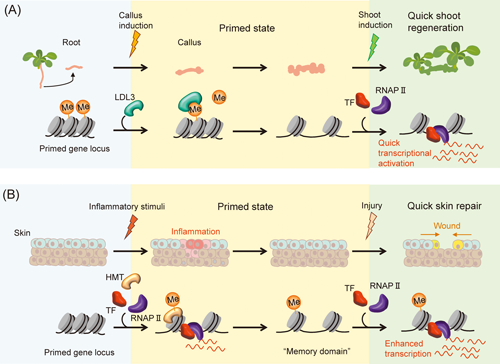
(A) Priming during callus formation by a histone demethylase, LDL3. (B) Priming of skin after inflammation by a histone methyltransferase, HMT. The chromatin structure remains open during the primed state by maintaining histone modification including methylation (Me). Transcription factors (TFs) and RNA polymerase II (RNAP II) can be rapidly recruited to the open chromatin, resulting in the rapid transcriptional activation or the enhanced transcrition.
Mammalian cells sense and respond to inflammatory, metabolic, and pathogenic stimuli and remain in a state of alert even after the initial stimuli have ceased. This state is known as the primed state, and cells in this state can respond more quickly to secondary triggers that provide anti-pathogenic and repair-promoting functions (Naik and Fuchs 2022). Priming is a typical mechanism of innate immune responses (Divangahi et al. 2021). The first stimulus improves the functional state of innate immune cells, and primes them for immune response, as evidenced by the activation of gene expression. The second stimulus elicits an additive response from the immune-primed cells. This enhanced transcriptional response may be due to more rapid recruitment of RNA polymerase II (RNAP II) to the promoters of primed genes.
In mammals, only a limited number of organs are capable of whole organ regeneration. However, the regeneration of skin tissue through wound repair is a typical process (Gurtner et al. 2008). Recent studies have shown that the transient application of acute inflammatory stimuli to the skin can enhance its subsequent wound healing ability (Naik et al. 2017). Epidermal stem cells have an epigenetic memory of the inflammatory experience, which is crucial for re-epithelialization during wound repair. The inflammatory stimulus induces epidermal stem cells into a primed state, activating genes that alter histone modifications and chromatin access status, including inflammatory, antimicrobial, and stress-related genes. Genes that are primed by inflammation maintain chromatin accessibility, even though their transcription returns to near baseline after the inflammation subsides. This is due to the persistence of H3K4me1 and H3K4me3 at the enhancer and proximal promoter genomic regions, respectively. These accessible genomic regions are known as ‘memory domains.’ This memory domain is believed to aid in the swift mobilization of RNAP II, enabling the rapid reactivation of primed genes for wound repair (Fig. 1B) (Larsen et al. 2021).
During the process of wound repair, stem cells located within the hair follicle migrate to the skin surface to promote skin healing. These stem cells remain in the area even after the wound has healed. Research has shown that stem cells in the hair follicle retain information about their original location, migration, and inflammation even after the wound has healed (Gonzales et al. 2021). Recently, researchers have discovered that the memory of the initial wound is maintained in stem cells located in distal hair follicles that are not involved in wound repair. These stem cells promote second wound repair (Levron et al. 2023). Further research is needed to analyze the extent of the regions that cause priming and the duration of memory maintenance.
There is still much to learn about the mechanism of the primed state of stem cells. Questions remain regarding which genes activated by changing histone modifications during initial reprogramming are maintained in the primed state, how primed cells are determined, and whether histone modifications alone suffice to maintain the memory domain in an open chromatin state. Recent discoveries have highlighted the role of the cytokine-activated transcription factor STAT and the stress-responsive transcription factor FOS in maintaining inflammatory memory in epidermal stem cells (Lau et al., 2018; Larsen et al., 2021). To better understand the molecular mechanisms of priming in regeneration, further research should focus on identifying priming-regulatory complexes. These complexes are likely to include chromatin remodeling factors, transcription factors, and histone modifying enzymes.
This work was supported by grants from MXT/JSPS KAKENHI (20H03297 and 22H00415), JST CREST (JPMJCR20S6), and ASPIRE to S.M.