2020 Volume 67 Issue 10 Pages 1023-1028
2020 Volume 67 Issue 10 Pages 1023-1028
Osteoporosis is one of the clinical features of women with Turner syndrome (TS). The reasons for low bone mineral density (BMD) and increased bone fragility are multifactorial, including estrogen deficiency, X-chromosome abnormalities, and environmental factors. Few, large-scale studies on bone mineral density in either adolescents or adults with TS have been done in Japan. The goal of the present study was to investigate spinal BMD in women with TS, assess its relationship with clinical parameters, especially estrogen replacement therapy, and investigate its longitudinal changes. The spinal BMD and clinical data of 149 Japanese women with TS aged 15 to 49 years who were followed at the four participating hospitals were retrospectively analyzed. The BMD Z-scores of the women with TS ranged from –5.30 to +1.89. Women with TS aged 15–39 years had lower BMD than healthy Japanese women (p < 0.01) while women with spontaneous menstruation had a significantly higher BMD Z-score than those without spontaneous menstruation (–0.73 ± 1.11 vs. –1.67 ± 1.18, p < 0.01). In women without spontaneous menstruation, BMD Z-scores correlated with the duration of their estrogen therapy (r = 0.167, p < 0.01). Women aged 15–39 years with TS had low BMD, which was associated with primary amenorrhea and short estrogen replacement therapy duration.
TURNER SYNDROME (TS), originally described by Turner et al. [1], is one of the most common sex chromosome aneuploidies, with an incidence of 1 per 2,000–2,500 women [2]. While the two main features of TS in women are short stature and gonadal failure, low bone mineral density (BMD) and an increased risk of fractures have also been reported [3-7]. The reasons for the low BMD and increased bone fragility are multifarious, including chronic estrogen deficiency, X-chromosome abnormalities (especially haploinsufficiency of the SHOX gene), and environmental factors, such as low/decreased physical activity due to skeletal muscular dysplasia [5, 8-10].
Estrogen replacement therapy (ERT) is a pivotal strategy for improving BMD in women with TS because chronic estrogen deficiency is one of the major reasons for bone loss in this disorder. Several studies indicated that ERT contributed to increasing/maintaining lumbar spine BMD [11-13], and that a delay in commencing ERT in primary amenorrhea was associated with lower BMD [6, 14-17]. Lumbar spine BMD is an important parameter because it is independently associated with the history and incidence of fractures and is a predictor of fracture risk in women with TS [16] as it is in the general female population [18].
To date, few, large-scale studies investigating BMD in adolescents and adults with TS have been done in Japan. Thus, this retrospective study aimed to (1) investigate the BMD of women with TS in Japan, (2) assess its relationship with clinical parameters, especially ERT, and (3) investigate longitudinal changes in BMD.
The present retrospective multicenter study in Japan enrolled women aged 15 to 49 years who had TS and underwent one or more dual-energy X-ray absorptiometry (DXA) scans between April 1994 and October 2018. Patients with the complications of anorexia nervosa or diabetes mellitus were excluded. First, a review of medical files identified 194 women with TS who fulfilled the criteria mentioned above. Second, BMD measurements after the administration of osteoporosis-specific drugs, such as bisphosphonates, were excluded. The remaining 449 BMD measurements were analyzed (Fig. 1).

Study flow
TS, Turner syndrome; BMD, bone mineral density
The following data were collected for each subject: karyotype, menstrual history, medication, and comorbidities. The primary outcome was the difference in BMD and BMD Z-score between the women with TS and healthy Japanese women reported previously [19]. The secondary outcomes were: (1) longitudinal changes in BMD and BMD Z-scores, (2) the difference in BMD Z-scores between a growth hormone (GH) therapy group and non-GH therapy group, (3) the difference between subjects with spontaneous menstruation and other subjects, and (4) the relationship between BMD Z-scores and the duration of cyclic estrogen and progesterone therapy (Kaufmann therapy) in TS women with primary amenorrhea. The duration of Kaufmann therapy was used as a marker for the duration of ERT in the present study.
Measurement of bone mineral densityFirst, a DXA scan was conducted using a single device (Hologic, Bedford, MA, USA). All women with TS had measurements of areal BMD (g/cm2) of the lumbar spine (L2-4). Second, the results were transformed into volumetric BMD following the method reported by Kroger et al. [20].
Statistical analysisContinuous, normally distributed data were expressed as the mean ± SD; continuous, non-normally distributed data were expressed as the median and interquartile range (IQR); and categorical data were expressed as a percentage. Intergroup comparisons were performed using Student’s unpaired t test (normally distributed data) or Mann-Whitney U test (non-normally distributed data) as appropriate. Frequency rates were compared using the χ2 test. Student’s paired t-test and regression analysis were performed to study longitudinal changes in BMD and the relationship of BMD with Kaufmann therapy, respectively. P < 0.05 was considered statistically significant. All statistical analyses were performed using Easy R (EZR) version 3.4.1. [21]
Volumetric BMD positively correlated with areal BMD (r = 0.84, p < 0.01; data not shown). The areal BMD and BMD Z-scores were used in all the subsequent analyses.
Baseline dataThe patient characteristics are shown in Table 1. The median year of birth of 194 subjects with TS was 1986 (range: 1963–2010). Of these, five had a past history of bone fracture, and 124 received/had received GH therapy. Thirty-four subjects had spontaneous menstruation, and the remaining 160 subjects required Kaufmann therapy. The median age at initiation of Kaufmann therapy was 16.7 years (range: 12–38 years). Nineteen of 194 subjects had Hashimoto disease (n = 16) or Grave’s diseases (n = 3). None had parathyroid disease. Thirteen subjects and one subject received vitamin D and K supplementation, respectively.
| Women with TS (n = 194) | ||
|---|---|---|
| Birth year | 1986 (1963–2010) | |
| Final height (cm) | 147.1 ± 5.7 (131–164) | |
| Karyotype | Monosomy | 39 |
| Structural abnormality | 89 | |
| Mosaic | 38 | |
| No precise information available | 28 | |
| History of bone fracture | Fracture (+) | 5 |
| Fracture (–) | 189 | |
| Medical history of GH therapy | GH (+) | 124 |
| GH (–) | 15 | |
| Unknown | 55 | |
| History of menstruation | Spontaneous menstruation | 34 |
| Primary amenorrhea | 160 | |
| Age at start of Kaufmann therapy in women with primary amenorrhea (years) |
16.7 (12–38) | |
| Complications | Hypothyroidism | 16 |
| Hyperthyroidism | 3 | |
| Inflammatory bowel disease | 1 | |
| Vitamin supplementation | Vitamin D | 13 |
| Vitamin K | 1 |
Data are expressed as the mean ± SD (range) or the median (range).
Four hundred forty-nine BMD Z-scores of women with TS fell in the range of –5.30 to +1.89. Of these, 307 subjects (68%) had a normal BMD Z-score (Z-score >–2.0). When analyzed by age increments of five years, each BMD measurement was significantly lower in women with TS than in healthy subjects (Table 2, Fig. 2). The BMD data after age 40 years were not used due to the small number of measurements, only 23.
| Age at measurement | TS women | Healthy subjects (Iki et al. [19]) | p value |
|---|---|---|---|
| 15–19 years | 0.819 ± 0.118 (n = 117) | 0.973 ± 0.094 (n = 243) | <0.001 |
| 20–24 years | 0.851 ± 0.138 (n = 102) | 1.003 ± 0.109 (n = 260) | <0.001 |
| 25–29 years | 0.870 ± 0.132 (n = 113) | 1.025 ± 0.100 (n = 288) | <0.001 |
| 30–34 years | 0.882 ± 0.120 (n = 53) | 1.038 ± 0.102 (n = 276) | <0.001 |
| 35–39 years | 0.870 ± 0.122 (n = 41) | 1.051 ± 0.115 (n = 283) | <0.001 |
| 40–44 years | 0.887 ± 0.090 (n = 17) | 1.035 ± 0.114 (n = 290) | N.A. |
| 45–49 years | 0.928 ± 0.115 (n = 6) | 1.022 ± 0.131 (n = 296) | N.A. |
Data are expressed as the mean ± SD. P values were determined by Student’s unpaired t test for women with TS and healthy Japanese women. N.A., not analyzed.
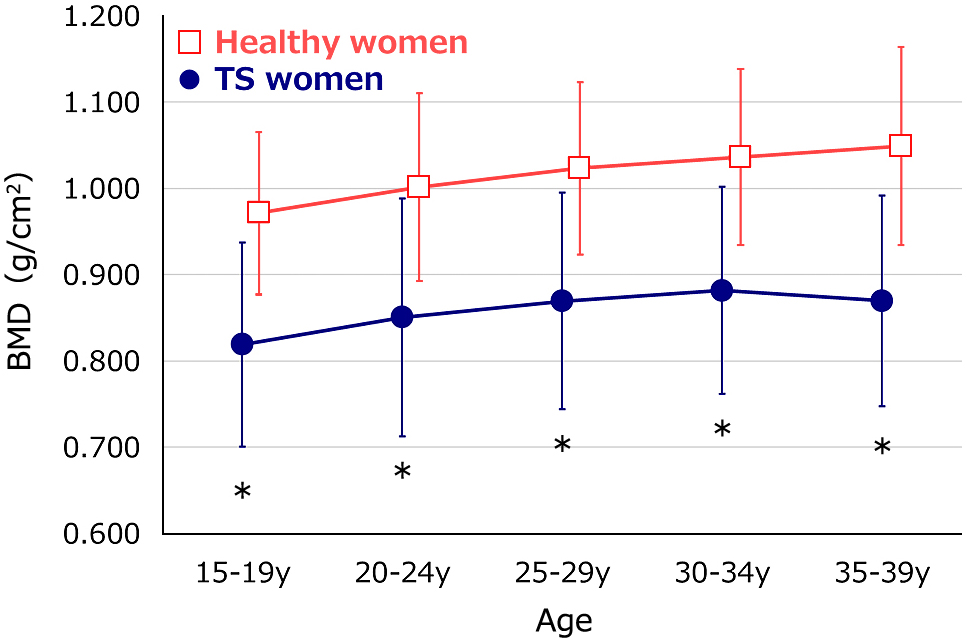
BMD of women with TS and healthy women
BMD values (g/cm2) were significantly lower in women with TS than in healthy Japanese women. *p < 0.05 versus healthy women of the same age-group.
In 17 women with TS, no longitudinal changes in BMD and BMD Z-score were observed (Fig. 3).

BMD of women with TS before and after age 35 years (n = 17)
No decrease in bone mass was observed before or after age 35 years.
There was no difference in the BMD of women with TS between the GH therapy group (n = 124) and non-GH therapy group (n = 14) although height differed significantly (p < 0.01) between the two groups (median: 147.6 cm and 144.8 cm, respectively). Women with spontaneous menstruation (n = 75) had a significantly higher BMD Z-score than those without spontaneous menstruation (n = 374) (–0.73 ± 1.11 vs. –1.67 ± 1.18, p < 0.01). In women without spontaneous menstruation, the BMD Z-scores correlated with the duration of Kaufmann therapy (Fig. 4). In 18 women with TS, a comparison of the BMD values before and during Kaufmann therapy showed an increase in BMD after the start of Kaufmann therapy (Fig. 5).

Relationship of BMD Z-scores and duration of Kauffman therapy
In women without spontaneous menstruation, BMD Z-scores were correlated with the duration of Kaufmann therapy on regression analysis (r = 0.167, p < 0.01). Note that the r value was not high.

BMD Z-scores of women with TS before and after starting Kaufmann therapy (n = 18)
BMD Z-scores during Kaufmann therapy (for at least two years) were higher than those before therapy.
The present study is one of the largest to be done on BMD in women with TS in Japan. Compared with healthy women, women with TS women had a low BMD at age 15–39 years. Similar results for BMD were confirmed in other countries as well [16, 22-25] .
Our study indicated the critical role played by estrogen in BMD in this age group. The significant difference was found in BMD between women with TS with and without spontaneous puberty. Furthermore, Kaufmann therapy increased BMD in women with TS in the present study. A similar effect was also reported by previous studies [11-13]. However, the age at which the BMD values start to decrease in TS remains unclear. Our previous report already demonstrated that BMD declines even in prepuberty in TS (Nanao et al. [14]).
Our data failed to show any earlier, age-related bone loss. Nguyen et al. [16] reported that age-related bone loss occurred earlier in women with TS than in healthy, premenopausal women described in other cohort studies [19, 26, 27], suggesting that this loss was accelerated by the disorder. Appropriate ERT may prevent the acceleration of bone loss. However, further studies are required to confirm that bone loss acceleration occurs in women with TS.
GH treatment did not contribute to any changes in BMD in our study although the number of subjects in the non-GH therapy group was small at just 14, making statistical analysis difficult. To date, the literature on the effects of GH therapy on BMD in women with TS is controversial. Some studies [6, 11, 23] have reported the beneficial effects of GH on BMD while others have not [13, 16, 28].
There are some limitations to this study. First, the age at the initiation of Kaufmann therapy was more advanced than that recommended in the guidelines for TS treatment. The subjects in the present study included a relatively large number of older women who received Kaufmann therapy before the current guidelines were published [29-31]. Second, the number of BMD measurements at or above age 40 years was too small to analyze longitudinal changes, particularly of age-related bone loss. Third, some women with TS with low BMD may have been excluded due to the exclusion of women with TS with BMD measurements taken during the administration of osteoporosis-specific drugs.
All women with TS aged 15–39 years had low BMD, which was associated with primary amenorrhea and a short duration of ERT.
We thank Mr. James R. Valera for his assistance with editing this manuscript.
Our study was approved by the ethical committee at all the participating institutions. The research was led by the Division of Endocrinology and Metabolism at Tokyo Metropolitan Children’s Medical Center (approval ID: H30b-191). Informed consent was not required due to the retrospective observational nature of the study, and the DXA scans were performed as part of routine clinical practice.
The authors have no conflicts of interest in connection with this article.