Abstract
Objectives: There are benefits of exercise-based cardiac rehabilitation (CR) in patients with heart failure (HF), but their underlying molecular mechanisms remain elusive. The effect of CR on the expression profile of circulating microRNAs (miRNAs), which are short noncoding RNAs that regulate posttranscriptional expression of target genes, is unknown. If miRNAs respond to changes following CR for HF, then serum profiling of miRNAs may reveal cardioprotective mechanisms of CR.
Methods: This study enrolled three hospitalized patients with progressed systolic HF and three normal volunteer controls. In patients, CR was initiated after improvement of HF, which included 2 weeks of bicycle ergometer and resistance exercises. Genome-wide expression profiling of circulating miRNAs was performed using microarrays for the patients (mean±SD age, 60.0±12.2 years) and controls (58.7±0.58 years). Circulating miRNA expression profiles were compared between patients with HF before and after CR and the controls.
Results: Expression levels of two miRNAs were significantly different in patients before CR compared with controls and patients after CR. The expression of hsa-miR-125b-1-3p was significantly downregulated and that of hsa-miR-1290 was significantly upregulated in patients before CR.
Conclusions: When performing CR, expression of certain circulating miRNAs in patients with HF is restored to nonpathological levels. The benefits of CR for HF may result from regulation of miRNAs through multiple effects of gene expression.
Introduction
Cardiac rehabilitation (CR) that includes exercise training results in a broad range of positive effects for patients with cardiac diseases. On the basis of strong evidence, CR is recommended for patients with heart failure (HF).1 In patients with mild to moderate chronic HF, exercise training improves their exercise tolerance and quality of life, as well as having the potential to reduce mortality and hospitalization.2 However, this recommendation is not sufficiently implemented in clinical practice. Clarification of the molecular mechanisms underlying the effect of CR could assist in an understanding of the benefit of CR, and lead to an increase in awareness of CR in clinical practice.
MicroRNAs (miRNAs) are a family of single-stranded, short, non-coding RNA sequences that comprise 19–23 nucleotides. These miRNAs act as important posttranscriptional regulators of genetic expression through translational repression or transcript cleavage resulting from binding to the target mRNA.3–5 The miRNAs were first reported as endogenous mediators in the worm Caenorhabditis elegans.6 Since this time, miRNAs have been shown to be involved in regulating various cellular processes, including cellular differentiation, proliferation, and apoptosis.7 A lot of evidence of the crucial roles of miRNAs in various diseases has accumulated over recent years,8–10 but their significance in molecular biological processes of cardiac diseases are only beginning to be recognized.11,12
The miRNAs exist in a stable form in the bloodstream and are referred to as circulating miRNAs. Circulating miRNAs could become useful biomarkers for various diseases,13,14 including cardiac diseases.15,16 The present study aimed to investigate whether the cardioprotective mechanisms of CR for patients with HF involve changes in circulating miRNAs.
Materials and Methods
Subjects, CR program, and blood sampling
Three patients with HF and three healthy controls were enrolled in the study. The patients were hospitalized for treatment of progressed HF with systolic left ventricular dysfunction from New York Heart Association classes I–II. The controls were volunteers who had no previous medical history and were recruited from staff at Fujita Health University Bantane Hospital. Written informed consent was obtained from all of the subjects and the protocol was approved by the Research Ethics Committee of Fujita Health University (HM16-203). In patients with HF, CR was initiated after recovery from New York Heart Association classes II to I by 1 week of treatment for HF. The CR exercise program comprised a bicycle ergometer at an anaerobic threshold intensity that was determined from a cardiopulmonary exercise test and resistance training for 20 min twice a day, 5 days a week. This exercise was performed daily for 2 weeks while the patient was hospitalized. Microarray analysis of circulating miRNAs was performed for these three patients before and after the CR program, as well as for the three controls. Blood samples were obtained in the morning in a calm environment, with the subject in a fasted state. A 20-mL aliquot of blood was drawn from a peripheral vein into a spitz tube containing serum separating agent. The serum was isolated by centrifugation at 3000 rpm for 10 min, transferred into an Eppendorf tube, and stored at –90°C.
RNA extraction and miRNA expression profiling
Total RNA was isolated from the serum using 3D-Gene RNA extraction reagent (Toray Industries, Kamakura, Japan) according to the manufacturer’s instructions. The extracted RNA was checked with a Bioanalyzer (Agilent, Santa Clara, CA, USA) and labeled with a 3D-Gene circulating miRNA labeling kit (Toray Industries). The labeled RNAs were hybridized onto a 3D-Gene Serum miRNA Oligo chip (Toray Industries). We ensured that the annotation and oligonucleotide sequences of the probes conformed with the miRBase miRNA database (miRbase.org). After stringent washing, the fluorescent signals were scanned with a 3D-Gene Scanner (Toray Industries) and analyzed using 3D-Gene Extraction software (Toray Industries). The raw data for each spot were normalized by substitution with the mean intensity of the background signal, which was determined from the 95% confidence intervals for the signal intensities of all of the brank spots. Measurements of spots were considered to be valid when the signal intensities were >2 standard deviations (SDs) from the background signal intensity. Relative expression levels of miRNAs were calculated by comparison with the signal intensities of the valid spots throughout the microarray analysis. The data were then globally normalized for each array by adjusting the median of the signal intensity to 25. The miRNA results for the patients before CR were compared with those of the controls and those of the patients after CR.
Statistical analysis
Continuous variables are presented as the mean±SD, and categorical variables are presented as numbers and percentages. Differences in the characteristics between patients with HF and controls were evaluated with unpaired t tests for continuous variables and chi-square analysis or Fisher’s exact test for absolute categorical variables. Differences in the characteristics between patients with HF before and after CR were evaluated with the paired t test for continuous variables. Statistical significance was set at p<0.05. In the microarray analyses, significant differential expression was defined by a mean fold difference of >2 or <0.5 relative to the controls or the patients after CR, with a p value adjusted by an FDR <0.05. All statistical analyses were performed with IBM SPSS statistics version 20.0 (IBM Corporation, Armonk, NY, USA).
Results
Subjects’ characteristics
The clinical characteristics of the patients with HF and the controls are shown in Table 1. The patients with HF and the controls were all men, and there was no significant difference in age between the patients and controls. Patients with HF had significantly higher levels of N-terminal pro-brain natriuretic peptide compared with controls. Table 2 shows comparison of the characteristics of the patients with HF before and after CR. Patients after CR had a significantly lower systolic blood pressure and were more likely to have a lower body weight, body mass index, diastolic pressure, and heart rate compared with patients before CR.
Table1
Clinical characteristics of patients with heart failure before cardiac rehabilitation and controls
| Characteristics |
Patients (n=3) |
Controls (n=3) |
p |
| Age (y) |
60.0±12.2 |
58.7±0.6 |
0.57 |
| Male sex |
3 (100%) |
3 (100%) |
— |
| BMI (kg/m2) |
31.0±12.6 |
22.4±2.2 |
0.59 |
| Pervious medical history |
| Hypertension |
1 (33%) |
0 (0%) |
— |
| Dyslipidemia |
1 (33%) |
0 (0%) |
— |
| Diabetes |
0 (0%) |
0 (0%) |
— |
| Smoking |
2 (66%) |
0 (0%) |
— |
| Medication |
| Diuretics |
3 (100%) |
0 (0%) |
— |
| ACEIs/ARBs |
3 (100%) |
0 (0%) |
— |
| Beta-blockers |
3 (100%) |
0 (0%) |
— |
| Hemoglobin (mg/dL) |
14.0±0.5 |
15.3±1.3 |
0.13 |
| Creatinine (mg/dL) |
0.85±0.23 |
0.89±0.12 |
0.85 |
| eGFR (mL/min/1.73 m2) |
76.7±23.8 |
69.2±10.2 |
0.85 |
| NT-pro BNP (pg/mL) |
2489±2015 |
52.7±44.1 |
0.02 |
ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; eGFR, estimated glomerular filtration rate; NT-pro BNP, N-terminal pro-brain natriuretic peptide.
Table2
Characteristics of the three selected patients with heart failure before and after CR
| Characteristics |
Before CR |
After CR |
p |
| Body weight (kg) |
88.7±44.8 |
74.6±30.1 |
0.24 |
| BMI (kg/m2) |
31.0±12.6 |
26.2±8.0 |
0.22 |
| Systolic BP (mmHg) |
142.7±4.0 |
102.7±2.5 |
<0.001 |
| Diastolic BP (mmHg) |
84.0±23.6 |
66.0±17.5 |
0.10 |
| Heart rate (bpm) |
78.3±28.0 |
60.7±6.4 |
0.30 |
| Hemoglobin (mg/dL) |
14.0±0.5 |
15.6±1.6 |
0.31 |
| Creatinine (mg/dL) |
0.85±0.23 |
0.89±0.26 |
0.32 |
| eGFR (mL/min/1.73 m2) |
76.7±23.8 |
73.7±24.2 |
0.20 |
| NT-pro BNP (pg/mL) |
2489±2015 |
1023±926 |
0.15 |
| Cardiac ultrasonography |
| LVDd (mm) |
60.6±7.4 |
63.4±7.5 |
0.38 |
| LVDs (mm) |
50.9±6.3 |
50.7±3.1 |
0.96 |
| LVEF (%) |
40.0±9.2 |
46.0±5.2 |
0.15 |
BMI, body mass index; BP, blood pressure; CR, cardiac rehabilitation; eGFR, estimated glomerular filtration rate; LVDd, left ventricular diastolic diameter; LVDs, left ventricular systolic diameter; LVEF, left ventricular ejection fraction; NT-pro BNP, N-terminal pro-brain natriuretic peptide.
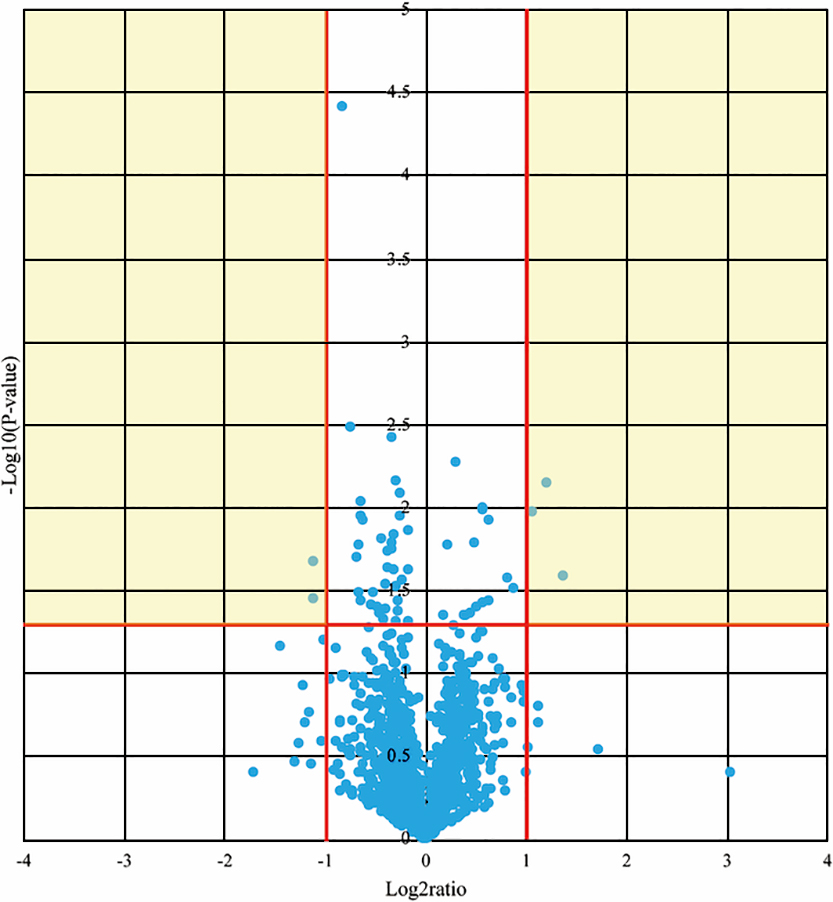
The p values and fold differences for all of the circulating miRNAs were compared between patients before CR and controls and are plotted in a volcano plot in Figure 1. Of these, 61 miRNAs met the criteria for a significant difference between patients before CR and controls. Expression levels of 41 miRNAs were significantly lower and those of 20 miRNAs were significantly higher in patients before CR compared with controls (Table 3).
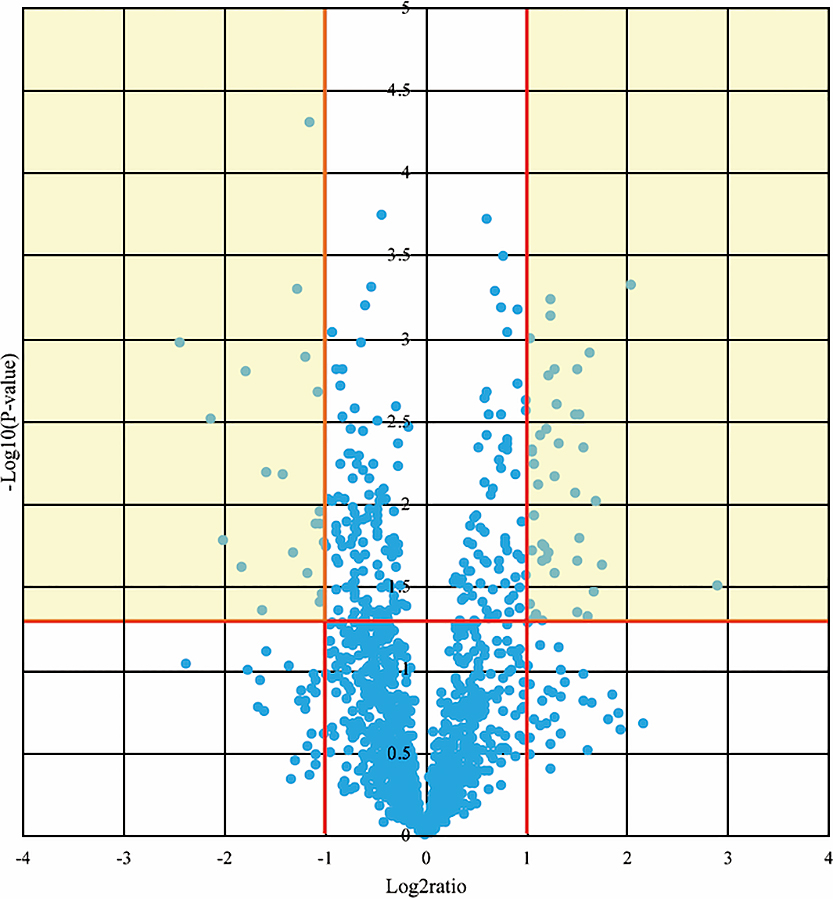
Table3
Circulating miRNAs that showed significantly different expression levels in patients with heart failure before cardiac rehabilitation compared with controls
| miRNAs |
Regulation |
miRNAs |
Regulation |
| hsa-miR-124-3p |
Downregulated |
hsa-miR-1240 |
Upregulated |
| hsa-miR-125a-3p |
Downregulated |
hsa-miR-1285-3p |
Upregulated |
| hsa-miR-125b-1-3p |
Downregulated |
hsa-miR-1290 |
Upregulated |
| hsa-miR-1470 |
Downregulated |
hsa-miR-135a-3p |
Upregulated |
| hsa-miR-151a-3p |
Downregulated |
hsa-miR-30b-3p |
Upregulated |
| hsa-miR-184 |
Downregulated |
hsa-miR-30c-1-3p |
Upregulated |
| hsa-miR-3155a |
Downregulated |
hsa-miR-3127-5p |
Upregulated |
| hsa-miR-3680-3p |
Downregulated |
hsa-miR-3945 |
Upregulated |
| hsa-miR-3714 |
Downregulated |
hsa-miR-4271 |
Upregulated |
| hsa-miR-3925-5p |
Downregulated |
hsa-miR-4673 |
Upregulated |
| hsa-miR-3936 |
Downregulated |
hsa-miR-4698 |
Upregulated |
| hsa-miR-4299 |
Downregulated |
hsa-miR-4733-3p |
Upregulated |
| hsa-miR-4300 |
Downregulated |
hsa-miR-5096 |
Upregulated |
| hsa-miR-4443 |
Downregulated |
hsa-miR-6081 |
Upregulated |
| hsa-miR-4444 |
Downregulated |
hsa-miR-665 |
Upregulated |
| hsa-miR-4448 |
Downregulated |
hsa-miR-6736-3p |
Upregulated |
| hsa-miR-4530 |
Downregulated |
hsa-miR-6812-3p |
Upregulated |
| hsa-miR-4538 |
Downregulated |
hsa-miR-7151-3p |
Upregulated |
| hsa-miR-4635 |
Downregulated |
hsa-miR-7641 |
Upregulated |
| hsa-miR-4681 |
Downregulated |
hsa-miR-92a-2-5p |
Upregulated |
| hsa-miR-4727-3p |
Downregulated |
|
|
| hsa-miR-4755-3p |
Downregulated |
|
|
| hsa-miR-4771 |
Downregulated |
|
|
| hsa-miR-4793-5p |
Downregulated |
|
|
| hsa-miR-520d-5p |
Downregulated |
|
|
| hsa-miR-520e |
Downregulated |
|
|
| hsa-miR-524-5p |
Downregulated |
|
|
| hsa-miR-525-5p |
Downregulated |
|
|
| hsa-miR-551b-5p |
Downregulated |
|
|
| hsa-miR-575 |
Downregulated |
|
|
| hsa-miR-593-5p |
Downregulated |
|
|
| hsa-miR-614 |
Downregulated |
|
|
| hsa-miR-6501-3p |
Downregulated |
|
|
| hsa-miR-6507-5p |
Downregulated |
|
|
| hsa-miR-6509-5p |
Downregulated |
|
|
| hsa-miR-6717-5p |
Downregulated |
|
|
| hsa-miR-6892-5p |
Downregulated |
|
|
| hsa-miR-7154-3p |
Downregulated |
|
|
| hsa-miR-920 |
Downregulated |
|
|
| hsa-miR-933 |
Downregulated |
|
|
The p values and fold differences for all of the circulating miRNAs were compared between before and after CR in patients with HF and are plotted in a volcano plot in Figure 2. Of these, five miRNAs met the criteria for a significant difference between before and after CR in patients with HF. Expression levels of three miRNAs were significantly lower and those of two miRNAs were significantly higher in patients before CR compared with patients after CR (Table 4).
Table4
Circulating miRNAs that showed significantly different expression levels in patients with heart failure before cardiac rehabilitation compared with patients with heart failure after cardiac rehabilitation
| miRNAs |
Regulation |
miRNAs |
Regulation |
| hsa-miR-125b-1-3p |
Downregulated |
hsa-miR-1290 |
Upregulated |
| hsa-miR-200c-3p |
Downregulated |
hsa-miR-196b-3p |
Upregulated |
| hsa-miR-3181 |
Downregulated |
|
|
When these results of the two volcano plots were combined, expression of two miRNAs was significantly different in patients before CR compared with controls and patients after CR as follows. Expression of hsa-miR-125b-1-3p was significantly downregulated and that of hsa-miR-1290 was significantly upregulated in patients before CR. Additionally, hsa-miR-24-3p and hsa-miR-3661 showed a trend of downregulation in patients before CR compared with controls and patients after CR (fold difference from 0.5–2.0, p<0.05), and hsa-miR-30c-1-3p, hsa-miR-196b-3p, hsa-miR-3945, and hsa-miR-7151-3p showed a trend of upregulation.
Discussion
This study used genome-wide microarray analyses to analyze expression levels of circulating miRNAs in three patients with HF before and after undergoing a 2-week program of CR in hospital. Our study showed for the first time that expression levels of two circulating miRNAs were significantly different before and after CR for HF. Before CR, miR-125b expression was significantly downregulated and miR-1290 expression was significantly upregulated compared with controls, and both of these miRNA levels returned to levels of controls after CR. These findings suggest that CR normalizes altered expression levels of some miRNAs in patients with HF.
Circulating miRNAs and exercise training
Circulating miRNAs in human serum and plasma were first identified in approximately 2008.17,18 The miRNAs are transported from the nucleus into the cytoplasm, where they become associated with various proteins and are secreted into the bloodstream. As well as circulating in association with proteins, miRNAs can be packaged into extracellular vesicles or high-density lipoproteins or released in apoptotic bodies. Circulating miRNAs are absorbed by remote tissues, where they regulate gene expression by translational repression, mRNA degradation, and deadenylation of mRNAs.19,20 Circulating miRNAs are secreted from various tissues, including the endothelium, in response to exercise. One potential mechanism underlying the change in miRNA profile with exercise is that increased shear stress along the endothelium may stimulate secretion of specific miRNAs from endothelial cells. The miRNAs miR-21, -126, -146a, and -210 are among the best described circulating miRNAs associated with the response to exercise in healthy men, and these are enriched in the endothelium.21 In patients with HF, circulating miR-146a levels do not change following acute exercise, whereas circulating miR-940 enriched in the myocardium is increased compared with healthy individuals.22 Therefore, the response of the circulating miRNA profile to exercise training in patients with HF requires careful evaluation to assess the differences to responses of healthy individuals. To the best of our knowledge, there have been no previous reports of changes in the profile of circulating miRNAs following CR that includes exercise training in patients with HF.
miRNAs associated with CR for HF
The miR-125 family has been implicated in various carcinomas and other diseases as either repressors or promoters.23–25 Members of this family play crucial roles in various cellular processes, including cell differentiation, proliferation, and apoptosis, by targeting many transcription factors,26 matrix metalloproteinase,27,28 and growth factors.29 miR-125 acts in different ways according to the cell context, such as playing important roles in the mitochondrial apoptosis pathway by targeting pro-apoptosis or anti-apoptosis genes.30 The pathological mechanisms of circulating miR-125 in the human heart have not been determined, although miR-125 was found to be upregulated in fibrotic heart tissue.31 In the present study, circulating miR-125b levels were significantly lower in patients with HF compared with controls and increased to normal levels after CR. Although the relationship between expression levels of miR-125 in cardiomyocytes and the blood has not been established, this result suggests that CR exerts protection against HF through changes in circulating and myocardial miR-125 expression levels.
miR-1290 plays a major role in initiation and progress of cancer. Studies have shown that increased miR-1290 levels are associated with proliferation, invasion, metastasis, and the clinical stage of cancer.32–34 A feature of the tumor microenvironment is regional severe hypoxia.35 Therefore, a potential role of miR-1290 is regulation of cell survival under hypoxic conditions. Recently, Wu et al.36 reported that the molecular mechanism underlying increased survival of cardiomyocytes with asiatic acid might be the negative relationship between expression levels of miR-1290 and the transcription factor hypoxia-inducible factor 3A in cardiomyocytes. A previous report showed that patients with HF who underwent CR improved their exercise tolerability.37 The relationship between expression levels of circulating and myocardial miR-1290 has not been clarified. However, our finding that circulating miR-1290 expression was significantly higher in patients before CR compared with controls with a decrease after CR suggests that miR-1290 contributes to increased exercise tolerability following CR through its molecular biological role of boosting resistance to cellular hypoxia.
In the present study, expression levels of circulating miR-24-3p, miR-196b, and miR-30c showed a tendency to be different in patients before CR compared with controls and patients after CR. Previous studies have reported interesting findings for the biological roles of these miRNAs. Qian et al.38 showed that miR-24 inhibited cardiomyocyte apoptosis in a mouse myocardial infarction model. Dahlmans et al.39 reported a correlation between miR-196b levels and in vivo mitochondrial function in human skeletal muscle. Duisters et al.40 reported that miR-30 directly downregulated connective tissue growth factor, a key molecule in the process of fibrosis, thereby regulating structural changes in the extracellular matrix of the myocardium. These findings are consistent with the positive effect of CR, such as reverse left ventricular remodeling,41 improvement of mitochondrial dysfunction in skeletal muscle,42 and a reduction in cardiac fibrosis.43
Clinical implications and limitations
The present results help to identify aspects of the biological mechanism of CR in patients with HF, such as improvement of mitochondrial dysfunction, an increased cellular resistance to hypoxic conditions, and a reduction in cardiomyocyte apoptosis and cardiac fibrosis. However, our study has several limitations. First, the number of subjects was small. Potential variation in circulating miRNAs among the three patients could have affected the results of the present study. Second, the possibility that treatment for HF affected the circulating miRNA expression profile cannot to be excluded, such as body weight loss and a decrease in blood pressure. Finally, the relationships between expression levels of circulating and intracellular miRNAs are unclear. However, to the best of our knowledge, this is the first study to evaluate the change in circulating miRNA profiles resulting from CR in patients with HF. The present results support the beneficial effect of CR for HF and could be the biological findings that enhance the evidence of CR. A large-scale study with polymerase chain reaction analysis is required to further investigate the roles of miRNAs in CR for HF at a genetic level.
Conclusion
In patients with HF, CR can restore expression of certain circulating miRNAs to nonpathological levels. Regulation of expression of multiple genes by these restored miRNAs may contribute to the beneficial effects of CR on HF.
Acknowledgments
Part of the data on this research was presented at the American Heart Association’s scientific sessions 2018, Chicago, 10–12 November.
Notes
Funding
This research was supported by JSPS KAKENHI (grant number: 19K08567 to Hideo Izawa).
Disclosures
Hideo Izawa has received grant support through his institution from Takeda, Shionogi, Otsuka, Pfizer, Teijin, and Daiichi-Sankyo, and honoraria for lectures from Otsuka and Daiichi-Sankyo.
References
- 1. JCS joint Working Group. Guidelines for rehabilitation in patients with cardiovascular disease (JCS 2012). Circ J 2014; 78: 2022–2093.
- 2. Dickstein K, Cohen-Solal A, Filippatos G, McMurray JJ, Ponikowski P, Poole-Wilson PA, Strömberg A, van Veldhuisen DJ, Atar D, Hoes AW, Keren A, Mebazaa A, Nieminen M, Priori SG, Swedberg K. ESC guidelines for diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the diagnosis and treatment of acute and chronic heart failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM). Eur J Heart Fail 2008; 10: 933–989.
- 3. Ambros V. The functions of animal microRNAs. Nature 2004; 431: 350–355.
- 4. Van Rooij E, Olson EN. MicroRNAs: Powerful new regulation of heart disease and provocative therapeutic targets. J Clin Invest 2007; 117: 2369–2376.
- 5. Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 2004; 116: 281–297.
- 6. Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 1993; 75: 843–854.
- 7. Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Caner 2006; 6: 857–866.
- 8. Voorhoeve PM, le Sage C, Schrier M, et al. A genetic screen implicates miRNA-372 and miRNA-373 as oncogenes in testicular germ cell tumors. Cell 2006; 124: 1169–1181.
- 9. Smirnova L, Grafe A, Seiler A, Schumacher S, Nitsch R, Wulczyn FG. Regulation of miRNA expression during neural cell specification. Eur J Neurosci 2005; 21: 1469–1477.
- 10. Jopling CL, Yi M, Lancaster AM, Lemon SM, Sarnow P. Modulation of hepatitis C virus RNA abundance by a liver-specific MicroRNA. Science 2005; 309: 1577–1581.
- 11. van Rooij E, Sutherland LB, Qi X, Richardson JA, Hill J, Olson EN. Control of stress-dependent cardiac growth and gene expression by a micro RNA. Science 2007; 316: 575–579.
- 12. Fujiwara W, Kato Y, Hayashi M, Sugishita Y, Okumura S, Yoshinaga M, Ishiguro T, Yamada R, Ueda S, Harada M, Naruse H, Ishii J, Ozaki Y, Izawa H. Serum microRNA-126 and -223 as new-generation biomarkers for sarcoidosis in patients with heart failure. J Cardiol 2018; 72: 452–457.
- 13. Mitchell PS, Parkin RK, Kroh EM, et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci U S A 2008; 105: 10513–10518.
- 14. Skog J, Würdinger T, van Rijn S, Meijer DH, Gainche L, Sena-Esteves M, Curry WT Jr, Carter BS, Krichevsky AM, Breakefield XO. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Bol 2008; 10: 1470–1476.
- 15. Dawson K, Wakili R, Ordög B, Clauss S, Chen Y, Iwasaki Y, Voigt N, Qi XY, Sinner MF, Dobrev D, Kääb S, Nattel S. MicroRNA29: A mechanistic contributor and potential biomarker in atrial fibrillation. Circulation 2013; 127: 1466–1475,1475e1-28.
- 16. Eitel I, Adams V, Dieterich P, Fuernau G, de Waha S, Desch S, Schuler G, Thiele H. Relation of circulating microRNA-133a concentrations with myocardial damage and clinical prognosis in ST-elevation myocardial infarction. Am Heart J 2012; 164: 706–714.
- 17. Chim SS, Shing TK, Hung EC, Leung TY, Lau TK, Chiu RW, Lo YM. Detection and characterization of placental microRNAs in maternal plasma. Clin Chem 2008; 54: 482–490.
- 18. Lawrie CH, Gal S, Dunlop HM, Pushkaran B, Liggins AP, Pulford K, Banham AH, Pezzella F, Boultwood J, Wainscoat JS, Hatton CS, Harris AL. Detection of elevated levels of tumor-associated microRNAs in serum of patients with diffuse large B-cell lymphoma. Br J Haematol 2008; 141: 672–675.
- 19. Boon RA, Vickers KC. Intracellular transport of microRNAs. Arterioscler Thromb Vasc Biol 2013; 33: 186–192.
- 20. Rayner KJ, Heneessy EJ. Extracellular communication via microRNA: lipid particles have a new message. J Lipid Res 2013; 54: 1174–1181.
- 21. Sapp RM, Shill DD, Roth SM, Hagberg JM. Circulating microRNAs in acute and chronic exercise: more than mere biomarkers. J Appl Physiol 2017; 122: 702–717.
- 22. Xu T, Zhou Q, Che L, Das S, Wang L, Jiang J, Li G, Xu J, Yao J, Wang H, Dai Y, Xiao J. Circulating miR-21, miR-378, and miR-940 increase in response to an acute exhaustive exercise in chronic heart failure patients. Oncotarget 2016; 7: 12414–12425.
- 23. Jiang L, Huang Q, Zhang S, Zhang Q, Chang J, Qiu X, Wang E. Hsa-miR-125a-3p and hsa-miR-125a-5p are downregulated in non-small cell lung cancer and have inverse effects on invasion and migration of lung cancer cells. BMC Cancer 2010; 10: 318.
- 24. Jiang F, Liu T, He Y, Yan Q, Chen X, Wang H, Wan X. MiR-125b promotes proliferation and migration of type Ⅱ endometrial carcinoma cells through targeting TP53INP1 tumor suppressor in vitro and in vivo. BMC Cancer 2011; 11: 425.
- 25. Ceribelli A, Yao B, Dominguez-Gutierrez PR, Nahid MA, Satoh M, Chan EK. MicroRNAs in systemic rheumatic disease. Arthritis Res Ther 2011; 13: 229.
- 26. Bousquet M, Nguyen D, Chen C, Shields L, Lodish HF. MicroRNA-125b transforms myeloid cell lines by repressing multiple mRNA. Haematologica 2012; 97: 1713–1721.
- 27. Bi Q, Tang S, Xia L, et al. Ectopic expression of MiR-125a inhibits the proliferation and metastasis of hepatocellular carcinoma by targeting MMP11 and VEGF. PLoS One 2012; 7: e40169.
- 28. Xu N, Zhang L, Meisgen F, Harada M, Heilborn J, Homey B, Grandér D, Ståhle M, Sonkoly E, Pivarcsi A. MicroRNA-125b down-regulates matrix metallopeptidase 13 and inhibits cutaneous squamous cell carcinoma cell proliferation, migration, and invasion. J Biol Chem 2012; 287: 29899–29908.
- 29. Ge Y, Sun Y, Chen J. IGF-Ⅱ is regulated by microRNA-125b in skeletal myogenesis. J Cell Biol 2011; 192: 69–81.
- 30. Sun YM, Lin KY, Chen YQ. Diverse function of miR-125 family in different cell context. J Hematol Oncol 2013; 6: 6.
- 31. Nagpal V, Rai R, Place AT, Murphy SB, Verma SK, Ghosh AK, Vaughan DE. MiR-125b is critical for fibroblast-to-myofibroblast transition and cardiac fibrosis. Circulation 2016; 133: 291–301.
- 32. Li M, He XY, Zhang ZM, Li S, Ren LH, Cao RS, Feng YD, Ji YL, Zhao Y, Shi RH. MicroRNA-1290 promotes esophageal squamous cell carcinoma cell proliferation and metastasis. World J Gastroenterol 2015; 21: 3245–3255.
- 33. Kim G, An HJ, Lee MJ, Song JY, Jeong JY, Lee JH, Jeong HC. Hsa-miR-1246 and hsa-miR-1290 are associated with stemness and invasiveness of non-small cell lung cancer. Lung Cancer 2016; 91: 15–22.
- 34. Li A, Yu J, Kim H, Wolfgang CL, Canto MI, Hruban RH, Goggins M. MicroRNA array analysis finds elevated serum miR-1290 accurately distinguishes patients with low-stage pancreatic cancer from healthy and disease controls. Clin Cancer Res 2013; 19: 3600–3610.
- 35. Reynolds TY, Rockwell S, Glazer PM. Genetic instability induced by the tumor microenvironment. Cancer Res 1996; 56: 5754–5757.
- 36. Wu K, Hu M, Chen Z, Xiang F, Chen G, Yan W, Peng Q, Chen X. Asiatic acid enhances survival of human AC16 cardiomyocytes under hypoxia by upregulating miR-1290. IUBMB Life 2017; 69: 660–667.
- 37. McKelvie RS, Teo KK, McCartney N, Humen D, Montague T, Yusuf S. Effects of exercise training in patients with congestive heart failure: a critical review. J Am Coll Cardiol 1995; 25: 789–796.
- 38. Qian L, Van Laake LW, Huang Y, Liu S, Wendland MF, Srivastava D. miR-24 inhibits apoptosis and represses Bim in mouse cardiomyocytes. J Exp Med 2011; 208: 549–560.
- 39. Dahlmans D, Houzelle A, Andreux P, Jörgensen JA, Wang X, de Windt LJ, Schrauwen P, Auwerx J, Hoeks J. An unbiased silencing screen in muscle cells identifies miR-320a, miR-150, miR-196b, and miR-34c as regulators of skeletal muscle mitochondrial metabolism. Mol Metab 2017; 6: 1429–1442.
- 40. Duisters RF, Tijsen AJ, Schroen B, Leenders JJ, Lentink V, van der Made I, Herias V, van Leeuwen RE, Schellings MW, Barenbrug P, Maessen JG, Heymans S, Pinto YM, Creemers EE. miR-133 and miR-30 regulate connective tissue growth factor: implications for a role of microRNAs in myocardial matrix remodeling. Circ Res 2009; 104: 170–178.
- 41. McGregor G, Gaze D, Oxborough D, O’Driscoll J, Shave R. Reverse left ventricular remodeling: effect of cardia rehabilitation exercise training in myocardial infarction patients with preserved ejection fraction. Eur J Phys Rehabil Med 2016; 52: 370–378.
- 42. Joseph AM, Adhihetty PJ, Leeuwenburgh C. Beneficial effects of exercise on age-related mitochondrial dysfunction and oxidative stress in skeletal muscle. J Physiol 2016; 594: 5105–5123.
- 43. Novoa U, Arauna D, Moran M, Nuñez M, Zagmutt S, Saldivia S, Valdes C, Villaseñor J, Zambrano CG, Gonzalez DR. High-Intensity Exercise reduces cardiac fibrosis and hypertrophy but does not restore the nitroso-redox imbalance in diabetic cardiomyopathy. Oxid Med Cell Longev 2017; 2017: 7921363.