Abstract
Background: Schwannomas are difficult to diagnose using imaging alone. Endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) is an effective and safe tissue sampling technique. Nevertheless, few reports have described EUS-FNA for schwannoma.
Objective: This study evaluates the efficacy of EUS-FNA for diagnosing schwannoma.
Methods: This retrospective study examined six consecutive schwannoma patients who were diagnosed as having schwannoma either from EUS-FNA results or from surgically resected specimens. The primary endpoint was diagnostic accuracy of EUS-FNA for schwannoma. The secondary endpoint was EUS-FNA safety.
Results: Based on cytomorphologic features and immunocytochemistry results after EUS-FNA, 4 out of 6 patients (66.7%) were diagnosed with schwannoma. The diagnoses before EUS-FNA were the following: 3 cases of gastric subepithelial lesion (SEL, suspicious for gastrointestinal stromal tumor), 1 case of intraperitoneal tumor, 1 case of retroperitoneal tumor, and 1 case of pancreatic tumor, with sizes of 15-44 mm (median 36 mm). No case was diagnosed as schwannoma solely based on image findings. Two cases of gastric SELs could not be diagnosed as schwannoma by EUS-FNA before surgery. Inadequate sampling and a lack of additional material for immunohistochemical studies could have engendered less-definite diagnoses in those cases. No procedural adverse events occurred.
Conclusion: The diagnostic accuracy rate of EUS-FNA for schwannoma is somewhat low. However, tissue samples were obtained safely using this method. Moreover, it is an important procedure for diagnosing schwannoma, which cannot be diagnosed solely from image findings.
Introduction
Schwannoma, a benign peripheral nerve sheath tumor originating from Schwann cells1), shows S-100 protein positivity when examined immunohistochemically. Schwannomas are difficult to diagnose solely based on imaging, such as computed tomography (CT) or endoscopic ultrasonography (EUS). For this reason, final diagnoses are usually made by surgical resection.
EUS-guided fine-needle aspiration (EUS-FNA) is an effective and safe tissue sampling technique for pancreatic tumors, gastrointestinal subepithelial lesions (SEL), and lymphadenopathy. Moreover, if the lesion is diagnosed as schwannoma using EUS-FNA, it is possible to avoid additional surgical resection because it is a benign tumor. Nevertheless, few reports in the literature describe the usefulness of EUS-FNA for schwannoma2-5).
This study was conducted to evaluate the efficacy of EUS-FNA for schwannoma.
Patients and Methods
Study design
From data collected January 2001-April 2013, we retrospectively reviewed 956 cases of patients who underwent EUS-FNA at Fukushima Medical University Hospital. Among them, six patients were diagnosed as having schwannoma. This study examined six cases that were ultimately diagnosed as schwannoma from EUS-FNA results or by observation of surgically resected specimens. These lesions were followed up until March 2015 (median: 65.5 months, range: 26-103 months).
For this study, the primary endpoint was the diagnostic accuracy rate of EUS-FNA for schwannoma. A secondary endpoint was the safety of EUS-FNA. This study conformed to the ethical guidelines of the 1975 Declaration of Helsinki. It was conducted with the approval of the Ethics Committee of Fukushima Medical University (approval No. 2399).
EUS-FNA
EUS-FNA was performed with linear or convex array echoendoscopes, GF-UCT240-AL5, GF-UC240P-AL5, GF-UCT 240, CF-UCT 260, and UCT-Y5 (Olympus Corp., Tokyo, Japan), using a 19-25 gauge manually operated needle device (Echotip ultra; Wilson-Cook Medical Inc., Tokyo, Japan, EZ-SHOT; Olympus Corp., Tokyo, Japan, Expect; Boston Scientific Corp., Tokyo, Japan) or a 22-gauge automated spring-loaded power shot needle device (NA-11 J-KB; Olympus Corp., Tokyo, Japan).
Written informed consent was obtained from all patients before EUS-FNA. All patients were sedated with pentazocine and midazolam before inserting echoendoscopes. After the target lesion was visualized on the monitor, a needle biopsy was performed while confirming a lack of blood flow on the puncture line. The needle was inserted through the gastrointestinal wall into the target lesion under EUS guidance with visualization of the needle in real time. Then, after being guided into the target lesion, the stylet was removed. The needle was moved back and forth 10-20 times within the mass while suction was applied using a 10-20 mL syringe. Finally, the suction syringe was released. The needle was withdrawn from the target lesion. Microscope slides were prepared from the biopsy sample and were stained using Cyto-Quick stain. Slides were assessed using rapid on-site cytological evaluation (ROSE)6). EUS-FNA was considered complete if the sample was evaluated as adequate by ROSE.
All patients were observed prospectively for adverse events based on the Common Terminology Criteria for Adverse Events v4.0 (CTC-AE).
Results
Patient characteristics and diagnostic imaging before EUS-FNA
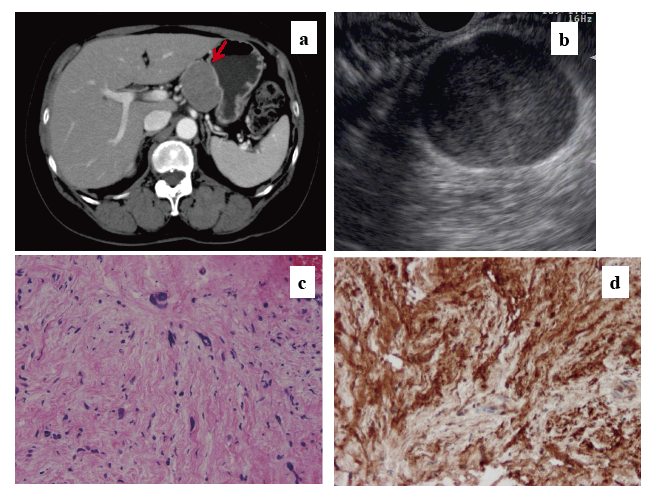
Table 1 presents summary information for these six patients. Images of representative cases are presented in Figs. 1 and 2. This series included three men and three women who were 36-71 years old (median 63 years). Tumors were 15-44 mm (median 36 mm). None of the patients had any symptoms. Tumors were detected using abdominal ultrasonography (US) as part of a medical checkup in two patients with a retroperitoneal tumor (case 1) and pancreatic tumor (case 2) and using an upper gastrointestinal tract radiologic examination in medical checkups for two patients with gastric SELs (cases 4 and 6). Case 3 included detection of an intraperitoneal tumor by CT when a gallbladder tumor in this patient was evaluated (Fig. 1-a). Tumors were detected for the first time in the other five cases. For case 5 (Fig. 2), EUS-FNA was used for additional examination of a gastric SEL after a 10-year follow-up because the lesion had become larger. No tumor was suspected as being schwannoma based on CT or EUS imaging. Three cases of gastric SELs were regarded as gastrointestinal stromal tumors (GIST).

The diagnostic accuracy rate of EUS-FNA for schwannoma was 66.7% (4/6). For six cases, sufficient specimens were obtained; immunohistochemical analyses were conducted in four cases (cases 1-3, 5). Schwannoma tumor cells were positive for immunohistochemical staining for S-100 (Figs. 1-d, 2-d) and negative for CD34, c-kit, SMA, and desmin. This immunohistochemical profile was consistent with the diagnosis of schwannoma in these four cases. In case 6, immunohistochemical staining for S-100 was not conducted because there was no suspicion of schwannoma. Case 4 did not undergo immunohistochemical staining for S-100 because no adequate specimen was obtainable. No anemia (Hb<10.0 g/dL), leukocytosis (>100,000/mm3), abdominal pain, perforation, or fever (>38°C) was observed in any patient after EUS-FNA, based on CTC-AE results.
Discussion
The results of this study suggest that EUS-FNA for schwannoma was useful for choosing a treatment plan, although the diagnostic accuracy rate was 66.7%. This accuracy rate is not very high, but EUS-FNA was nevertheless an important procedure for diagnosing schwannoma because it was done solely by image findings.
Schwannomas are tumors that originate from Schwann cells, showing a spindle cell tumor with S-100 protein positivity. Schwannoma patients are most often middle-aged, and the tumors are most often found as a painless mass. The most common sites of origin are the retroperitoneum, inclusive of the pelvic extra-peritoneal space, and the posterior mediastinum. The tumor median size is 6.2 cm.1,7) Most tumors are typically solitary and benign. Valentin et al.8) reported the analysis of 353 patients with malignant schwannoma. In their study, malignant schwannomas occurred in 37% of patients with NF1 and 59% of patients with sporadic tumors. However, gastric malignant schwannomas are extremely rare, and only a few cases have been reported.9-11) Based on diffusely visible mitosis9) or vascular invasion and lymph node metastasis10), the tumor was diagnosed as a malignant schwannoma. Malignant schwannoma has no specific histological marker. Malignant schwannomas are distinguished from benign schwannomas based on histological and clinical features.
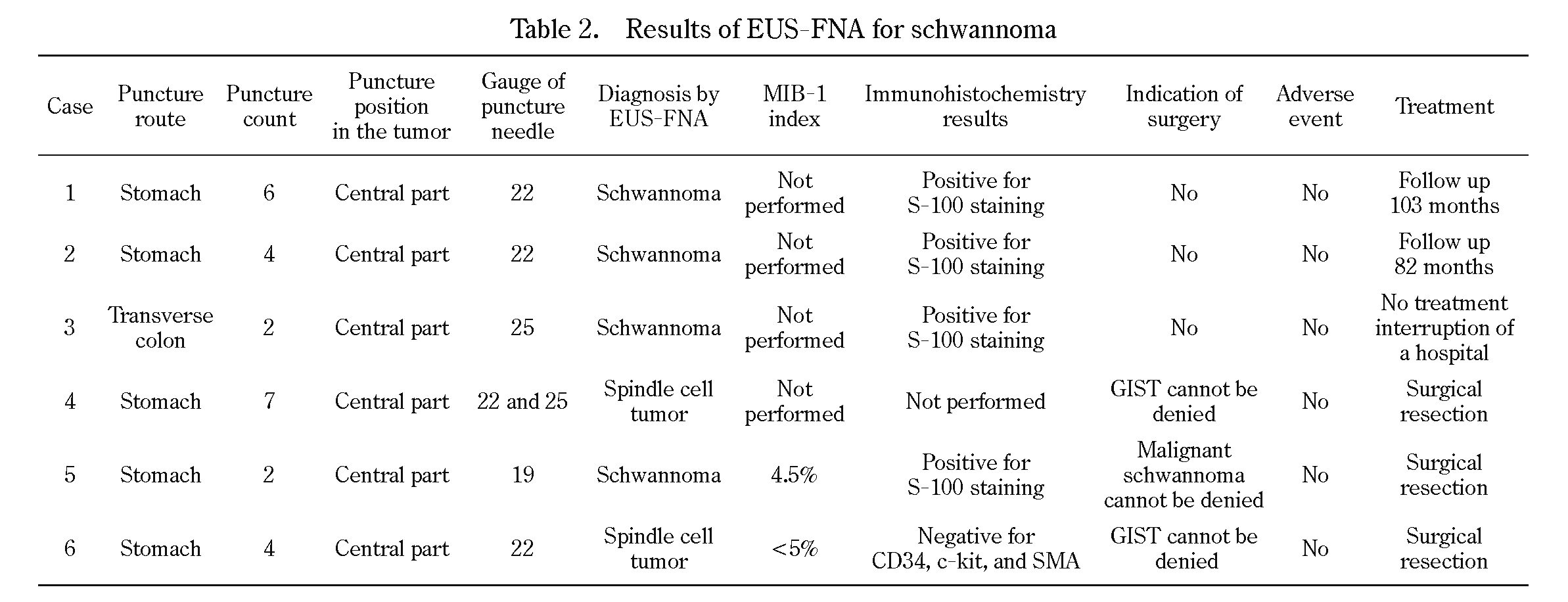
Schwannoma can be diagnosed using EUS-FNA if a sufficiently large specimen is obtained. However, EUS-FNA is not a perfect procedure for diagnosing schwannoma. Two cases of gastric SELs were diagnosed only as spindle cell tumors using EUS-FNA. There are two reasons they were not diagnosed as schwannoma using EUS-FNA. First, in case 4 and case 6, immunohistochemical studies of S-100 were not performed. Furthermore, the possibility of these lesions being GISTs could not be eliminated. In the gastrointestinal tract, GISTs are the largest group of mesenchymal tumors, while schwannomas are rare, representing approximately 1.4-6.3% of gastric mesenchymal tumors12-13). Therefore, we selected surgery for use with these cases. Second, it was somewhat difficult to obtain a specimen with EUS-FNA because of the histological features of schwannoma. Histologically, schwannoma consists of spindle cells in a hypercellular palisade arrangement area (Antoni type A) and myxomatous cells in a hypocellular organized area (Antoni type B)5). It is difficult to insert a needle into the hypocellular organized area and collect a specimen by EUS-FNA. In the gastric schwannoma case, the gastric wall escapes from the needle after the puncture. Therefore, it is difficult to move the needle in the tumor. It is apparently sometimes difficult to obtain tissue samples when using EUS-FNA for gastric schwannomas. Case 5 was diagnosed as schwannoma. However, the patient underwent surgery because the tumor size was increasing. Nevertheless, the FNA specimen of the case revealed no mitosis (Figs. 2c and 2d) and exhibited no metastasis or invasion. Therefore, this case might have avoided surgery. We used 19-25 gauge EUS-FNA needles, but the needle size did not seem to influence the accuracy (Table 2).
This study had some limitations. First, the sample size was small. Second, this study was retrospective and was conducted at a single institution. Finally, only 3 of 6 patients had undergone surgical resection, and the final diagnosis is usually determined by a resected specimen. We note, however, that schwannoma does not require resection. It can be followed up because schwannoma is fundamentally a benign tumor.
In conclusion, the results showed that the diagnostic accuracy rate of EUS-FNA for schwannoma is somewhat low. However, it is a safe and widely used procedure to obtain tissue and diagnose schwannoma, which cannot be diagnosed solely through imaging. Immunohistochemical studies are important to distinguish schwannomas from other gastrointestinal mesenchymal tumors. Adequate sampling is necessary to perform immunohistochemical staining to diagnose schwannoma using EUS-FNA. Furthermore, improvement in the accuracy of EUS-FNA is expected through the development of puncture needles and other components.
Acknowledgments
We express our gratitude to all the endoscopy medical staff members of the Endoscopy Department of Fukushima Medical University Hospital for their collaboration and assistance with the EUS-FNA procedures.
Conflict of interest disclosure
The authors have no conflicts of interest, financial or otherwise, in relation to this study.
References
- 1. White W, Shiu MH, Rosenblum MK, et al. Cellular schwannoma. A clinicopathologic study of 57 patients and 58 tumors. Cancer, 66: 1266-1275, 1990.
- 2. McGrath KM, Ballo MS, Jowell PS, et al. Schwannoma of the mediastinum diagnosed by EUS-guided fine needle aspiration. Gastrointest Endosc, 53: 362-365, 2001.
- 3. Young PE, Oosterveen S, Hanna N, et al. Presacral schwannoma diagnosed by EUS-guided FNA. Gastrointest Endosc, 67: 383-385, 2008.
- 4. Pakseresht K, Reddymasu SC, Oropeza-Vail MM, et al. Mediastinal schwannoma diagnosed by endoscopic ultrasonography guided-fine needle aspiration cytology. Case Rep Gastroenterol, 5: 411-415, 2011.
- 5. Kudo T, Kawakami H, Kuwatani M, et al. Three cases of retroperitoneal schwannoma diagnosed by EUS-FNA. World J Gastroenterol, 17: 3459-3464, 2011.
- 6. Hikichi T, Irisawa A, Bhutani MS, et al. Endoscopic ultrasound-guided fine-needle aspiration of solid pancreatic masses with rapid on-site cytological evaluation by endosonographers without attendance of cytopathologists. J Gastroenterol, 4: 322-328, 2009.
- 7. Fletcher CDM, Davies SE, McKee PH. Cellular schwannoma: A distinct pseudosarcomatous entity. Histopathology, 11: 21-35, 1987.
- 8. Valentin T, Le Cesne A, Ray-Coquard I, et al. Management and prognosis of malignant peripheral nerve sheath tumors: The experience of the French Sarcoma Group (GSF-GETO). European Journal of Cancer (Oxford, England: 1990), 56: 77-84, 2016.
- 9. Takemura M, Yoshida K, Takii M, et al. Gastric malignant schwannoma presenting with upper gastrointestinal bleeding: a case report. Journal of Medical Case Reports, 6: 37, 2012.
- 10. Loffeld RJ, Balk TG, Oomen JL, van der Putten AB. Upper gastrointestinal bleeding due to a malignant Schwannoma of the stomach. Eur J Gastroenterol Hepatol, 10: 159-162, 1998.
- 11. Bees NR, Ng CS, Dicks-Mireaux C, Kiely EM. Gastric malignant schwannoma in a child. Br J Radiol, 70: 952-955, 1997.
- 12. Atmatzidis S, Chatzimavroudis G, Dragoumis D, et al. Gastric schwannoma: a case report and literature review. Hippokratia, 16: 280-282, 2012.
- 13. Mekky MA, Yamao K, Sawai A, et al. Diagnostic utility of EUS-guided FNA in patients with gastric submucosal tumors. Gastrointest Endosc, 71: 913-919, 2010.