2018 Volume 64 Issue 2 Pages 73-81
2018 Volume 64 Issue 2 Pages 73-81
Background: Cervical spondylotic radiculopathy (CSR) is a relatively common neurological disease caused by the mechanical compression of nerve roots. Limaprost, a prostaglandin E1 derivative, functions as a vasodilator and has been used in the treatment of lumbar spinal stenosis in Japan. However, the effects of limaprost in cervical radiculopathy remain unclear. Our aim was to compare the efficacy of limaprost with that of pregabalin, which is widely used for the treatment of neuropathic pain.
Methods: In this randomized trial, patients with CSR received either limaprost or pregabalin orally for 8 weeks, along with nonsteroidal anti-inflammatory drugs. The primary outcomes were assessed using a numerical rating scale of pain and numbness, both at rest and during movement. Secondary outcomes were assessed using Short Form-36, provocation tests, painDETECT questionnaire, and subjective global assessment. The obtained data were evaluated according to the per-protocol analysis principle.
Results: A total of 46 patients were enrolled in this study, and 35 were available for analysis. A greater reduction in pain score was observed in neck pain during movement, and scapular and arm pain both at rest and during movement in the pregabalin-treated group up to 4 weeks. In the limaprost-treated group, numbness of the arm during movement showed a marked alleviation compared to the pregabalin-treated group at 8 weeks. There were no apparent differences between the two groups in terms of the secondary outcomes.
Conclusions: Although pregabalin provided an earlier pain relief than limaprost, limaprost was superior to pregabalin in treating arm numbness. Limaprost might be one of the effective therapeutic options for CSR in primary care settings.
Cervical spondylotic radiculopathy (CSR) is a widespread disease caused by nerve root compression due to foraminal space narrowing secondary to spondylarthrosis and degenerative disc disease. The general clinical characteristics include neck and scapular pain irradiating to the arm and fingers corresponding to the affected nerve root, although absence of radiating arm pain does not preclude nerve root impairment. Evaluation of patients’ history, physical examination, and imaging modalities, including plain radiographs, magnetic resonance imaging (MRI), and/or computed tomography (CT), are necessary to assist this diagnosis; however, no universally accepted criteria are currently available1-3). Cervical radiculopathy is typically self-limited and its prognosis is favorable. Approximately 70-90% of patients reported symptomatic alleviation with conservative therapies4,5). In practice, analgesics are the standard primary care in CRS, unless some signs of myelopathy or significant motor weakness are detected.
Nerve root compression underlying cervical radiculopathy is supposed to result in both nociceptive and neuropathic pain components6,7). Nonsteroidal anti-inflammatory drugs (NSAIDs) could alleviate nociceptive pain as first-line agents in acute settings. Some patients may benefit from opioids, such as tramadol, or oral steroids3,8). For the management of neuropathic pain, pregabalin, a calcium channel alpha2-delta ligand, is well-accepted for most neuropathic pain conditions and its efficacy in painful cervical radiculopathy was reported as well9-12). Considering the pathogenesis underlying neuropathic pain, nerve tissue ischemia seems to play a crucial role in the development of neuropathic pain13-15). Prostaglandin E1 (PGE1) derivative, a vasodilator and an inhibitor of platelet aggregation, improves neuropathic pain and neural dysfunction by acting on the impaired nerve blood flow as shown in animal studies using neuropathic pain models16-18). Clinical studies reported that oral administration of PGE1, limaprost, improved neurological symptoms, walking ability, and quality of life (QOL) in patients with lumbar spinal stenosis (LSS)19-21). In addition, a recent randomized controlled trial showed that limaprost was not inferior to pregabalin in LSS22). These findings lead us to propose a therapeutic potential of limaprost for CSR, taking into account the similar neural pathogenesis caused by nerve root compression. However, to our knowledge, the effects of limaprost in the treatment of CSR-related symptoms have not been previously investigated, indicating a lack of evidence regarding PGE1 effects in cervical radiculopathy. Therefore, we aimed to assess how limaprost affects CSR-related symptoms compared to pregabalin that is generally used against neuropathic pain state.
Study participants were prospectively recruited from patients diagnosed with CSR at our hospital between January 2014 and March 2016. Patients should have symptoms of radiating arm pain or numbness in the distribution of an ipsilateral specific nerve root. Plain radiographs and MRI were used to assist in CSR diagnosis and rule out other diseases, such as disc herniation, infection, and tumor. Exclusion criteria included a history of treatments within the past year, previous cervical spine surgery, surgery needed due to progressive and severe neurological deficits, antiplatelet or anticoagulant agents use, severe cardiovascular, hepatic, or renal disorders, pregnancy, cerebral infarction, history of gastric ulcers or intestinal bleeding, and psychogenic disorders. Informed consent which included that limaprost was currently an unapproved agent for cervical radiculopathy in Japan was obtained from all eligible participants of this study. Ethical approval was obtained from the institutional review board of our hospital (ID: 25092701).
InterventionsThe participants were randomly assigned to limaprost plus NSAIDs (L + N) and pregabalin plus NSAIDs (P + N) treatments groups to compare the efficacy and tolerability of treatments for CSR-induced radicular pain and associated symptoms. Block randomization was performed by an independent member of our institution, using random number charts and the results were presented to each subject by sealed envelopes. The treatment allocation was concealed until the patient was entered into the trial where, after allocation, the patient or physician was not blinded to the medication administered. The L + N group received 5 µg limaprost three times daily and a physician-determined NSAID with the recommended standard dose. The P + N group received 25 mg pregabalin during the first week and then 75 mg from second dose twice daily as well as the aforementioned NSAID. Each subject also received a concomitant 100 mg rebamipide with NSAID for gastric mucosal protection. Each treatment lasted 8 weeks. Additional therapies, including physical therapy, immobilization by cervical collar, and any type of injections for pain relief were prohibited throughout the study period.
Clinical outcome assessmentsFor the primary outcomes, numerical rating scale (NRS) in pain and numbness were assessed to compare the therapeutic effectiveness after treatments between the two groups. The secondary outcomes were health-related QOL, pain provocation tests, presence of a neuropathic pain component, and subjective satisfaction. All clinical outcomes were conducted by a self-reported ques–tionnaire forms that were distributed and collected by the nurse at outpatient clinic.
NRS:All items of NRS in pain (neck, scapula, arm) or numbness intensity were required for scores in worst during a few days and valued on an 11-point intensity, where 0 = no symptom and 10 worst possible symptoms, periodically in 2, 4, and 8 weeks after treatments. These CSR-associated symptoms were assessed both at rest and during movement.
SF-36:The health-related QOL were assessed using the Short Form-36 (SF-36), Japanese version. This questionnaire was administered at the first and at the final visit to assess physical, social, and mental well-being. Scores were transformed to a scale of 0 to 100, with a higher score indicating better QOL.
Provocation tests:Spurling and Jackson tests to detect nerve root involvement were performed by a physician in charge. A positive test determined radiating pain in the affected arm. The tests were performed at 2, 4, and 8 weeks after treatments.
PainDETECT:A score ≤ 12 indicated that pain was unlikely to have a neuropathic component (negative), while a score ≥ 19 suggested that pain was likely to have a neuropathic component (positive). A score between these values indicated an uncertain result (unclear)23). The scores after treatments were compared between two groups at 2, 4, and 8 weeks after treatments.
Subjective satisfaction:The overall subjective global satisfaction with treatment was determined and compared between treated groups. The subjective satisfaction was measured on NRS (0-10 points) as well; a higher score meant higher satisfaction.
Statistical AnalysisThe overall differences between different treatments in NRS scores (mean ± standard deviation), PainDETECT (mean ± standard deviation), and subjective satisfaction (mean ± standard deviation) were assessed using analysis of variance for repeated measures and then the Mann-Whitney test was used for comparisons between two groups at each time point. Statistical differences in provocation tests and SF-36 subscales (mean ± standard deviation) between different treatments at specific time points were assessed using the χ2 test and un-paired t-test, respectively. Demographic data of the evaluated subjects were also assessed using the χ2 test and un-paired t-test. Data were analyzed with JMP® software (SAS Institute Inc., Cary, NC, USA). It was calculated that a sample size in each group would have 80% power to detect a mean difference of two points on the NRS, assuming a common standard deviation of 2.0. As the result of calculation, a number of subjects above 17 in each group was warranted in the current study. The per-protocol analysis population included all subjects who fulfilled the study protocol. P values < 0.05 were considered statistically significant.
A total of 35 patients of 46 enrolled participants were ultimately available for the protocol analysis (Fig. 1). Involved nerve roots were C6 (n = 17), C7 (n = 14), C5 (n = 3), and C8 (n = 1), respectively.
No significant differences were found between the two groups in demographic characteristics (Table 1). In the P + N group, 2 patients were withdrawn from this study protocol because of dizziness and somnolence. No serious adverse events occurred in either treatment group. NSAID use in this study was follows: 60 mg loxoprofen 3 times daily (n = 18), 100 mg celecoxib 2 times daily (n = 15), and 4 mg lornoxicam 3 times daily (n = 2).
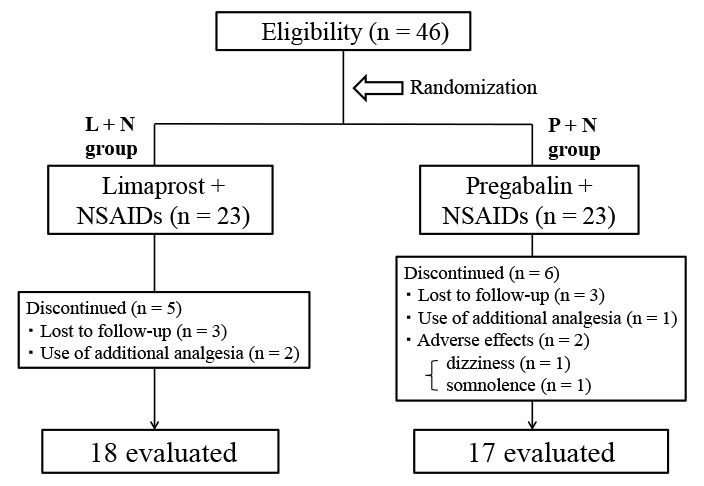
Study flow diagram
Of 46 eligible participants, six were lost to follow-up, three needed additional analgesia, and two discontinued because of adverse effects. The remaining 35 participants completed this study.
NSAIDs, nonsteroidal anti-inflammatory drugs; L + N, limaprost plus NSAIDs; P + N, pregabalin plus NSAIDs

Demographic characteristics of the included patients
Variable data: mean (SD); categorical data: number of cases
BMI, body mass index
P values showing comparison between the groups
Neck, scapular, and arm pain
At rest, NRS of the scapular pain showed a significant reduction in the P + N group at 2 and 4 weeks after treatment compared to the L + N group. During movement, the pronounced reductions in score were also detected in the P + N group at 2 weeks in the neck, and at 2 and 4 weeks in the scapula and in the arm, respectively. An insignificant difference was confirmed between the two treatment groups at week 8. Changes in score from baseline values showed the marked reductions at 2 weeks in the arm pain at rest in the P + N group (Table 2).
Arm numbness
No significant differences in numbness at rest were detected between the two groups throughout the observation period. A remarkable alleviation from pre-treatment value in numbness during movement was detected at 8 weeks in the L + N group compared to the P + N group. The differences in score from baseline at 8 weeks were -5.2 ± 2.6 in the L + N group and -2.9 ± 2.5 in the P + N group, respectively (P < 0.05) (Table 2).

NRS at rest and during movement
Values are presented as mean ± SD
P values showing comparison between the groups
*P < 0.05, significant difference between the groups followed by a repeated measures ANOVA
An extent of increment in score was noticeable in body pain as a physical component summary over time. Conversely, the vitality, social function, and mental health did not show a trend toward amelioration. Comparisons of any subscales between two groups failed to reach statistical significance (Table 3).

SF-36 subscale scores
Values are presented as mean ± SD
P values showing comparison between the groups
The initial positive provocation tests were confirmed at the rate of 29% (10/35 patients) for the Jackson test and 69% (24/35 patients) for the Spurling test, respectively. At final follow-up, the rates decreased to 11% (4/35 patients) for both tests. No apparent differences in the positive rate were detected over time course between the two groups (Table 4).

Provocation tests
Values are presented as the number of positive results in each group
P values showing comparison between the groups at each time point
The average painDETECT score was 12.5 ± 4.6 at the first visit. The number of positive, unclear, and negative scores were 4 (11.4%), 13 (37.1%), and 18 (51.4%), respectively. No significant differences were seen between two groups at every time points (Table 5).

Scores of painDETECT
Values are presented as mean ± SD (95% confidence interval)
P values showing comparison between the groups at each time point
The scores of satisfaction gradually increased in both groups with time course. At final follow-up, the scores were 7.5 ± 2.6 in the L + N group and 6.4 ± 2.4 in the P + N group, respectively, suggesting a modest trend favoring the limaprost-treated group (P = 0.06) (Table 6).

Scores of subjective satisfaction
Values are presented as mean ± SD (95% confidence interval)
P values showing comparison between the groups at each time point
The outcome of NRS in the present trial showed an earlier reduction in neck pain during movement, and scapular and arm pain both at rest and during movement at 2 weeks in the pregabalin-treated group compared to the limaprost-treated group. However, the beneficial effect did not outlast over 4 weeks. With regards to arm numbness, a greater improvement was confirmed in the limaprost-treated group at 8 weeks. Provocation test, painDETECT, SF-36, and subjective satisfaction did not reach significant changes between the two treatment groups.
CSR is a disorder involving compressed cervical nerve roots ischemia that is generally treated by conservative therapies, in which pharmacotherapy, as a part of a multimodal approach, is the initial step for therapeutic strategies. Limaprost, an oral PGE1 analog, improves cauda equina and sciatic nerve blood flow in chronic LSS animal models24,25) and thereby has been used for the treatment of leg pain, leg numbness, and intermittent claudication in patients with LSS in Japan19-21). The difference in the response to PGE1 treatment might exist between cervical and lumbar nerve roots because of morphological and physiological features, however the nerve roots compression due to degenerative spondylotic changes is thought to be a common scenario in cervical and lumbar radiculopathy. In our trial settings, limaprost administration to CSR was compared to pregabalin, a worldwide recognized agent for neuropathic pain. Pregabalin was started at a low dose of 50 mg/day to reduce the withdrawal due to adverse events and increased to 150 mg/day after 1 week. Additionally, NSAIDs were simultaneously administrated for nociceptive pain component in both treatment groups.
The NRS reduction in CSR-related pain and numbness were commonly confirmed in each treatment group over time. The positive rate of provocation tests was also reduced in response to NRS changes. With regards to pain intensity, the pregabalin treatment resulted in more pronounced pain reduction beginning with 2 weeks after treatment with a clinical meaningful difference, suggesting a change over 2 points in NRS score26). However the superiority of pregabalin did not last throughout all observational period. A recent double-blind, randomized study showed that the efficacy of limaprost for LSS-induced leg pain was similar to that of pregabalin at time points of 4 and 8 weeks with an improvement from baseline over time22). That temporal superiority for pregabalin in the current study implies the possibility of a gradual analgesic effect of limaprost or just mirroring natural history of CSR with the treatment of NSAIDs; however, the exact explanation is beyond the obtained results. The observed greater alleviation of numbness during movement in the limaprost-treated group at 8 weeks was considered to be related to improved peripheral blood circulation in the affected nerve root. The reason for this might be that the blood flow impairment caused by dynamic factor is likely to be a reversible pathophysiological change, and therefore is more reactive to the limaprost treatment than that of a statically compressed condition. An experimental entrapment neuropathy model revealed that PGE1 led to not only an inhibition of nerve growth factor (NGF) associated with initiation and maintenance of neuronal excitability, but also with an enhancement of vascular endothelial growth factor (VEGF) attributed to an improvement of blood flow at the compressed nerve root lesion17). Furthermore, it was reported that in clinical practice, limaprost significantly improved leg numbness, but not low back pain or leg pain, in patients with LSS after an 8-week intervention in comparison with NSAIDs20). These facts are partly in accordance with the observed findings in the current study. An insignificant difference in SF-36 score in an 8-week observational period indicates that the improved numbness could not influence QOL, though suggested a trend toward better overall satisfaction in the limaprost treatment. In the current study, 48.5% of patients with CSR were confirmed to have a positive or ambiguous neuropathic pain component according to the painDETECT, whereas the rate was lower than that of approximately 90% reported by a previous study in degenerative cervical radiculopathy27). The difference in rate was likely to be due to the duration from the disease onset, because an average duration in an aforementioned study was 7.6 months, which was longer than approximately 6 weeks shown in our trial. Taken these acquired findings together, pregabalin for CSR-related symptoms induced an earlier response than limaprost in pain sensation and the later response of limaprost was confirmed especially in numbness sensation. Subsequently, the comparisons of health-related QOL could not reflect such somatosensory changes.
There are some limitations of this trial. First, our trial was performed in acute clinical setting according to the disease duration; it is therefore considered that NSAIDs might be even more effective for such condition that is different from chronic pain state. Second, the design flaws included the absence of the control (placebo plus NSAIDs group), which implies that a favorable natural course of CSR with NSAIDs treatment cannot be ruled out to explain the observed effects. Third, the required sample size based on our preliminary research would be small if a common standard deviation was assumed at 3.0 in NRS score. Fourth, the variability in the selection of concomitant NSAIDs would influence on the resultant effects, though there is an evidence that various types of NSAIDs including COX-2 inhibitors are equally effective for low back pain28). Fifth, the dosage of pregabalin was limited to 150 mg/day and thus there is a possibility that a greater dose of pregabalin could result in the further analgesic effects. On the other hand, it should be noted that pregabalin produces dose-dependent side effects as observed in this study9). Even under our condition, pregabalin-treated group provided a rather rapid pain relief as clinically beneficial aspect. In terms of arm numbness, the effect of limaprost was interestingly more notable than CSR-related pain, indicating that an improvement of numbness sensation might be susceptible to the changes of nerve blood flow. Comprehensively, the present randomized study was the first trial that assessed the efficacy of limaprost in cervical radiculopathy as compared to pregabalin. It is plausible that an amelioration of impaired blood flow in the nerve root is a pivotal target for neuropathic pain state caused by the compressed nerve root.
In this context, under conditions of routine clinical practice in primary care setting, limaprost seems to be a viable option for the management of CSR, in particular, for numbness and, thereby, minimize residuals. Future research is needed to elucidate whether limaprost exerts an additional advantage to the prolonged cervical radiculopathy or a form of intractable pain as next steps.
The authors thank Dr. Atsuko Ogoshi for data collection.
The authors declare no conflict of interest in this work.