Article ID: 24-00010
Article ID: 24-00010
Human herpesvirus 7 (HHV-7) is ubiquitous and infects most children. Severe HHV-7 infection was considered to be rare. In this case series, we report the clinical findings and clinical courses of three immunocompetent children who had severe HHV-7 infection: two fatal cases of encephalopathy and one patient with severe sequelae after myocarditis. In all three patients, HHV-7 DNA was detected in acute phase specimens, including serum by real-time PCR. In the myocarditis case, HHV-7 DNA was also detected in myocardial tissue, suggesting that HHV-7 was the cause. Patient 1 was a 6-year-old Japanese girl with encephalopathy who died one day after onset. Patient 2 was a 4-year-old Japanese girl with encephalopathy whose absence of brainstem reflexes was confirmed and died 29 days after onset. Patient 3 was a 22-month-old Japanese girl with myocarditis who managed with extracorporeal membrane oxygenation and survived but was left with severe neurological sequelae. Because HHV-7 can cause serious outcomes in children, a virological search for HHV-7 in severe infections needs to be aggressive, and cases should be accumulated.
Human herpesvirus 7 (HHV-7) belongs to the genus Roseolovirus of the Betaherpesvirinae subfamily, Orthoherpesviridae family1). After initially infecting via saliva or other secretions, HHV-7 remains latent in the individual and potentially reactivates in immunocompromised patients2-4). HHV-7 infection primarily occurs in early childhood, and approximately 100% of adults are estimated to be infected5-7). The typical clinical manifestations of HHV-7 infection include exanthema subitum (ES), fever without rash, febrile convulsions, and upper respiratory inflammatory symptoms, with good prognosis8-10).
The progression of initial HHV-7 infection is good, and only a few severe and fatal cases have been reported. However, we encountered two fatal cases of encephalopathy and one patient with severe sequelae after myocarditis, all of them had HHV-7 infection. In this case series, we report the clinical findings and disease course of these three patients.
All three patients had no history or family history of immunodeficiency.
We defined the time of illness onset as the time when the patient first developed a fever (>37.5℃), and we defined this day as day 0.
Nasopharyngeal swabs (NPS), rectal swabs (RecS), and whole blood, serum, and urine samples were collected from all patients. Cerebrospinal fluid (CSF) samples were collected from patients 1 and 2. Myocardial tissue samples were also collected from patient 3. These clinical samples were used for quantitative real-time polymerase chain reaction (q-PCR) assays to detect herpes simplex virus 1, herpes simplex virus 2, cytomegalovirus, varicella zoster virus, Epstein–Barr virus (EBV), human herpes virus 6 (HHV-6), HHV-7, adenovirus, human parvovirus B19, and Mycoplasma pneumoniae. Simultaneously, we performed quantitative real-time reverse transcription-polymerase chain reaction assay (qRT-PCR) to detect RNA viruses (enterovirus, respiratory syncytial virus–A/B, human rhinovirus (HRV), human metapneumovirus, parainfluenza virus type 3, influenza virus- A/B, mumps virus, and human parechovirus) using the same specimens. However, only HRV was detected at 5.74 × 10⁵ copies/mL from the NPS of case 2. These assays were performed using a QuantStudio 6 Flex system in the laboratory of the Department of Pediatrics at Fukushima Medical University. The primers and probes used in this study have been previously described (S1 Table)11-26). The cutoff was set at 40 threshold cycles. In all three patients, the serum HHV-7 immunoglobulin (Ig) G titer during hospitalization was measured using an indirect fluorescent antibody assay27).
We obtained informed consent from the patients’ parents or guardians.
Patient 1 was a 6-year-old Japanese girl with a history of febrile convulsions.
On day 0, she developed a fever measuring 38 ℃ and vomiting. Two hours later, she was rushed to a nearby hospital because she developed a generalized tonic seizure. Her seizure stopped after treatment with diazepam; however, >2h later, she went into cardiac arrest. After administering cardiopulmonary resuscitation for 30 min, spontaneous circulation was restored. The patient was then transported to our hospital with continuous intravenous adrenaline administration.
Physical examination revealed a heart rate of 147 beats per minute (BPM), blood pressure of 69/30 mmHg, and no spontaneous respiration. Both pupils were mydriatic without a light reflex.
Biochemical tests revealed elevated serum aspartate aminotransferase, alanine aminotransferase, and creatine kinase (Table 1). Brain computed tomography (CT) showed total brain edema. Electroencephalography (EEG) showed mostly flat activity waves. Bacterial culture tests of CSF and blood samples yielded negative results (Fig. 1). Serum HHV-6 IgG and HHV-7 IgG titer were 128- and 32-fold higher, respectively. HHV-7 DNA was detected in the nasopharyngeal swabs, whole blood, and serum samples (Table 2).
Based on these findings, we diagnosed the patient with acute encephalopathy and treated her with hydrocortisone, mannitol, recombinant thrombomodulin-α, adrenalin, and blood transfusion. Despite intensive and aggressive treatment, the coagulopathy progressed, and the patient died on day 1.
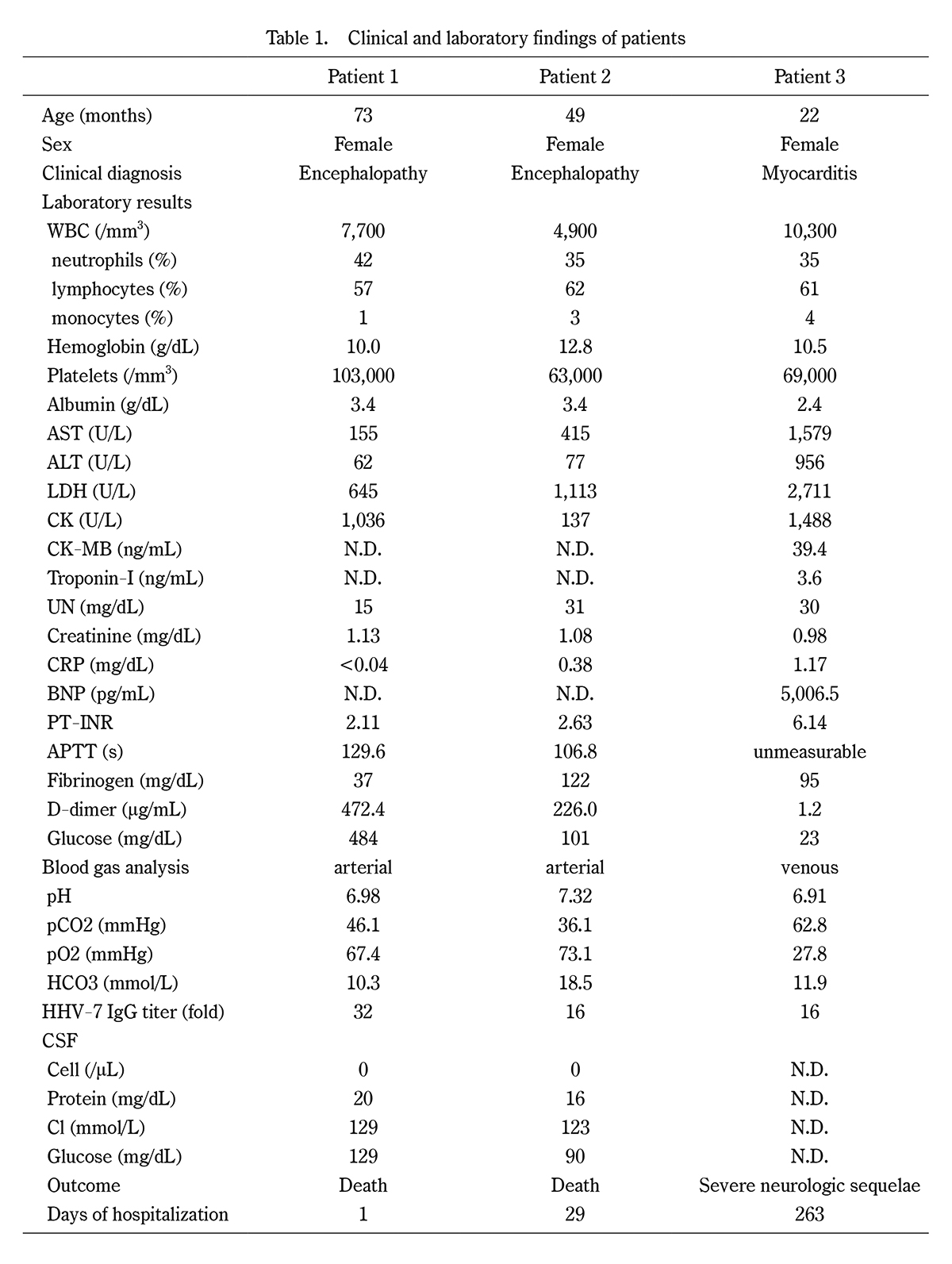
Clinical and laboratory findings of patients
WBC, white blood cell; AST, aspartate aminotransferase; ALT, alanine aminotransferase; LDH, lactate dehydrogenase; CK, creatine kinase; UN, urea nitrogen; CRP, C-reactive protein; BNP, brain natriuretic peptide; PT-INR, prothrombin time-international normalized ratio; APTT, activated partial thromboplastin time; CSF, cerebrospinal fluid; IgG, immunoglobulin G.

Case 1. (a) Cranial computed tomography shows total brain edema. (b) Electroencephalography shows mostly flat activity waves.

Results of real-time polymerase chain reaction for DNA pathogens
The level of detected pathogens is represented as copies/mL of the exponential notation. The cutoff was set at 40 threshold cycles. NPS, nasopharyngeal swab; RecS, rectal swab; CSF, cerebrospinal fluid; HSV-1, herpes simplex virus 1; HSV-2, herpes simplex virus 2; CMV, cytomegalovirus; VZV, varicella zoster virus; EBV, Epstein-Barr virus; HHV-6, human herpesvirus 6; HHV-7, human herpesvirus 7; AdV, adenovirus; PVB19, human parvovirus B19; M. pneumoniae, Mycoplasma pneumoniae; EnV, Enterovirus; RA, right atrial; LA, left atrial, N.D., not done. Clinical samples were used for quantitative real-time polymerase chain reaction assays to detect DNA pathogens (HSV-1/2, CMV, VZV, EBV, HHV-6/7, AdV, PVB19, and M. pneumoniae. While, in the quantitative real-time reverse transcription-polymerase chain reaction assay to detect RNA viruses (enterovirus, respiratory syncytial virus-A/B, human rhinovirus, human metapneumovirus, parainfluenza virus type 3, influenza virus-A/B, mumps virus, and human parechovirus) conducted using the same specimen, only HRV was detected at a level of 5.74 × 10⁵ from the NPS of Case 2.
Patient 2 was a 4-year-old Japanese girl with a history of ES and febrile convulsions at the age of 1 year.
On day 0, she presented to a nearby hospital with repeated generalized clonic seizures and a fever of 38 ℃. Thereafter, she was transferred to our hospital.
Physical examination revealed a heart rate of 160 BPM, blood pressure of 67/27 mmHg, disturbances in consciousness, and peripheral circulation disorders. Her pupils were 3-4 mm in diameter and showed slow light reflexes.
Biochemical tests revealed elevated aspartate aminotransferase and alanine aminotransferase levels (Table 1). Brain CT showed cerebral edema. EEG showed generalized high-amplitude slow waves (Fig. 2). Bacterial culture tests of CSF and blood samples yielded negative results. Serum HHV-7 IgG titer was 16-fold higher. HHV-7 DNA was detected in NPS and RecS samples, whole blood, and serum samples (Table 2). SARS-CoV-2 RNA was not detected in the NPS.
Based on these clinical findings, we diagnosed the patient with acute encephalopathy and treated her with methylprednisolone-pulse therapy, γ-globulin, mannitol, recombinant thrombomodulin-α, noradrenalin, and mitochondrial cocktails (ascorbic acid, benfotiamine, pyridoxine, and cyanocobalamin) therapy.
However, her cerebral edema progressed, and the EEG showed flat and no activity waves on day 5. On day 8, a rash appeared on her trunk, suggestive of ES. The absence of brainstem reflexes was confirmed on day 12, and the patient died on day 29.

Case 2. (a) Cranial computed tomography shows cerebral edema. (b) Electroencephalography shows generalized high-amplitude slow waves.
Patient 3 was a 22-month-old Japanese girl with no history of ES.
On day 0, she developed a high fever of 40 ℃, vomiting, diarrhea, and transient loss of consciousness. On day 1, she was transferred from a nearby hospital to our hospital because she was suspected of having acute myocarditis.
Physical examination revealed a heart rate of 94 BPM, weak heart sounds, disturbances in consciousness, hepatomegaly, and peripheral circulation disorders. The blood pressure could not be measured.
Biochemical tests revealed elevated levels of brain natriuretic peptides and myocardium-derived enzymes (Table 1). Chest radiography revealed cardiomegaly (cardiothoracic ratio 65%) and pulmonary congestion (Fig. 3). Echocardiography revealed severe hypokinesis of the left ventricle, and the ejection fraction could not be measured. She was clinically diagnosed with acute myocarditis and was placed on extracorporeal membrane oxygenation (ECMO).
A small portion of the myocardial tissue from the right and left atrial tissues was removed when the ECMO was placed. The tissue was cut to a size of <125 mm3, thoroughly washed with saline, immersed in saline overnight to remove blood, and used for PCR testing. The HHV-7 DNA load in myocardial tissues was equal to or greater than that in serum.
The cardiac function recovered; therefore, ECMO was discontinued on day 5. However, on day 9, brain CT showed cerebral edema, and EEG indicated a loss of brain activity. She underwent a tracheostomy on day 88 and was discharged from the hospital under mechanical ventilation on day 263.
The serum HHV-7 IgG titer was 16-fold at the time of admission and increased 32-fold after 80 days. At the time of admission, serum EBV capsid antigen (CA) IgG levels were 40-fold. Serum EBV-CA IgM, EBV early antigen (EA) IgG, EBV-EA IgM, and EBV nuclear antigen IgG levels were undetectable. HHV-7 DNA was detected in NPS and RecS samples and in whole blood, serum, urine, and myocardial tissue obtained after the introduction of ECMO (Table 2). SARS-CoV-2 RNA was not detected in the NPS.

Case 3. Chest radiography shows cardiomegaly (cardiothoracic ratio 65%) and pulmonary congestion.
Nearly all humans are infected with HHV-7 in early childhood and show a mild disease course; only a few severe cases of HHV-7 infection have been reported.
HHV-7 infects CD4+ T lymphocytes and epithelial cells of the salivary glands, and some HHV-7 proteins have been detected in the lungs, skin, and mammary glands28,29). Therefore, although HHV-7 DNA detection in white blood cells and tissues does not affirm the acute phase of infection, HHV-7 detection in serum or plasma may indicate active replication of HHV-7 and viremia.
We encountered two patients (cases 1 and 2) with acute encephalopathy who tested positive for HHV-7 DNA PCR results in both whole blood and serum samples. Moreover, the low titer of HHV-7 IgG also indicated that these two patients had primary HHV-7 infection at 6 and 4 years of age, respectively. The pathogenesis of HHV-7-associated encephalopathy remains unclarified, and delayed primary HHV-7 infection may be a risk factor for subsequent neurological complications30).
In case 2, EBV was detected in the whole blood but not in the serum. The EBV activity was considered low, and EBV may not have contributed to the development of acute encephalopathy.
Case 3, which involved acute myocarditis, was the most notable. As observed in cases 1 and 2, the high level of HHV-7 DNA in the serum of case 3 indicated acute viremia in case 3. Due to the emergent nature of open chest biopsy and the insufficient amount of myocardial tissue obtained, we could not perform a histopathological examination. However, we diagnosed the patient with acute myocarditis based on clinical findings and detection of viral DNA in the myocardial tissue. Although removing blood or serum-derived HHV-7 DNA from myocardial tissue specimens is impossible, high levels of HHV-7 DNA in the myocardial tissue suggest that HHV-7 may directly infect the myocardium and cause acute myocarditis. In this case, EBV was detected in the whole blood, serum, and right arterial tissue. The antibody pattern (positive for EBV capsid antigen IgG but negative for EBV capsid antigen IgM and EBV nuclear antigen antibody) indicated that the patient might have been infected with EBV several weeks before HHV-7 infection. These findings suggest that EBV infection in case 3 is more likely to have contributed to the severity of HHV-7 infection than that in case 2.
In both cases 1 and 2, CSF examinations showed neither pleocytosis nor HHV-7 DNA. This led us to diagnose the patients with encephalopathy due to an indirect immune response rather than encephalitis caused by direct HHV-7 infection of the central nervous system. Additionally, hypoxic-ischemic encephalopathy could not be ruled out in any of the cases, and in case 3, we could not rule out hypoglycemic encephalopathy. In cases 1 and 2, we did not perform autopsies in accordance with the families’ wishes. Furthermore, we performed PCR testing for herpes viruses on acute-phase specimens only, and we could not assess changes in viral load. Further studies are required to address these limitations.
Generally, HHV-7 infection requires no treatment, no treatment for severe HHV-7 infection is established. β-herpesviruses do not synthesize thymidine kinase, so viral thymidine kinase-dependent drugs such as acyclovir are ineffective. Ganciclovir, foscarnet, and cidofovir, which are antivirals against human cytomegalovirus, may also be effective against Roseolovirus31), but ganciclovir is reportedly ineffective against HHV-732). In these cases, we treated the patients with immunoglobulin, steroids, blood transfusion, anti-disseminated intravascular coagulation therapy, and ECMO, but not with antiviral drugs. Establishing treatment protocols for severe HHV-7 infections is desirable.
The factors contributing to the severity of HHV-7 infection and the mechanisms by which the virus affects the central nervous system and myocardium remain unclear30,33-35). However, it is crucial to highlight that HHV-7 has been underrecognized as a significant pathogen in severe infectious diseases, including encephalopathy and acute myocarditis. To improve our understanding and ensure timely diagnosis, HHV-7 should be actively detected in younger children presenting with severe infectious diseases, particularly by analyzing clinical samples such as serum.
The authors thank Professor Tetsushi Yoshikawa, Fujita Health University School of Medicine, for measuring HHV-7 IgG.
The authors have no conflicts of interest relevant to this article to disclose.
The project was done with no specific support.
CT, computed tomography; EBV, Epstein–Barr virus; ECMO, extracorporeal membrane oxygenation; EEG, electroencephalography; ES, Exanthema Subitum; FluV, influenza virus; HHV-6, human herpesvirus 6; HHV-7, human herpesvirus 7; NPS, nasopharyngeal swab; PCR, polymerase chain reaction; RecS, rectal swab; RSV, respiratory syncytial virus.
Dr. Hisao Okabe and Dr. Masatoki Sato conceptualized and designed the study, drafted the initial manuscript, and critically reviewed and revised it.
Sakurako Norito performed the quantitative real-time reverse transcription-polymerase chain reaction assays and critically reviewed and revised the manuscript.
Dr. Kazufumi Yaginuma and Dr. Yasushi Saito designed the data collection instruments, collected the data, and critically reviewed and revised the manuscript.
Dr. Tetsushi Yoshikawa measured HHV-7 IgG, and critically reviewed and revised the manuscript.
Dr. Mitsuaki Hosoya conceptualized and designed the study, coordinated and supervised the data collection, and critically reviewed and revised the manuscript for important intellectual content.
All authors have approved the final manuscript as submitted and agreed to be accountable for all aspects of this work.