Abstract
Background: The COVID-19 pandemic has had a significant impact on healthcare, including cancer management. This study aimed to investigate the prognostic impact of the COVID-19 pandemic, throughout its duration, on patient care for pancreatic ductal adenocarcinoma (PDAC).
Methods: We collected clinical data of patients with unresectable PDAC who underwent palliative chemotherapy at five medical facilities in Fukushima, Japan. The patients were divided into two groups: group A (nonpandemic cohort) and group B (pandemic cohort). Survival analysis was performed for progression-free survival (PFS) and overall survival (OS) via the Kaplan‒Meier method with the log-rank test.
Results: In total, 249 patients were selected for the analysis. Patients in Group B had significantly greater serum CA19-9 levels and proportions of patients selected for combination therapy; however, we did not find significant differences between the two groups in PFS (Group A vs. Group B: 5.6 months vs. 4.4 months, HR = 0.82 (95% CI, 0.6–1.1), p = 0.17) or OS (14.8 months vs. 12.3 months, HR = 0.81 (95% CI, 0.7–1.3), p = 0.81) in the survival analysis.
Conclusions: We did not observe a negative impact on the prognosis of patients with unresectable PDAC throughout the COVID-19 pandemic.
Introduction
The novel coronavirus SARS-CoV-2 was first identified in December 2019 and has rapidly spread worldwide1). On January 30, 2020, the World Health Organization (WHO) declared COVID-19 a “Public Health Emergency of International Concern (PHEIC)” because of global health risks associated with this novel coronavirus infection. On March 11, 2020, a pandemic was declared on the basis of the global spread of SARS-CoV-2 and its severe consequences2). As of November 2023, there have been more than 772 million confirmed COVID-19 cases and nearly 7 million deaths worldwide3). In Japan, the first domestic case was confirmed in January 2020; infection control measures were implemented in accordance with the spread of the virus until May 2023, when the state of emergency officially ended4).
During this period, the healthcare system, along with life in general, underwent significant disruptions. Healthcare providers faced challenges in delivering medical services amid various constraints while responding to COVID-194-10). Cancer management was particularly impacted by delays in early detection and initiation of treatment, leading to an increase in advanced cases11-13). For unresectable cancer patients undergoing palliative chemotherapy, continuation of treatment was advised, albeit with modified guidelines suggesting reduced dosages and extended treatment intervals owing to increased infection risks14,15).
Disruptions caused by the COVID-19 pandemic were extreme, particularly in the early stages, when the Wuhan strain and Delta variant had high fatality rates, and effective measures, including vaccines, were lacking. Consequently, most reports on the impact of the COVID-19 pandemic on cancer management are limited to these periods, with few analyses addressing effects of the longer pandemic. Therefore, this study aimed to elucidate the impact of the COVID-19 on the management of unresectable pancreatic adenocarcinoma (PDAC) cases throughout the pandemic. Furthermore, we examined differences in the prognoses of these cases during the highly chaotic period from January 2020 to June 2021 and the relatively stable period thereafter.
Patients and methods
Patients
In this multicenter retrospective study, data from patients who received palliative chemotherapy for advanced PDAC at Fukushima Medical University Hospital and four associated facilities between January 2017 and May 2023 were analyzed. After rare pancreatic neoplasms were excluded, patients were divided into two groups on the basis of when their first treatment was initiated: group A (nonpandemic cohort: January 2017 to December 2019) and group B (pandemic cohort: January 2020 to May 2023). Group B was further divided into early-stage (January 2020 to June 2021) and late-stage (July 2021 to May 2023) subgroups on the basis of the prevailing pathogenicity of the COVID-19 variants16,17). The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the institutional ethics committee of Fukushima Medical University (Fukushima, Japan; IRB number #29254). The need for informed consent was waived. Since we collected information from patients who not only received palliative chemotherapy for advanced PDAC but also underwent surgery or best supportive care, we evaluated the rates of clinical staging and treatment as supplemental information for the entire cohort.
Treatment
All patients were chemotherapy naïve and received standard treatment with gemcitabine (GEM), S-1, gemcitabine plus S-1, gemcitabine plus radiation therapy, gemcitabine plus nab-paclitaxel (GnP) therapy or FOLFIRINOX (FFX). Treatment with gemcitabine or S-1 alone was defined as monotherapy, and the other regimens were defined as combination therapy.
Subjects for analysis
Variables
Clinical characteristics before the initiation of chemotherapy (including age, sex, tumor stage, Eastern Cooperative Oncology Group performance status [PS], body mass index [BMI], serum total bilirubin [TB], albumin [Alb], C-reactive protein [CRP] and tumor markers (carcinoembryonic antigen [CEA], cancer antigen 19-9 [CA19-9]) were collected. Additionally, we compared the time from the first hospital visit to the first treatment between the 2 groups. Progression-free survival (PFS) and overall survival (OS) were calculated from the date of the initial day of chemotherapy to the date of disease progression or any cause of death, respectively.
Statistics
Continuous variables are reported herein as means with standard errors (SEs). For categorical data, the chi-square test or Fisher’s exact test was performed, as appropriate. Continuous variables were compared via either the Mann–Whitney test or the Kruskal–Wallis test. Survival analysis was performed via the Kaplan‒Meier method with the log-rank test. Statistical analyses were performed using SPSS version 29.0 for Windows (SPSS Inc., Chicago, IL, USA), and figures were generated via Prism 9.0 (GraphPad, San Diego, CA, USA). A p value < 0.05 was considered to indicate statistical significance.
Results
Baseline characteristics
The study included 249 patients, whose average age was 69.3 years, with 53.8% male and 81.5% having an ECOG PS of 0. Combination therapy was chosen as the first-line treatment for 83.1% of patients (Table 1). The median PFS was 5.1 months, and the median OS was 13.4 months (Fig. 1A and B).
Comparison of treatment outcomes before and during the COVID-19 pandemic
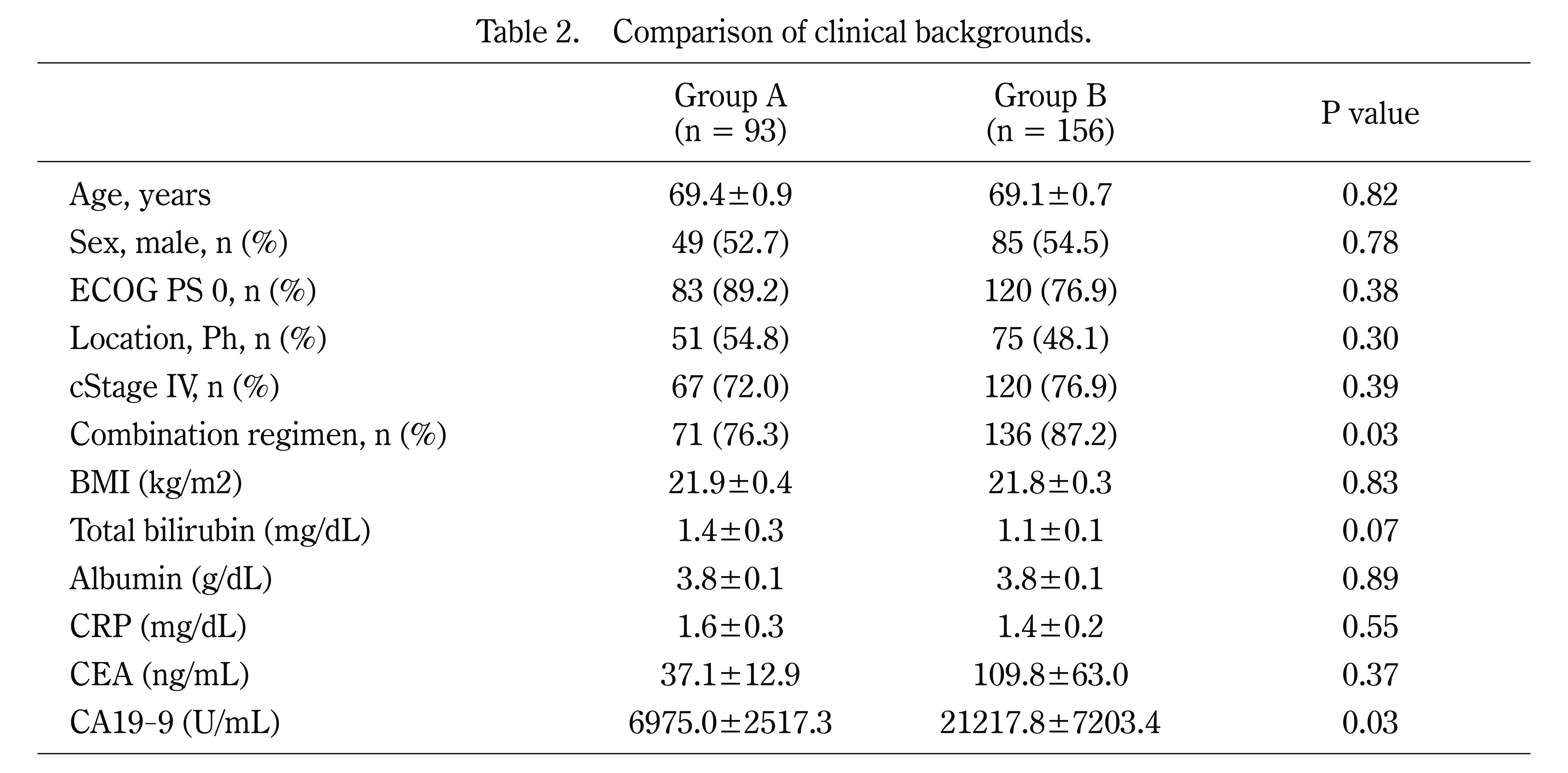
We summarized the characteristics of the patients in Group A (n = 93) and Group B (n = 156) (Table 2). Among them, the combination regimen was selected more often in Group B than in Group A (76.3% vs. 87.2%, p = 0.03), and serum CA19-9 was significantly greater in Group B than in Group A (6975.0 vs. 21217.8 ng/mL, p = 0.03). Regarding the time from the first hospital visit to the first treatment, we did not observe a significant difference between the 2 groups (42.0 days vs. 34.0 days, p = 0.42). Survival analysis revealed no significant differences in PFS (Group A vs. Group B: 5.6 months vs. 4.4 months, HR = 0.82 (95% CI, 0.6–1.1), p = 0.17) or OS (14.8 months vs. 12.3 months, HR = 0.81 (95% CI, 0.7–1.3), p = 0.81) between the 2 groups (Fig. 2A and B).
Next, we compared the patients’ characteristics between early-stage and late-stage disease, but there was no significant difference of any variable (Table 3). Survival analysis revealed no significant differences in PFS (early-stage vs. late-stage: 4.3 months vs. 4.7 months, HR = 0.98 (95% CI, 0.7–1.4), p = 0.91) or OS (14.4 months vs. 9.0 months, HR = 0.82 (95% CI, 0.5–1.2), p = 0.82) between the 2 groups (Fig. 3A and B).
Discussion
This retrospective study was aimed to ascertain the clinical impact of the COVID-19 pandemic on pancreatic cancer treatment. Data from 249 patients with unresectable PDAC across five facilities in Fukushima Prefecture, Japan, were analyzed. The study findings indicate that clinical outcomes, such as progression-free survival (PFS) and overall survival (OS), during the COVID-19 pandemic were comparable to those in the antecedent nonpandemic period. Importantly, even during the pandemic’s early stages, which were characterized by high pathogenic COVID-19 variants and considerable disruptions in healthcare delivery, the clinical outcomes of patients with unresectable PDAC were not adversely affected. To our knowledge, this is the first study to evaluate the clinical impact of the COVID-19 pandemic over its entire duration in Japan. These results suggest that effective infection control measures can enable the continuation of palliative chemotherapy without compromising its efficacy, providing valuable insights for future pandemics.
Recommended guidelines include continuing palliative chemotherapy with caution to minimize COVID-19 infection since this is the only treatment used to improve patients’ prognosis14,15). The following methods were proposed as countermeasures: i) consider treatment delay or a switch to less immunosuppressive therapies in the case of a low risk of recurrence during postoperative chemotherapy, ii) consider switching to oral medications, iii) consider changing to a regimen with longer dosing intervals, and iv) consider the use of G-CSF preparations and prophylactic antibiotics. With respect to PDAC management, prior studies have investigated the prognostic impact of the COVID-19 pandemic. In the Netherlands, Graus et al. reported that there was no difference in treatment type between nonpandemic and pandemic periods, and the overall survival rates of the two groups were comparable16). Hall et al. reported similar results in a cohort study from the UK17). Kasuga et al. reported the clinical impact of the pandemic on unresectable PDAC in Japan and reported that the median overall survival was similar between the groups (pandemic vs. nonpandemic, 12.6 vs. 11.9 months, p = 0.174)18). Many other studies have investigated the short-term impact of the pandemic on cancer survival, presumably because of the catastrophic impact of the early-stage COVID-19 pandemic in 2020. However, we experienced restrictions in medical care even in the late stage of the pandemic. This prompted our cohort study to clarify the prognostic impact of the COVID-19 pandemic throughout its entire duration.
Some limitations should be acknowledged. This retrospective analysis had a relatively small patient cohort, and comprehensive data on specific countermeasures implemented at each facility were lacking. Additionally, potential biases (such as higher serum CA19-9 levels in Group B) existed, which suggested the possibility of more advanced disease stages despite similar clinical staging proportions. Although the increased use of combination therapy in Group B might have mitigated the overall impact on patient prognosis, larger-scale studies would be necessary to fully understand these aspects.
In conclusion, our study revealed no significant negative impact on prognosis in patients with unresectable PDAC during the COVID-19 pandemic.
Availability of data and material:
The data that support the findings of the present study are available from the corresponding author upon reasonable request.
Funding:
No funding was received for this study.
Conflict of interest disclosure:
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
Ethics approval statement and patient consent statement:
The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki, and was approved by the institutional ethics committee of Fukushima Medical University (Fukushima, Japan; IRB number #29254). The need for informed consent was waived.
Authorship statement:
All the authors meet the ICMJE authorship criteria. R.S., H.A., N.K., K.W., H.I., Y.W., M.S., Y.S., H.I., T.T. and H.O. served as medical experts and were involved in primary manuscript writing. R.S. contributed to the study design. All the authors contributed to the final version of the manuscript. R.S. had final responsibility for the decision to submit for publication.
References
- 1. Sachs JD, Karim SSA, Aknin L, Allen J, Brosbol K, Colombo F, et al. The Lancet Commission on lessons for the future from the COVID-19 pandemic. Lancet, 400: 1224-1280, 2022.
- 2. McVernon J, Liberman J. WHO keeps covid-19 a public health emergency of international concern. BMJ, 380: 504, 2023.
- 3. WHO COVID-19 Dashboard. Geneva: World Health Organization, 2020. Available online: https://covid19.who.int/ (last cited: [November 30, 2023]). 2020.
- 4. Hirae K, Hoshina T, Koga H. Impact of the COVID-19 pandemic on the epidemiology of other communicable diseases in Japan. Int J Infect Dis, 128: 265-271, 2023.
- 5. Tan BYQ, Chew NWS, Lee GKH, Jing M, Goh Y, Yeo LLL, et al. Psychological Impact of the COVID-19 Pandemic on Health Care Workers in Singapore. Ann Intern Med, 173: 317-320, 2020.
- 6. Chutiyami M, Cheong AMY, Salihu D, Bello UM, Ndwiga D, Maharaj R, et al. COVID-19 Pandemic and Overall Mental Health of Healthcare Professionals Globally: A Meta-Review of Systematic Reviews. Front Psychiatry, 12: 804525, 2021.
- 7. Shah SA, Brophy S, Kennedy J, Fisher L, Walker A, Mackenna B, et al. Impact of first UK COVID-19 lockdown on hospital admissions: Interrupted time series study of 32 million people. EClinicalMedicine, 49: 101462, 2022.
- 8. Adapa S, Chenna A, Balla M, Merugu GP, Koduri NM, Daggubati SR, et al. COVID-19 Pandemic Causing Acute Kidney Injury and Impact on Patients With Chronic Kidney Disease and Renal Transplantation. J Clin Med Res, 12: 352-361, 2020.
- 9. Carbone L, Raffone A, Travaglino A, Saccone G, Di Girolamo R, Neola D, et al. The impact of COVID-19 pandemic on obstetrics and gynecology hospitalization rate and on reasons for seeking emergency care: a systematic review and meta-analysis. J Matern Fetal Neonatal Med, 36: 2187254, 2023.
- 10. Dale CE, Takhar R, Carragher R, Katsoulis M, Torabi F, Duffield S, et al. The impact of the COVID-19 pandemic on cardiovascular disease prevention and management. Nat Med, 29: 219-225, 2023.
- 11. Al-Quteimat OM, Amer AM. The Impact of the COVID-19 Pandemic on Cancer Patients. Am J Clin Oncol, 43: 452-455, 2020.
- 12. Jazieh AR, Akbulut H, Curigliano G, Rogado A, Alsharm AA, Razis ED, et al. Impact of the COVID-19 Pandemic on Cancer Care: A Global Collaborative Study. JCO Glob Oncol, 6: 1428-1438, 2020.
- 13. Morris EJA, Goldacre R, Spata E, Mafham M, Finan PJ, Shelton J, et al. Impact of the COVID-19 pandemic on the detection and management of colorectal cancer in England: a population-based study. Lancet Gastroenterol Hepatol, 6: 199-208, 2021.
- 14. You B, Ravaud A, Canivet A, Ganem G, Giraud P, Guimbaud R, et al. The official French guidelines to protect patients with cancer against SARS-CoV-2 infection. Lancet Oncol, 21: 619-621, 2020.
- 15. Burki TK. Cancer guidelines during the COVID-19 pandemic. Lancet Oncol, 21: 629-630, 2020.
- 16. Graus M, de Hingh I, Besselink MG, Bruno MJ, Wilmink JW, de Meijer VE, et al. Population-based impact of COVID-19 on incidence, treatment, and survival of patients with pancreatic cancer. HPB (Oxford), 25: 1195-1202, 2023.
- 17. Hall LA, McKay SC, Halle-Smith J, Soane J, Osei-Bordom DC, Goodburn L, et al. The impact of the COVID-19 pandemic upon pancreatic cancer treatment (CONTACT Study): a UK national observational cohort study. Br J Cancer, 128: 1922-1932, 2023.
- 18. Kasuga A, Nojima M, Okamoto T, Ishitsuka T, Yamada M, Nakagawa H, et al. Impact of the COVID-19 Pandemic on the Management and End-of-life Care of Unresectable Pancreatic Cancer. Intern Med, 61: 3641-3649, 2022.