Abstract
Transgrafting, a grafting technique that uses both genetically modified (GM) and non-GM
plants, is a novel plant breeding technology that can be used to improve the efficiency of
crop cultivation without introducing foreign genes into the edible parts of non-GM plants.
This technique can facilitate the acquisition of disease resistance and/or increased
yield. However, the translocation of low-molecular-weight compounds, ribonucleic acid
(RNA), and proteins through graft junctions raises a potential safety risk for food crops.
Here, we used a transgenic tobacco plant expressing a firefly luciferase gene
(LUC) to examine the translocation of the LUC protein beyond the graft
junction in grafted plants. We observed the bi-directional translocation of LUC proteins
in transgrafted tobacco plants, i.e., from the rootstock to scion and vice versa.
Transcriptomic analysis revealed that transcripts of the LUC gene were undetectable in
non-GM plant bodies, indicating that the LUC protein itself was translocated. Moreover,
the movement of the LUC protein is an episodic (i.e., non-continuous) event, since non-GM
samples showing high LUC activity were flanked by non-GM samples showing no apparent LUC
activity. Translocation from the GM to non-GM part depends on the characteristics of GM
plant bodies; here, the enhanced translocation of the LUC protein into the non-GM scion
was observed when LUC-expressing rootstocks with hairy roots were used. Moreover, the
quantity of translocated LUC protein was far below the level that is generally required to
induce an allergenic response. Finally, since the LUC protein levels of plants used for
transgrafting are moderate and the LUC protein itself is relatively unstable, further
investigation is necessary regarding whether the newly expressed protein in GM plants is
highly stable, easily translocated, and/or highly expressed.
1. Introduction
Grafting is a traditional technique in which two different plant bodies are merged into one
plantlet via bonding along the cutting surface. This technique has been used for thousands
of years for the cultivation of fruit crops1). Wild plants or cultivars that are resistant to biotic and
abiotic stresses are often used as rootstocks, and cultivated varieties that possess good
food properties—but which may be sensitive to abiotic and/or biotic stress—are used as
scions. In the 1850s, a soil-dwelling insect pathogen originating in the United States,
phylloxera, invaded European grape plants, thereby causing serious damage to the grape and
wine industries. This pest was overcome via grafting of phylloxera-sensitive European grape
cultivars onto phylloxera-resistant American grape rootstocks2). Moreover, grafting has also been employed to reduce
farm labor requirements. For example, the dwarf apple rootstock has been used to improve
fruit harvest efficiency, namely by altering tree morphology to modify shoot elongation and
blanch angle3). In this case,
phenotypic alteration to the scion is likely established by the exchange of
biomolecules—including small-molecule chemicals, RNAs, and proteins—between the rootstock
and scion via the graft junction4).
Transgrafting is a technique that produces a plant consisting of a genetically modified
(GM) rootstock and a non-GM scion, or vice versa5), and is a key new plant breeding technology (NPBT)6,7). GM rootstocks with increased resistance to abiotic and/or
biotic stresses are designed to attenuate decreases in crop production under suboptimal
conditions. Moreover, since fruits and crops obtained from the non-GM plant parts of the
transgrafted plant do not contain transgene sequences in their genome, the consumption of
these foods is expected to be exempted from regulations for GM plants. However, as mentioned
above, the possible exchange of biomolecules between the rootstock and scion raise concerns
regarding the safe use of foods obtained from grafted plants. For example, one incident
occurred in which eggplant (Solanum melongena) fruits were harvested from
plants grafted onto Datura metel rootstock; when served they caused severe
food poisoning in Japan8). This
incident was caused by the transport of toxic alkaloids from the Datura
rootstock to the eggplant fruits on the scion. Alkaloids, frequently produced by members of
the Solanaceae family, are known to be synthesized in the roots then transported throughout
the plant9). Therefore, if toxic
compounds are produced in a transgene-dependent manner, the fruits and crops obtained from
the non-GM scions of transgrafted plants may cause health risks if consumed.
In addition to the translocation of low-molecular compounds via the graft junction, protein
translocation has been also observed. Two types of protein movement have been studied:
short-distance movement between neighboring cells via plasmodesmata and long-distance
movement in which proteins are translocated to distal tissues via the vascular system.
Plasmodesmata are tubes with diameters ranging from 30–60 nm; this tube size is often
regulated by the deposition of β-1,3-glucan polymers (i.e., callose) to cell walls
containing plasmodesmata. Plant cells control the permeability of biomolecules through
plasmodesmata to regulate their cell-to-cell transport10). Moreover, it has been observed that plasmodesmata can form
across a graft junction11).
Green fluorescent protein (GFP) is used as a soluble reporter, and has been found to be
capable of diffusive spreading into neighboring cells via plasmodesmata12). Long-distance protein movement
was first demonstrated in Arabidopsis, in which the floral signaling
protein FLOWERING LOCUS T (FT) was found to be transported over long distances via the
phloem13). Subsequently, FT
homologous proteins were found and their translocation tracked in various plant species,
including potato14),
tomato15), and
squash16). Moreover,
grafting was frequently used to demonstrate the long-distance translocation of FT proteins.
In addition, long-distance movement of GFP-tagged chloroplast transit peptides from the
scion to the rootstock has also been detected in transgrafted Arabidopsis
plants17).
In previous studies we performed omics analyses of the edible parts of homo-transgrafted
plants18,19). Homo-transgrafting refers to a
grafting technique in which a non-GM scion and GM rootstock, or vice versa, are prepared
from the same plant species. The GM tomato18) and GM potato19) plants used in previous transgrafting experiments harbored
transgenes encoding β-glucuronidase (GUS) and a potato FT homolog protein, respectively. In
the resulting tomato fruits and potato tubers, the newly expressed GUS and FT proteins were
not detected by proteomic analyses. Next, we subjected tomato fruits harvested from
hetero-transgrafted plants to omics analysis20). In this case, hetero-transgrafting was established using a
GM tobacco rootstock and a non-GM tomato scion. Here, a proteomic analysis of tomato fruits
detected two kinds of tobacco proteins. This result and a previous report by Paultre
et al. (2016) raised the possibility of the long-distance movement of
newly expressed proteins (NEPs) produced in the GM parts of transgrafted plants. Since the
allergenicity of NEPs is the most important assessment issue for the guidelines for GM foods
prepared by the Codex Alimentarius Commission (CAC)21), the understanding of the long-distance movement of NEPs
from GM plant parts—and especially the levels of translocated NEPs and their distribution
patterns in non-GM parts—is critically important to establish the safety of transgrafted
plants. In this study, we used a GM tobacco plant that expresses firefly luciferase (LUC)
gene for producing a homo-transgrafted tobacco plant. Since LUC activity can be detected
with quite high sensitivity, even very low levels of the LUC protein are easily detected. In
addition, the short turnover of LUC proteins makes them ideal for monitoring translocation,
where the LUC protein produced in the GM plant body accumulates at specific parts in the
non-GM plant body. The results obtained here show that the LUC protein moved from the
rootstock to the scion, and vice versa. Next, we investigated the effects of so-called
“mentor-grafting22)” on LUC
protein translocation. Mentor grafting is a technique in which the growth of scion parts is
preferentially dependent on the supply of nutrients from the rootstock by the removal of all
scion leaves except young leaves near the scion’s shoot apical meristem. The transport of
biomolecules from rootstock to scion is expected to be enhanced under mentor-grafting
conditions relative to conventional grafting. For example, the enhanced movement of small
RNAs has been observed in mentor-grafted tomato plants23). Furthermore, we also addressed the translocation of LUC
protein when the roots of the rootstock were replaced with hairy roots. Hairy roots are the
transformed organ formed following infection by Agrobacterium rhizogenes
(Rhizobium rhizogenes). A modified morphological phenotype has been
reported in cherry scions grafted onto a rootstock that had been regenerated from hairy
roots24). Therefore, the
effects of the rootstock on the phenotype of the scion may be greater when using a rootstock
with hairy roots than when using a rootstock with normal roots. Here, this transgrafting
study used a GM tobacco plant expressing the LUC gene to reveal that the
translocation of the LUC protein beyond the graft junction is dependent on the grafted
condition and is also markedly affected by rootstock characteristics. The intermittent
localization of LUC proteins in the scion indicated that the potential risks of the
transgene product should be assessed even if the transgene products are not detected in the
samples obtained from the non-GM plant bodies of the transgrafted plants.
2. Materials and Methods
2.1 Plant Materials and Grafting
A transgenic tobacco line expressing the LUC gene (Uniprot Accession:
P08659) was produced as previously described25). This transgenic plant was named the “LUC plant.”
Transgrafted plants were prepared using LUC plants and corresponding non-GM, wild-type
(WT) tobacco plants (i.e., Nicotiana tabacum cv. SR1). Transgrafting was
performed using a conventional grafting method. Briefly, seeds of WT and LUC plants were
sown under sterile conditions and one month later the resulting seedlings were transferred
to soil. Rootstocks were prepared by cutting the stem at a position 10−20 cm above the
soil, and shoots with three mature leaves were used as scions. The grafted junctions were
then fastened with surgical tape. Combinations of grafted WT and LUC plants are described
using the format “scion/rootstock.”
In WT (scion)/LUC (rootstock) grafted plants (Fig.
1), axillary buds that formed on the rootstock were removed so that nutrients
from the rootstock were efficiently transported to the scion. We prepared a total of 13
transgrafted plants. Then, approximately 3–8 weeks after grafting (WAG), we examined the
LUC activities of stem samples collected at 10 mm intervals from the graft junction. As a
control, LUC activity was also measured in the leaves and stems of 2 month-old WT plants.
We also harvested and evaluated the LUC activity of all scion leaves.
We prepared a total of eight transgrafted plants (i.e., LUC (scion)/WT (rootstock)
plants; Fig. 2). At 3 WAG, non-GM stem samples
were collected along a transect at each 10 mm interval from the graft junction. Moreover,
we also collected samples from the stems of axillary buds formed on the rootstock at
intervals of 10 mm. All leaves from the axillary buds were sampled to measure LUC
activity.
2.2 Transgrafting Using the Mentor Grafting Method
Mentor grafting is a method in which all leaves on the scion except the youngest, near
the shoot apical meristem, are removed22). A total of 10 scions were grafted onto LUC rootstocks, and
for five of these plants all developed leaves were then removed (i.e., mentor grafting).
The remaining five grafted plants were grown as negative controls (i.e., conventional
grafting) (Fig. 3). Stem and leaf
samples from all plants were collected at 3 WAG. Any axillary buds formed on the scion
during this period were removed.
2.3 Preparation of WT/LUC Transgrafted Plants with Hairy Roots
Hairy roots were generated via infection of Agrobacterium tumefaciens
R-1000 strain. This strain carries a hairy-root-inducing Ri plasmid, pRiA4b, instead of Ti
plasmids26,27). For this experiment,
two-month-old LUC plants grown in plant culture vessels were infected by pricking using a
syringe needle containing Agrobacterium cells. The prick occurred on the
stem 2−3 cm above the root, and hairy roots usually formed two weeks after infection.
Next, normal roots were removed by cutting the stems of LUC plants just below the site of
hairy root formation. The resulting LUC plants with hairy roots (called “hr-LUC plants”)
were transferred to a Murashige-Skoog solid medium containing 750 mg/L Augmentin
(GlaxoSmithKline, Brentford, UK) then grown in plant culture vessels. After 3 weeks,
aseptically grown WT scions were grafted onto hr-LUC rootstocks (Fig. 4). Similarly, LUC plants grown in plant culture
vessels were used for grafting onto WT plants. The numbers of the WT/hr-LUC and WT/LUC
transgrafted plants were 5 and 13, respectively. The leaves of two WT/hr-LUC plants were
harvested at 3 WAG. The other three WT/hr-LUC plants were transferred on soil on the same
day, then subsequently cultured for another three weeks. We obtained leaf and stem samples
on 4, 6, and 8 WAG, and subjected these samples to measurements of LUC activity.
All sampled tissues were immediately measured for LUC activity. We used the Luciferase
Assay System (Promega, WI, USA) to quantify LUC activity. Briefly, 200 µL of fivefold
diluted Cell Culture Lysis Reagent was added to each sample. After homogenization, all
samples were centrifuged at 15,000 rpm for 10 min at 4°C. The LUC activity of the
supernatant was determined as previously described28). The assay was performed in 1 sec raw format using a
Lumat3 LB7608 (Berthold Technologies GmbH & Co. KG, Bad Wildbad, Germany). All results
are shown as relative light units (RLUs). The calibration curve for luciferase protein
quantification was prepared by using a purified luciferase protein standard (Merck
Millipore, Billerica, MA, USA, Code number: L9420-1MG).
2.5 Protein Quantification
Protein quantification was performed using a DC protein assay (Bio-Rad Laboratories, Inc.
CA, USA). Calibration curves were prepared via stepwise dilution of bovine serum albumin
in sterile water. The assay system involved a small-scale modification of the micro assay
protocol; briefly, 20 µL of reagent S was added to 1 mL of reagent A to create reagent A’.
Next, 70 µL of reagent A’ was added to 140 µL of diluted sample and vortexed.
Subsequently, 560 µL of reagent B was added and vortexed well before being incubated at
room temperature for 15 min. The absorbance at 750 nm was then measured with a
spectrophotometer (UV-1800, SHIMADZU, Kyoto, Japan).
2.6 Transcriptomic Analysis of the Transgrafted Scion
To perform transcriptomic analyses, total RNA was first extracted from frozen leaf and
petiole samples of WT/LUC plants prepared by a conventional grafting method. Leaves and
petioles were frozen in liquid nitrogen immediately after sampling. They were then used
for RNA extraction. Extraction was carried out using a FavorPrep Plant Total RNA Mini Kit
(Favorgen Biotech Corp., Taiwan). The outsourcing service of Eurofins Genomics (Tokyo,
Japan) constructed the RNA library and obtained mRNA sequencing data. Briefly, the mRNA
was purified as poly(A)+ RNA, and paired-end 150-base sequencing data was
generated using a NovaSeq 6000 platform (Illumina Inc., San Diego, CA, USA). The mRNA-seq
dataset (BioProject ID: PRJDB11010, Experiment ID: DRX411439-40) contained a total of
161.7 million reads. Adapter sequences were then trimmed, and low-quality reads containing
poly-N sequences and/or that were shorter than 50 bp in length were discarded using fastp
version 0.23.4. The bowtie2 version 2.5.1 alignment tool was then used to search for
LUC gene transcripts and align reads to Nicotiana
tabacum cDNA (Ntab-TN90_AYMY-SS_NGS.mrna.annot.fasta).
3. Results
3.1 Detection of LUC Activities in the Scion of WT/LUC Plants
A homozygous transgenic tobacco line expressing the firefly LUC gene was
used for transgrafting experiments. When the LUC plants were used as the rootstock and WT
plants as the scion, the resulting grafted plants were called WT/LUC plants. Plants grown
on the soil were used for grafting. Moreover, we harvested scion samples 3, 4, 6 and 8
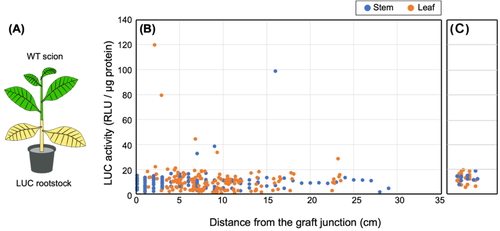
WAG, and all LUC activities of the scion were determined collectively (Fig. 1). Interestingly, we detected
weak LUC activity in the (WT) scion, and this activity was distinguishable from the
background luminescence of the WT plants (i.e., approximately 0−20 RLU/μg protein). The
highest LUC activity in the scion was observed 4 WAG; thereafter the LUC activity was
detected only at a relatively low level (Fig.
S1). The highest LUC activity (119 RLU/μg protein) was observed in
leaves that had been harvested 3 cm above the graft junction. Furthermore, despite the
fact that the scion stem samples (1 cm in length) were prepared in series from the graft
junction, the strength of LUC activity did not fade in a linear manner but rather had
seemingly random peaks. In fact, the second highest LUC activity (99 RLU/μg protein) was
observed in a stem sample prepared 16 cm above the graft junction. In addition, we did not
detect increased LUC activity in seeds produced by the scion of WT/LUC plants (Fig. S2).
Transcriptomic analysis was then carried out on the scion leaves and petioles of WT/LUC
plants at 6 WAG. Transcriptomic data for two samples were generated, and we obtained
approximately 80 M reads for each. Next, alignment of these reads to the cDNA database
generated using Nicotiana tabacum genome data resulted in alignment rates
of around 90% for each sample (Table S1;
WT-LUC-1 read data was obtained from petiole samples and WT-LUC-2 was obtained from a mix
of petiole and leaf samples). The remaining 10% of reads could not be aligned to any
tobacco cDNAs and these sequence reads may correspond to RNA species such as tRNA, rRNA
and so on or RNAs isolated from environmental contaminants such as bacteria and fungi in
the samples. The sequencing of the Nicotiana tabacum genome is not yet
complete. The number of publicly available tobacco genes is very large because the tobacco
database contains redundant sequences. A total of 189,413 genes (as cDNA references) can
be found in the tobacco cDNA database, and the transcriptomic reads from the scion samples
were mapped to the 137,645 genes, indicating that the transcriptomic reads covered
approximately 73% of the tobacco cDNAs. Since the scion samples consisted of petiole and
leaf tissues, the genes preferentially expressed in the reproductive organs and roots
would not be detected in the transcriptomic reads of the scion samples. Under this
condition, no transcriptomic reads were aligned to the LUC genes.
However, the possibility remains that LUC gene transcripts are
translocated from the rootstock to the WT scions of WT/LUC plants.
3.2 LUC Activity in the Rootstocks of LUC/WT Plants
Next, we determined which direction—i.e., from rootstock to scion or vice versa—was more
common for LUC protein translocation. For this purpose, a LUC scion was grafted onto a WT
rootstock, and this grafted plant was called a “LUC/WT plant.” Next, LUC activity was
measured in the stem part of the rootstock and in new axillary buds that formed on the
rootstock after grafting (Fig. 2).
Interestingly, a number of stem and leaf samples obtained from the axillary buds on the
rootstock showed very high LUC activities (i.e., more than 500 RLU/μg protein) (Fig. 2C). In fact, the highest LUC
activity (2,200 RLU/μg protein) observed in the axillary buds was 18-fold higher than the
highest activity observed in the scion of the WT/LUC transgrafted plants (Fig. 1). In contrast, stem samples
taken from directly below the graft junction showed almost no LUC activity and resembled
the background luminescence level (Fig.
2B). Therefore, the LUC protein produced in the scion was observed to move
through the stem of the rootstock and accumulated in young tissues such as axillary
buds.
We then assessed whether the LUC protein moved more frequently from scion to rootstock
than vice versa. Although the LUC gene is transcribed under the control
of the Cauliflower mosaic virus 35S promoter and is therefore constitutively expressed
throughout all plant tissues, transcription itself is more active in young than in old
tissues. Therefore, actively growing, young tissues are expected to show higher LUC
activity than older tissues. When LUC activity was quantified in the LUC scion and LUC
rootstock that were used for transgrafting, the scion showed increases in LUC activity of
approximately 1.4-fold and 2.6-fold for stem and leaf samples, respectively, relative to
corresponding samples from the rootstock (Fig.
S3). In contrast, the LUC activities detected in the axillary buds of the
rootstocks of LUC/WT plants were significantly higher (maximum 2,200 RLU/μg protein) than
those detected in the scion samples of the WT/LUC plants (maximum 119 RLU/μg protein).
Therefore, even though LUC protein production was expected to be higher in the scion than
the rootstock, it is likely that the LUC protein moves more easily from the scion to the
rootstock than vice versa.
3.3 Effects of the Mentor Grafting Technique on the Movement of LUC Protein
When using the mentor grafting technique, scion growth largely depends on the nutrient
supply from the rootstock. Next, we determined whether movement of the LUC protein is
enhanced in mentor-grafted WT/LUC plants. Compared to the LUC activity of WT/LUC plants
prepared using the conventional grafting, we detected slightly higher LUC activities in
the scions of WT/LUC plants produced by mentor grafting (Fig. 3). Specifically, we observed a mean value of
10.2 RLU/μg protein for conventional grafting and 18.3 RLU/μg protein for mentor grafting.
Furthermore, in the mentor grafting plants we detected a significantly high LUC activity
(360 RLU/μg protein) in stem samples taken 3 cm above the graft junction. Taken together,
these results indicate that the movement of the LUC protein is influenced by the grafting
method, but its effect on the quantity of LUC protein that is moved remains quite
limited.
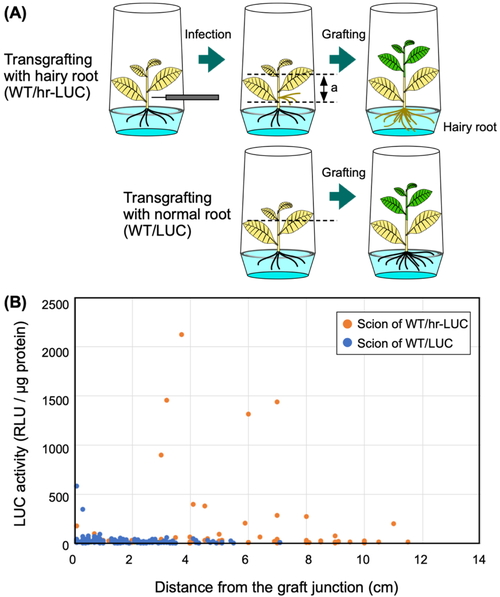
3.4 LUC Activities of WT Scions Grafted onto LUC Rootstocks with Hairy Roots
Hairy roots are root organs transformed with the Ri plasmid. The characteristics of hairy
roots include rapid growth and a high capacity for secondary metabolite and recombinant
protein production29,30). Next, we studied whether or not the movement of LUC
protein from the rootstock to the scion was strengthened by the hairy root phenotype. To
do so, LUC plants were first infected with Agrobacterium harboring an Ri
plasmid. After generating hairy roots at the infection site, normal roots were removed and
the resulting LUC plants with hairy roots (called “hr-LUC plants”) were used as a
rootstock (Fig. 4A). We used LUC
plants with normal roots as a control. We detected LUC activity measurements above 200
RLU/μg protein in scion samples of WT/hr-LUC plants taken from more than 3 cm above the
graft junction. The highest LUC activity (i.e., 2,118 RLU/μg protein) was detected at a
distance of 3.7 cm from the graft junction (Fig.
4B). Furthermore, we also detected high (201 RLU/μg) LUC activity in a
scion sample taken from 11 cm above the graft junction. We note that in this experiment,
grafting was carried out using aseptically grown, young plants (Fig. 4A), which may enhance the movement of the LUC
protein. Two high LUC activities (i.e., 345 and 583 RLU/μg protein) were detected in
control samples prepared from the stem tissues close to the graft junction.
The highest LUC activity observed in the scion of WT/hr-LUC plants (Fig. 4B) was comparable to the highest value observed in the
axillary buds that emerged from the rootstock of LUC/WT plants (Fig. 2C). In stem samples prepared from the scions of WT/hr-LUC
plants, we found that proximal samples and distal samples flanking other samples with
extremely high LUC activity did not consistently show high LUC activity (Fig. S4). These results confirms that LUC protein
movement across the graft junction was episodic instead of continuous.
4. Discussion
Risk assessments for the safe use of GM foods are mandatory in most countries, including
Japan. Moreover, the evaluation of the allergenic and toxic potential of NEPs in GM foods is
critically important31). Thus,
the ability to detect transgene sequences is a prerequisite for adequate risk management of
GM foods. Therefore, when particular fruits obtained from the scion parts of transgrafted
plants do not contain any transgene sequences in their genome, these foods should be out of
scope of risk management strategies. However, if NEPs move beyond the graft junction, we
must consider their allergenic and toxic potential before the use of food products obtained
from transgrafted plants is sanctioned. Here, we showed that a NEP (i.e., LUC protein) was
detected in WT plant bodies of transgrafted plants.
Small molecular compounds, such as alkaloids, can be quantitatively detected, and the
effects of transgrafting on their abundance in fruits has also been observed. For example,
α-tomatine, a toxic substance found in tomato, was less abundant and nicotine, a toxic
substance found in tobacco, was more abundant in tomato fruits grown on transgrafted plants
in which a non-GM tomato scion was grafted onto a GM tobacco rootstock20). In contrast, our data showed that
the LUC protein was episodically detected in the WT plant bodies of transgrafted plants. In
fact, the non-GM stem samples that showed extremely high LUC activity were flanked by stem
tissues that did not show any significant LUC activity (Fig. S4). Similar results have been reported for the translocation of GFP fusion
proteins from transgenic scions to the specific tissues of the non-GM roots17). These results suggest that the
movement of the LUC protein beyond the graft junction occurred episodically, and not
continuously, in transgrafted plants. The firefly LUC protein is relatively unstable and has
a half-life of approximately 3−4 h32). Due to this relative short half-life, LUC has been used for
various circadian studies33).
If so, we detected the LUC protein relatively shortly after translocation into non-GM plant
bodies from the GM parts of transgrafted plants. Although the route of LUC protein movement
has not yet been determined, the LUC protein most likely moves via phloem, as does the FT
protein. The velocity of FT in the phloem has been estimated to be 30–50 cm h–1,
which is comparable to the estimated velocity of the phloem flux34,35). Therefore, it is likely that the export of LUC protein from
GM plant bodies is an episodic event that occurs frequently. In contrast, the preferential
detection of LUC activity in the axillary buds of the rootstock and not in stem tissues that
directly abutted the GM scion (Fig. 2) suggest
that the LUC protein moved beyond the graft junction to accumulate at specific “sink
tissues.” Indeed, Paultre et al. have suggested that GFP fusion proteins translocated
through the phloem are “unloaded” from the phloem in specific tissues17). In addition, the tissues in which
the translocated proteins accumulated differed among the different GFP fusion proteins,
suggesting that phloem unloading may be regulated differently for each protein17). If such a protein export and
unloading mechanism is associated with most of the translocated protein types, the
discontinuously detected LUC protein may be explained by phloem unloading at the specific
plant body and may not by the episodic export from the GM plant body.
In WT-LUC transgrafted plants, we observed that the mentor grafting technique showed only a
limited effect on the transport of LUC protein from the rootstock (Fig. 3). Moreover, high levels of LUC protein were detected in non-GM
scions when the hr-LUC rootstock was used for grafting (Fig. 4). These results suggest that the level of NEPs in the non-GM scion was
dependent on the characteristics of the rootstock that produced the NEPs. In contrast, the
effect of scion condition on NEP translocation was nearly negligible, since mentor grafting
has quite a limited effect. Hairy roots are well known to grow vigorously and produce
secondary metabolites and NEPs29,30). However, our results also suggest that hairy roots can
systemically export NEPs more efficiently than normal roots. In conventional grafting, those
rootstocks that show maximum stress-resistance and rooting vigor are preferably chosen,
especially for heterografting. Our results indicate that the NEP levels of non-GM scions can
be affected by the GM rootstock, and it is therefore necessary to assess what kind of GM
rootstock is used for grafting, especially for hetero-transgrafting.
In this study, the average measured LUC activity was approximately 280,000 RLU/μg protein
in the young leaves of LUC plants (Fig. S3), and
the highest detected LUC activity in non-GM plant bodies was approximately 2,000−2,500
RLU/μg protein (Figs. 2 and 4). When we used purified firefly luciferase as a calibration
standard for protein quantification, we obtained a maximum estimate of 0.51 pg total
luciferase protein per sample for the non-GM samples (stem or leaf samples) which showed the
highest LUC activity of all samples tested. The lowest observed adverse-effect level (LOAEL)
has been estimated for many IgE-dependent food allergies, and LOAELs are commonly in the
range of 1-2 mg of natural foods, representing a few hundred micrograms of protein36). Therefore, the amount of LUC
protein that moved though the graft junction is very small when compared with the LOAELs of
IgE-dependent food allergens. In addition, although LUC activity was not detected in seeds
harvested from the non-GM scions, tobacco rootstock-derived proteins have been detected in
tomato fruits in our previous study20). As discussed above, it is possible that the tissue in which
the translocated proteins accumulate differs among the translocated protein types.
Therefore, necessity of evaluation for the potential risk, such as allergenicity, for the
NEPs produced in the GM plant body of the transgrafted plants remains to be further
discussed.
5. Conclusion
Here, we showed that a cytosolic LUC protein can be translocated beyond the graft junction
of a grafted plant, both from the rootstock to scion and vice versa. A previous analysis of
phloem exudates showed that the majority of proteins ranged in size from 20−70 kDa, and the
translocation of these mobile proteins is controlled by plasmodesmata at the
pericycle-endodermis boundary17). Since the size of the LUC protein is 60 kDa, the
translocation of LUC protein is therefore predictable. Because the structure and
permeability of plasmodesmata can be modified by both pathogens and the host plant37), further study of NEP
translocation is necessary to establish the safety of using transgrafting fruits. One
important point is that translocation from the GM plant body is not a continuous event and
is dependent on the characteristics of GM plant bodies. Given these factors, it is difficult
to statistically assess degree of NEP translocation in the transgrafted plants. In addition,
as mentioned in our previous study20), small unfavorable metabolites are transferred from the GM
plant body to non-GM plant body. Therefore, the potential risks of the transgene product and
small molecules of concerned toxicity should be assessed when the transgene-free fruits
obtained from the non-GM plant bodies of the transgrafted plants are commercialized.
Acknowledgments
We thank Prof. Yoshihiro Ozeki for his kind advice related to this research. This study was
supported by a grant of Research Program for Risk Assessment Study on Food Safety (No. 1902
and 2101) from the Food Safety Commission, Cabinet Office, Government of Japan.
Transcriptome analysis was partially performed using the NIG supercomputer at the ROIS
National Institute of Genetics.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.Habibi F, Liu T, Folta K, Sarkhosh A.
Physiological, biochemical, and molecular aspects of grafting in fruit trees.
Hortic Res. 2022; 9: uhac032. .PMID:35184166,
https://doi.org/10.1093/hr/uhac032
- 2.Melnyk CW, Meyerowitz EM. Plant grafting.
Curr Biol. 2015; 25(5): R183–R188.
https://doi.org/10.1016/j.cub.2015.01.029.
- 3.Tworkoski T, Miller S. Rootstock effect on
growth of apple scions with different growth habits. Sci Hortic
(Amsterdam). 2007; 111(4): 335–343.
.https://doi.org/10.1016/j.scienta.2006.10.034
- 4.Jeynes-Cupper K, Catoni M. Long distance
signalling and epigenetic changes in crop grafting. Front Plant Sci.
2023; 14: 1121704. .PMID:37021313,
https://doi.org/10.3389/fpls.2023.1121704
- 5.Albacete A, Martínez-Andújar C, Martínez-Pérez
A, Thompson AJ, Dodd IC, Pérez-Alfocea F. Unravelling rootstockxscion interactions to
improve food security. J Exp Bot. 2015;
66(8): 2211–2226. .PMID:25754404,
https://doi.org/10.1093/jxb/erv027
- 6.Lusser M, Parisi C, Plan D, Rodríguez-Cerezo E.
Deployment of new biotechnologies in plant breeding. Nat Biotechnol.
2012; 30(3): 231–239. .PMID:22398616,
https://doi.org/10.1038/nbt.2142
- 7. Zaidi SSA, Vanderschuren H, Qaim M, et al. New
plant breeding technologies for food security. Science. 2019;
363(6434): 1390–1391. .PMID:30923209,
https://doi.org/10.1126/science.aav6316
- 8.Oshiro N, Kuniyoshi K, Nakamura A, Araki Y,
Tamanaha K, Inafuku Y. A case of food poisoning due to ingestion of eggplant, Solanum
melongena, grafted on Devil’s trumpet, Datura metel [In Japanese]. Shokuhin
Eiseigaku Zasshi. 2008; 49(5): 376–379.
.PMID:19029791, https://doi.org/10.3358/shokueishi.49.376
- 9. Nakajima K, Hashimoto T. Two tropinone
reductases, that catalyze opposite stereospecific reductions in tropane alkaloid
biosynthesis, are localized in plant root with different cell-specific patterns.
Plant Cell Physiol. 1999; 40(11):
1099–1107. .PMID:10635114,
https://doi.org/10.1093/oxfordjournals.pcp.a029494
- 10. De Storme N, Geelen D. Callose homeostasis at
plasmodesmata: molecular regulators and developmental relevance. Front Plant
Sci. 2014; 5: 138. .PMID:24795733,
https://doi.org/10.3389/fpls.2014.00138
- 11. Melnyk CW, Schuster C, Leyser O, et al. A
developmental framework for graft formation and vascular reconnection in
Arabidopsis thaliana. Curr. Biol. 2015;
25(10): 1306–1318.
https://doi.org/10.1016/j.cub.2015.03.032.
- 12. Oparka KJ, Roberts AG, Boevink P, et al. Simple,
but not branched, plasmodesmata allow the nonspecific trafficking of proteins in
developing tobacco leaves. Cell. 1999; 97(6):
743–754. .PMID:10380926, https://doi.org/10.1016/S0092-8674(00)80786-2
- 13. Corbesier L, Vincent C, Jang S, et al. FT protein
movement contributes to long-distance signaling in floral induction of Arabidopsis.
Science. 2007; 316(5827): 1030–1033.
.PMID:17446353, https://doi.org/10.1126/science.1141752
- 14. Navarro C, Abelenda JA, Cruz-Oró E, et al.
Control of flowering and storage organ formation in potato by FLOWERING LOCUS T.
Nature. 2011; 478(7367): 119–122.
.PMID:21947007, https://doi.org/10.1038/nature10431
- 15. Lifschitz E, Eviatar T, Rozman A, et al. The
tomato FT ortholog triggers systemic signals that regulate growth and flowering and
substitute for diverse environmental stimuli. Proc Natl Acad Sci USA.
2006; 103(16): 6398–6403. .PMID:16606827,
https://doi.org/10.1073/pnas.0601620103
- 16. Yoo SC, Chen C, Rojas M, et al. Phloem
long‐distance delivery of FLOWERING LOCUS T (FT) to the apex. Plant J.
2013; 75(3): 456–468. .PMID:23607279,
https://doi.org/10.1111/tpj.12213
- 17. Paultre DSG, Gustin MP, Molnar A, Oparka KJ. Lost
in transit: long-distance trafficking and phloem unloading of protein signals in
Arabidopsis homografts. Plant Cell. 2016;
28(9): 2016–2025. .PMID:27600534,
https://doi.org/10.1105/tpc.16.00249
- 18. Kodama H, Miyahara T, Oguchi T, et al. Effect of
transgenic rootstock grafting on the omics profiles in tomato. Food
Safety. 2021; 9(2): 32–47. .PMID:34249588,
https://doi.org/10.14252/foodsafetyfscj.D-20-00032
- 19. Miyahara T, Nishiuchi T, Fujikawa N, et al. Omics
profiles of non-GM tubers from transgrafted potato with a GM scion. Food
Safety. 2023; 11(1): 1–20. .PMID:36970308,
https://doi.org/10.14252/foodsafetyfscj.D-22-00010
- 20. Ogawa T, Kato K, Asuka H, et al. Multi-omics
analyses of non-GM tomato scion engrafted on GM rootstocks. Food Safety.
2023; 11(3): 41–53. .PMID:37745161,
https://doi.org/10.14252/foodsafetyfscj.D-23-00005
- 21.Codex Alimentarius Commission
(CAC). Joint FAO/WHO Food Standards Program.
https://apps.who.int/iris/handle/10665/136259. Accessed on August 28,
2023.
- 22. Goldschmidt EE. Plant grafting: new mechanisms,
evolutionary implications. Front Plant Sci. 2014; 5: 727.
.PMID:25566298, https://doi.org/10.3389/fpls.2014.00727
- 23. Nakamura S, Hondo K, Kawara T, et al. Conferring
high‐temperature tolerance to nontransgenic tomato scions using graft transmission of RNA
silencing of the fatty acid desaturase gene. Plant Biotechnol J. 2016;
14(2): 783–790. .PMID:26132723,
https://doi.org/10.1111/pbi.12429
- 24. Rugini E, Silvestri C, Cristofori V, Brunori E,
Biasi R. Ten years field trial observations of ri-TDNA cherry Colt rootstocks and their
effect on grafted sweet cherry cv Lapins. Plant Cell Tissue Organ Cult.
2015; 123(3): 557–568.
.https://doi.org/10.1007/s11240-015-0860-x
- 25.Kodama H, Iwasa H, Hirai S, et al.
Amplification of small interfering RNAs in transgenic plants. Agriculture Research and
Technology. Bundgaard K, Isaksen L, eds. New York, USA: Nova Science Publishers; 2010:
379–395.
- 26. Moore L, Warren G, Strobel G. Involvement of a
plasmid in the hairy root disease of plants caused by Agrobacterium rhizogenes.
Plasmid. 1979; 2(4): 617–626. .PMID:231271,
https://doi.org/10.1016/0147-619X(79)90059-3
- 27. White FF, Taylor BH, Huffman GA, Gordon MP,
Nester EW. Molecular and genetic analysis of the transferred DNA regions of the
root-inducing plasmid of Agrobacterium rhizogenes. J Bacteriol. 1985;
164(1): 33–44. .PMID:4044524,
https://doi.org/10.1128/jb.164.1.33-44.1985
- 28. Koizumi M, Shimotori Y, Saeki Y, Hirai S, Oka S,
Kodama H. Effects of the 2b protein of Cucumber mosaic virus subgroup IB strain IA on
different transgene-induced RNA silencing pathways. Plant Mol Biol Rep.
2017; 35(2): 265–272.
.https://doi.org/10.1007/s11105-016-1020-0
- 29. Giri A, Narasu ML. Transgenic hairy roots. recent
trends and applications. Biotechnol Adv. 2000;
18(1): 1–22. .PMID:14538116,
https://doi.org/10.1016/S0734-9750(99)00016-6
- 30. Aragão MM, Alvarez MA, Caiafa L, Santos MO.
Nicotiana hairy roots for recombinant protein expression, where to start? A systematic
review. Mol Biol Rep. 2023; 50(5): 4587–4604.
.PMID:36917368, https://doi.org/10.1007/s11033-023-08360-1
- 31. McClain S, Herman RA, Islamovic E, et al. Allergy
risk assessment for newly expressed proteins (NEPs) in genetically modified (GM) plants.
J Reg Sci. 2021; 9(1): 67–75.
.https://doi.org/10.21423/JRS-V09I1MCCLAIN
- 32. Leclerc GM, Boockfor FR, Faught WJ, Frawley LS.
Development of a destabilized firefly luciferase enzyme for measurement of gene
expression. Biotechniques. 2000; 29(3):
590–591, 594–596, 598 passim. .PMID:10997273,
https://doi.org/10.2144/00293rr02
- 33.Noguchi T, Golden S. Bioluminescent and
fluorescent reporters in circadian rhythm studies. The Bioclock Studio,
Univ. California, San Diego, CA, USA, Mar. 2017.
https://ccb.ucsd.edu/the-bioclock-studio/education-resources/reporter-review/ReporterReviewPDF.pdf.
Accessed on August 28, 2023.
- 34. Takeba G, Takimoto A. Translocation of the floral
stimulus in Pharbitis nil. Bot Mag Tokyo. 1966;
79(942): 811–814.
.https://doi.org/10.15281/jplantres1887.79.811
- 35. Savage JA, Zwieniecki MA, Holbrook NM. Phloem
transport velocity varies over time and among vascular bundles during early cucumber
seedling development. Plant Physiol. 2013;
163(3): 1409–1418. .PMID:24072581,
https://doi.org/10.1104/pp.113.225359
- 36. Moneret-Vautrin DA, Kanny G. Update on threshold
doses of food allergens: implications for patients and the food industry. Curr
Opin Allergy Clin Immunol. 2004; 4(3): 215–219.
.PMID:15126945, https://doi.org/10.1097/00130832-200406000-00014
- 37. Liu J, Zhang L, Yan D. Plasmodesmata-involved
battle against pathogens and potential strategies for strengthening hosts. Front
Plant Sci. 2021; 12: 644870. .PMID:34149749,
https://doi.org/10.3389/fpls.2021.644870