2014 Volume 2 Issue 3 Pages 37-54
2014 Volume 2 Issue 3 Pages 37-54
Norovirus gastroenteritis remains a leading cause of morbidity and is still a major public health problem worldwide. The virus usually causes mild and self-limiting gastroenteritis symptoms in all age groups, mainly through the fecal-oral transmission route. However, the virus is highly contagious, relatively stable in the environment, and has long virus-shedding duration. These characteristics result in frequent outbreaks and make the control of the outbreaks difficult. In addition, the diversity of norovirus genetic characteristics and the emergence of new variants every one or two years may make the virus to escape the immunity. Moreover, the human immune response to norovirus infection still remains unclear and recurrent infection is possible. The diagnosis of norovirus infection is mainly based on reverse transcription polymerase chain reaction (RT-PCR), Loop-mediated isothermal amplification (LAMP), and immunochromatography (IC). There are no specific anti-norovirus drugs and treatment focuses mainly on supportive care, especially preventing and treating dehydration. Development of a norovirus vaccine has many difficulties and limitations, but some progress and some vaccine candidates have passed a phase II clinical trial. To prevent norovirus infection, hygiene is still the key point. Moreover, laws, regulations, and official guidelines are issued to help to manage norovirus infection and outbreak. This review provides an overview of norovirus infection and further discusses key characteristics of the virus, along with pathogenesis, clinical manifestation, diagnosis, treatment, prevention and control of the virus infection. We also view the virus through the lens of foodborne illnesses, and cover the current situation of the disease in Japan and research progress made so far.
In our daily lives, our basic needs are paramount: food, clothing, and shelter. Food is the most important, as we cannot live without food. However, food safety issues are always thought to be more vital than eating food.
Foodborne illness, also called “food poisoning,” occurs because of eating food contaminated with bacteria, viruses, and toxic or hazardous substances; it induces symptoms of nausea, vomiting, abdominal pain, diarrhea, fever, paralysis, rash, and other symptoms.
Viral foodborne illness is caused by norovirus, sapovirus, astrovirus, Aichivirus, rotavirus, hepatitis A virus, adenovirus, and so on, in the order of importance and incidence of outbreaks. These viruses can only grow or produce in the cells of humans and animals, but not in food1). The virus is excreted in the stool from human and animal hosts, and then contaminates river/sea water through sewage, either directly or by means of a sewage treatment facility. Many enteric viruses are excreted by humans and animals, and are frequently detected in fecal contaminated water. Hepatitis E also causes foodborne illness, and is found in the raw meat and blood of wild animals and pigs.
Norovirus or Campylobacter jejuni/coli has caused the highest number of food poisoning incidents in Japan in recent years (Fig.1), but the highest number of patients has been observed in illnesses caused by norovirus (Fig 2)2,3). Norovirus infections are widespread and clearly associated with almost all outbreaks. When the outbreak of norovirus infection increases, food-related norovirus infection is increased as well. In this article we mainly focus on norovirus and norovirus-related infection, especially foodborne infection. However, other viruses are also discussed.

The number of incidents of food poisoning by various causal agents in Japan from 1998 to 2012. The numbers by Norovirus and Campylobacter incidents are high. The incidents of Vibrio parahaemolytics and Salmonella species are in decline.

The number of patients of food poisoning by various causal agents in Japan from 1998 to 2012. The number of patients with norovirus food poisoning is remarkably high in comparison with other pathogens.
Noroviruses belong to the Caliciviridae family that includes five genera: Norovirus (NoV), Sapovirus (SaV), Lagovirus (LaV), Vesivirus (VeV) and Nebovirus4). In each genus, one or more species have been recognized. The first two genera contain mainly human viruses, while the other genera represent animal viruses. Although some additional genera are also reported, e.g., Tulane virus (simian), they have not yet been approved by the International Committee on Taxonomy of Viruses (ICTV)4,5).
Viruses of this family are non-enveloped and icosahedral of 23 to 40 nm in diameter including T = 1 to T = 3. The capsid includes the major VP1 protein around 60k Dalton and the minor VP2 protein. Genome is positive-sense single stranded RNA, which is around 7 to 8 kb in length, non-segmented, and polyadenylated at the 3′-terminal (Fig 3).
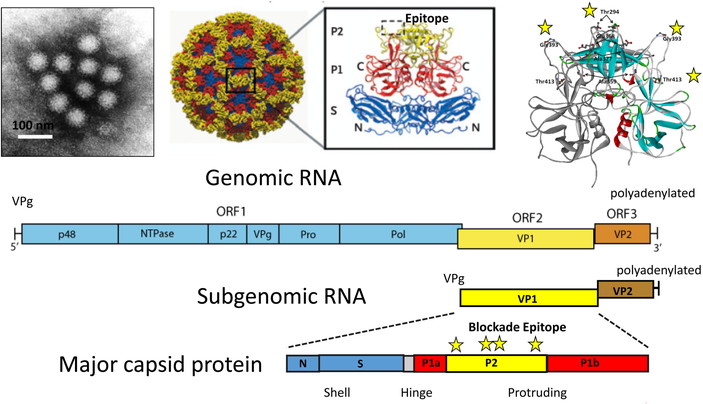
Structure of norovirus from stool samples by immune electron microscope
(left upper side); Norwalk VLP reconstruction (middle upper side, Ref 4). Epitope region of VP1 which interacted with carbohydrates of histo-blood group antigens, are shown as dotted squares; Three dimensional structure of norovirus Sydney strain and blockade epitope regions are shown as stars (upper right); Genomic RNA, subgenomic RNA and major capsid proteins are shown at lower side.
Viral gastroenteritis has been known since the 1940s, when unglazed pottery-filterable stool samples from infectious patients caused the disease in volunteers after ingestion. Norwalk virus, the prototype of norovirus, was first detected by using an immune electron microscope (IEM) in 1972 from the patients of the 1968 outbreak at an elementary school in Norwalk, Ohio6). Later, other gastroenteritis viruses, e.g., rotavirus, sapovirus, astrovirus and so on, were discovered1). Reference strains of norovirus, such as Hawaii virus, Snow Mountain virus and others, were discovered after the Norwalk virus and thought to have a large variability within the norovirus genus7).
Human norovirus is particularly difficult to study. Unlike other viruses, it is not possible to grow human norovirus using cultured cells or to infect animals. Human norovirus can only infect humans and proliferate only in human intestines. The virus cannot be proliferated even in human cultured cells. Therefore, research has not been well advanced on human norovirus.
Many researchers have tried to culture human norovirus, however only murine norovirus (Genogroup V in Norovirus) was cultured successfully in 20048). Recently, Tulane virus was too5). The complete RNA genome sequence of Norwalk virus was analyzed in 19909), and three open reading frames were identified: ORF1 (encoding nonstructural proteins, such as proteinase and polymerase), ORF2 (encoding the major capsid protein, VP1) and ORF3 (encoding the minor capsid protein, VP2) in the genome.
After the success of complete genome sequencing of norovirus, common primers were designed and used in reverse transcription polymerase chain reaction (RT-PCR) for clinical diagnosis7). Norovirus capsid protein, virus-like particle (VLP), was artificially produced by using a baculovirus expression system in 199210). Norovirus VLPs were used not only in basic research and diagnostic tools11) but also in vaccine trials for humans since 1996 with limited success. Thus, development of effective anti-norovirus agents for prevention and/or therapy still remains a big challenge today.
2–2. Characteristics and ReplicationNorovirus virion is non-enveloped. The capsid is composed of 180 VP1 proteins (90 dimers) with about 38–40 nm in diameter and T = 3 icosahedral symmetry. Small empty virions are about 23 nm in diameter, and composed of 60 VP1 proteins with T = 1 icosahedral symmetry. Its genome is single stranded positive sense RNA of 7.3 to 8.3 kb. Its 5′-end is covalently linked to VPg (viral genome-linked protein), and 3′-terminus is polyadenylated. The virion RNA is infectious and serves as viral messenger RNA. Cleavage of ORF1 polyprotein by the virus-encoded 3C-like cysteine proteinase yields the mature nonstructural proteins. Subgenomic RNA encodes the capsid protein (VP1) and VP2 minor structural protein. VP2 is expressed through RNA termination-reinitiation (Fig 3)7,12,13).
Lifecycle of norovirus is as follows (Fig 4); Norovirus attaches to host cells using carbohydrate receptor and probably another receptor, and enters into cells through clathrin- and caveolin-independent endocytosis. After that, the virus is uncoated and viral genomic RNA is released into the cytoplasm. VPg is then removed and viral RNA is translated into a processed ORF1 polyprotein to yield the replication proteins. A dsRNA genome is synthesized from the viral ssRNA(+) which is then transcribed and replicated to yield viral mRNAs and new ssRNA(+) genomes, respectively. Subgenomic RNA is translated to form major capsid protein (VP1) and VP2. Finally, new virus particles are assembled and released by cell lysis7,12,13).

1) Norovirus life cycle and change of host cell (Left side)
(1) Binding to the receptor (2) uncoating (3) translation of nonstructural polyprotein (4) cleaving of nonstructural protein (NSP) (5) reconstruction of NSP (6) transcription of (7) and (8) (7) replication of genomic RNA (8) replication of subgenomic RNA (9) translation for structural protein (10) assembly for virus structure (11) release of new virus (12) transportation of electrolyte and water (13) apoptosis (14) interaction with enteric nervous system (15) norovirus invasion to blood vessels
2) Inhibition of norovirus growth and symptoms (Right side)
[A] attachment inhibitor, [B] inhibitor against host factors which enhances norovirus growth, [C] protease inhibitor, [D] polymerase inhibitor, [E] compensation of electrolytes, [F] antiemesis
Norovirus RNA is replicated from 5′ end to produce entire complimentary RNA. The genome characteristically contains pGpU sequence at 5′ end that is covalently linked to a small protein, VPg. A short conserved region (CR) at the 5′ end is repeated internally in the genome near the beginning of a subgenomic-sized RNA transcript that is co-terminal with the 3′ end of the genome. When the cells are co-infected with two different strains, there is a possibility of starting replication of a strain A from 5′end and switch to strain B at the CR region. Chimeric virus may appear in nature. Like other single stranded RNA viruses, mutation also occurs in norovirus7,12). These chimeric viruses and mutant viruses cause outbreak so often7,14).
Capsid protein is analyzed by x-ray crystallography and computer analysis. Two proteins of shell (S) domain (inner part of the capsid) and protruding (P) domain (outer part and protrudes to the surface of the virus) are recognized. P domain is subdivided into P1 (P1a and P1b) and P2. P domain, particularly P2 subdomain is more variable in amino acid sequence. P2 region on the surface plays an important role as major antigenic and receptor binding site. P domain becomes dimer, P particle or small P particle. They are important for structure analysis, antigen–antibody recognition, carbohydrate binding and vaccine development. Recently, it has been shown that other regions on capsid also have minor antigenic properties7,13).
2–3. Virus ClassificationGenus Norovirus is classified into five genogroups (I to V). GI derives from human, GII from human and porcine, GIII from bovine, GIV from human, feline and canine and GV from murine. GI, GII and GIII are further subdivided into 9, 22, and 2 genotypes or more, respectively7,15). There is no evidence of zoonosis in nature16), however, gnotobiotic porcine can be infected with human norovirus.
Norovirus strain is designated like norovirus II/Hu/FR/2004/GII.P12_GII.3/Paris23[a: norovirus genogroup/b: host: Hu (human), Bo (bovine), Mu (murine), Po (porcine), Ca (canine)/ c: country code (ISO 2-letter code/d: year of sampling/e: genogroup and genotype (ORF1 and ORF 2)/f: variant name, city if necessary followed by a serial number]15).
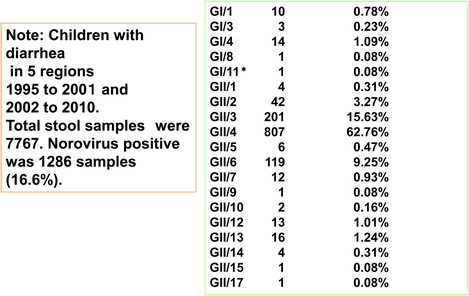
GII noroviruses, especially GII.4 followed by GII.3 genotype, are frequently detected in human stools17). GII norovirus is major but GI norovirus is also rarely detected from stools (Table 1). However, the detection rate of GI is higher in environment18,19). Animal noroviruses are also found from environment16).
GII.4 variant is very common worldwide and it spreads very rapidly within one or two years. Camberwell, Grimsby, Farmington Hills, Hunter, Sakai, Den Haag, New Orleans 2009 and Sydney 2012 were spread in 1987–1994, 1995–2001, 2002, 2004, 2004–2007, 2006–2008, 2009–2012, and 2012–2013, respectively17). Outbreak of one type strain often spreads for years. These outbreaks are related to not only viral mutation but also host immune activities17).
2–4. Histo-Blood Group AntigensHisto-blood group antigens (HBGAs) are thought to be the receptors or co-receptors of norovirus that allow the virus particle to attach and enter permissive cells20,21). HBGAs are a diverse family of carbohydrates that are expressed on red blood cells, vascular endothelial cells, and mucosal epithelia of the gastrointestinal, urogenital and respiratory tracts. They are often found in soluble form in biological fluids, such as blood, saliva, gastrointestinal secretes and milk.
These HBGAs are also found in some other animals like pigs22). HBGA like substances are also found on shellfish, plants and bacteria23,24,25). However, the expression of HBGAs seems to vary in different organs, mucosal tissues and fluids in humans and animals. Midgut gland cells of clams, mussels and oyster express HBGA like substances, which cause bio-accumulation of norovirus on the cell surface23). Even in oysters, expression of HBGA related substances is different in the oysters from different production areas26). Anti-norovirus antibody and HBGA like substances are used for a fixture to accumulate norovirus to clean the environment.
The HBGA binding interfaces, corresponding to the most outer surface of the capsid, are located at the top of the P dimer (Fig 3). The carbohydrate binding pockets involve several scattered amino acid residues in the P domain that form extensive hydrogen bond network with individual saccharides, and thus stabilizing the binding of HBGAs to the capsid protein27).
2–5. Pathogenesis of InfectionAfter norovirus was taken orally to the body, it replicates in the upper intestinal tract (duodenum and upper jejunum) (Fig 4). A broadening and blunting of the villi of the intestine occurs. Norovirus infection alters enzyme activity in infected intestinal cells. Transient malabsorption of fat, d-xylose, and lactose is reported during experimentally induced norovirus illness7,28).
Major shedding of norovirus from infected cells goes to intestinal lumen, but norovirus RNA is detected in serum and cerebrospinal fluid also29). Although the usual/actual method of norovirus pathogenesis is yet needed to be elucidated, it is thought that enteric infection in gnotobiotic piglets and primates when administered by the intravenous or oral route causes gastroenteritis28,30).
Expression of HBGA on intestinal cells is needed for virus attachment. Relation between HBGA and norovirus is complicated: the types of HBGA, norovirus strains, volume of norovirus and binding activities in host are interrelated. Non secretor of HBGA is less susceptible to norovirus infection. GII.4 strains, such as GII.4 2006b (Den Haag) and GII4 2012 (Sydney) strains, which cause outbreak worldwide, usually bind to all types of HBGA. However, how HBGA facilitates norovirus to enter into target cells, whether it is an attachment ligand only, and whether it plays a critical role of functional receptor, is not yet clear7,17,31).
Human immune response to norovirus infection also remains unclear. One reason for this obscurity is that human norovirus does not grow in cell culture. Unlike rotavirus infection, adults are found susceptible to recurrent norovirus infection even they have antibodies to norovirus. Probably, neutralizing antibodies to norovirus do not persist for long time, or antibodies to one genotype/strain may not be effective against others. Paradoxical evidence has demonstrated that norovirus infected volunteers suffering from diarrhea showed high level of antibody in convalescent sera compared to those without diarrhea32). Recent study showed that antibody avidity and blocking antibody are surrogate measurement of protecting norovirus7,32,33,34).
Cell-mediated immune response is also important for protection from the diseases. Both VLPs and norovirus induce γ-interferon. The dominant response of Th-1 is a characteristic of cell-mediated immunity. However, innate immune response against norovirus is also found in mice and humans7,35).
Norovirus is released from the enteric tract in feces and vomitus. Norovirus RNA are found in stools for several days and weeks. Average duration of norovirus shedding was 14 days with some individuals shedding up to 32 days. Infants often shed norovirus for longer time than adults. Immunocompromised patients release the virus for more than 30 days. When the immunocompromised condition is proved by treatment and organ transplantation, norovirus infectious condition is improved. Death is often reported in norovirus infection due to malnutrition.
2–6. Transmission 2–6-1. Transmission RouteNoroviruses are transmitted primarily through the fecal-oral route, either by direct person-to-person spread (88%) or by ingestion of norovirus contaminated food (10%) or water (1.5%)36). Noroviruses can also spread via a droplet route from vomitus. However, almost all infection in children is caused by unknown reasons (Fig 5). The outbreaks of norovirus are mainly reported in winter. The infection spreads in a family by person-to-person contact and then transmitted to kindergarten or other public institutions to cause outbreak.

Routes of norovirus infection: human and environment.
After the first person infected with norovirus in a community (primary infection), sometimes the other persons are infected via the first person (secondary infection). Usually, in the person-to-person infection outbreaks, the numbers of patients increase and then decrease gradually. However, outbreaks caused by food-borne infection occur at once and intensively, since the cohort is mostly exposed to the contaminated food at the same time.
Waterborne infection is caused by using municipal water, stored water, swimming pools, and so on. Chlorination of water is not enough to protect against norovirus infection. The foodborne norovirus outbreaks are often associated with the intake of shellfish including oysters. Vegetables and fruits are rarely contaminated with norovirus by water. However, the main cause of foodborne norovirus infection is by food handlers through their hands or clothes12,14).
The numbers of norovirus particles are sometimes more than 108 to 1010 in one g stool, and 104 to 105 particles in one g vomitus7). Norovirus is stable in environments: at pH 2.7 for 3 hours, at 4 °C with 20% ether for 18 hours, at 60 °C for 30 min37). Norovirus is more resistant to chlorination than poliovirus type 1, human rotavirus (Wa) and simian rotavirus (SA11)38). Higher concentrations of chlorination are needed (10 mg/L) to disinfect water from norovirus contamination. Norovirus RNA has been detected in the mouths of patients for several days after symptoms subsided, suggesting that norovirus can be transmitted through oral contact39), which also may play a vital role for outbreak.
2–6-2. InfectivityNoroviruses are highly infectious: as few as ten live virus particles are thought to be sufficient to cause infection. The symptoms may vary from asymptomatic to severe diarrhea, vomiting, and dehydration even after similar exposure to infectious units due to the complicated relationship of virus binding to host cells7).
2–7. Epidemiology 2–7-1. Age DistributionUnlike rotavirus, norovirus infections are seen in all age groups, although severe outcomes and longer durations of illness are most likely to be reported among the elderly, particularly in those living in institutional settings such as nursing homes7,40). In contrast, norovirus accounted for 12% of severe gastroenteritis cases (hospitalized cases) among children at less than five years of age and 12% of mild and moderate diarrhea cases (outpatient cases) among persons of all ages41). About 30 to 50% of diarrheal patients are thought to be infected with norovirus from our study in children in Japan17). The percentage is becoming higher in recent years as the prevalence of rotavirus is decreasing day by day after implementation of rotavirus vaccination.
2–7-2. Morbidity and MortalityPrecise numbers of mortality due to norovirus infection in the world are not certain. Data from the WHO estimated that approximately 200,000 deaths occur per year in children less than five years of age in the developing countries41). In contrast, most cases of death in Japan were among the elderly. During the outbreak in 2006 in Japan, almost 100,000 individuals were infected. At an outbreak in a nursing home, there was the report that some cases of the elderly died42). In addition to norovirus infection in adult and children, neonatal infection was also reported, especially preterm infants with necrotizing enterocolitis43,44).
Statistically, norovirus is recognized as the major cause of nonbacterial gastroenteritis outbreaks in United States and Europe37). In Japan, norovirus is also a major cause of viral gastroenteritis across the country2).
Norovirus infection is one of the causes of travelers’ diarrhea45,46). Norovirus transmissions in airplanes and cruise ships have been reported. Therefore, norovirus new strains or new variants are potentially easy to spread globally47,48).
2–7-3. SeroepidemiologyGastroenteritis is one of the most common diseases in humans. It is estimated that children have diarrhea around six times during the first two years. Almost all children have had norovirus infection before five years old12,14). One report noted that 91% of children have developed specific GII.4 norovirus antibodies at the age of five years old in Finland49). In Georgia, United States, 16% of the population in the community and 12% of outpatients were infected by norovirus50).
2–7-4. Molecular EpidemiologyMolecular analysis for norovirus detection has been developed after the full-genome sequence was determined first in 19909). Then, PCR and sequencing methods accelerated molecular epidemiological studies. In addition to capsid region, polymerase region and whole genome sequence are also used for classification of the viruses. Genogroup, genotype, subgenotype (cluster) and mutations were determined (2–2, 2–3). Although GI.1 was the first report9), GII.4 and GII.3 are major genotypes circulating in Japan (Table 2)17). Mixed infection among different norovirus strains have also been recorded. Recombination may be induced after mixed infection between different genogroups, genotypes and subgenotypes, especially GII.3. Recently, GII.4 variants have been reported as the major cause of several outbreaks worldwide. The emergence of new norovirus variants occurs every one or two years17). The comprehensive norovirus surveillance networks, including information, epidemiology, and databases, have been established at both the national and international levels, such as GatVirusWeb (Japan), CaliciNet (United States) and NoroNet (the Netherlands).
| Year | GI | GII.1 | GII.2 | GII.3 | GII.4 | GII.5 | GII.6 | GII.7 | GII.8 | GII.10 | GII.12 | GII.13 | GII.14 | GII.15 | GII.17 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1998–1999 | 0 | 0 | 0 | 28.33 | 51.67 | 0 | 18.33 | 0 | 0 | 0 | 1.67 | 0 | 0 | 0 | 0 |
| 1999–2000 | 1.11 | 0 | 5.56 | 18.89 | 64.44 | 2.22 | 5.56 | 0 | 0 | 0 | 1.11 | 0 | 1.11 | 0 | 0 |
| 2000–2001 | 5.63 | 1.41 | 0 | 19.72 | 56.34 | 2.82 | 5.63 | 0 | 2.82 | 1.41 | 1.41 | 0 | 1.41 | 1.41 | 0 |
| 2002–2003 | 5.66 | 0 | 0.94 | 3.77 | 75.47 | 0.94 | 11.32 | 0 | 0 | 0 | 1.89 | 0 | 0 | 0 | 0 |
| 2003–2004 | 1.72 | 0 | 13.79 | 43.1 | 34.48 | 0 | 6.9 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2004–2005 | 1.22 | 1.22 | 0 | 12.2 | 80.49 | 0 | 4.88 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2005–2006 | 0 | 1.96 | 1.96 | 47.06 | 43.14 | 0 | 3.92 | 1.96 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2006–2007 | 2.06 | 0 | 2.06 | 6.19 | 86.6 | 0 | 0 | 3.09 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2007–2008 | 5.6 | 0 | 0 | 4.8 | 73.6 | 0 | 4.8 | 0 | 0 | 0 | 0 | 0 | 11.2 | 0 | 0 |
| 2008–2009 | 0 | 0.8 | 3.1 | 5.4 | 71.3 | 0 | 14 | 0.8 | 0 | 0 | 2.3 | 0 | 2.3 | 0 | 0 |
| 2009–2010 | 0 | 0 | 11.43 | 14.29 | 64.29 | 0 | 0.48 | 2.86 | 0 | 0 | 2.38 | 0.48 | 3.33 | 0 | 0.48 |
| 2010–2011 | 0.47 | 0 | 7.91 | 27.91 | 54.88 | 0 | 0.93 | 0.47 | 0 | 0 | 0.47 | 0.93 | 6.51 | 0 | 0 |
| 2011–2012 | 0 | 0 | 3.72 | 15.96 | 70.21 | 0 | 1.6 | 0 | 0 | 0 | 0 | 2.66 | 5.85 | 0 | 0 |
| 2012–2013 | 2.17 | 0 | 3.72 | 0.62 | 86.38 | 0 | 1.86 | 1.86 | 0 | 0 | 0.31 | 0.62 | 2.79 | 0 | 0 |
Viral gastroenteritis is generally a mild and self-limiting illness. Patients sometimes feel devastating illness for 24 to 48 hrs. Severe dehydration occurs in high-risk individuals, such as infants, the elderly, and immunocompromised hosts4,7,14). Norovirus infection is rarely fatal due to malnutrition and dehydration.
Fever, diarrhea, vomiting, abdominal discomfort, anorexia, headache, and myalgia are common. The severity is different in individuals depending on infectious doses of the virus, host immunity, and so on. Usually the patients recover within a few days, but norovirus shedding continues for long time (14 days to one month).
There is no evidence of clinical severity differences among different strains of GII.4 by volunteer’s study51).
Co-infection with other viruses and bacteria, such as rotavirus and Campylobacter, is occasionally observed in norovirus-infected patients17,52).
General laboratory tests are usually normal in numbers of white blood cells, CRP, and electrolyte levels in mild diarrheal cases. Leukocytosis, mild elevation of CRP, imbalance of electrolytes and ketosis are recognized. Vesikari score for rotavirus is also useful for the scale of severity of norovirus infection53).
Norovirus RNA has been detected in human serum suggesting that norovirus invades from the gastrointestinal tract to blood circulation54). In addition, complications of convulsion, encephalopathy, necrotizing enterocolitis and nephropathy are also recognized29,44,55,56).
2–9. Diagnosis 2–9-1. Introduction of DiagnosisThe clinical manifestations of patients with diarrhea are generally not indicative of specific pathogen. Diagnosis of norovirus infection is difficult from clinical symptoms only. Norovirus infection is suspected by watery diarrhea, severe vomiting, age distribution, seasonality, outbreak, and intake of risky food4,12,14). The patient with gastroenteritis due to any pathogens is diagnosed as “infectious gastroenteritis.”
2–9-2. Laboratory DiagnosisGenetic diagnosis: Originally norovirus was found and diagnosed by IEM from stool samples6). However, this method is time consuming and requires highly skilled techniques. It is not suitable for routine use in clinics and hospitals. RT-PCR has been widely used since the 1990s57). Moreover, nested PCR and real time PCR are useful tools for detecting small amounts of the viruses of less than 100 genome copies. RT-PCR is available for further analysis of genome sequencing58). Commercially available PCR methods have been developed. Loop-mediated isothermal amplification (LAMP), transcription-reverse transcription concerted reaction (TRCR), Seeplex-MultiNA are commercially available12). Multiplex RT-PCR for detection of norovirus together with other gastroenteritis viruses is a useful method for detection of wide viral pathogens59).
Immunological diagnosis: In recent years, baculovirus expressed norovirus capsid proteins have been established to produce recombinant VLPs. The VLPs were useful for immunization of different animal species to produce monoclonal and polyclonal antibodies. A number of enzyme immunoassay (EIA) and immunochromatography (IC) kits for detection of norovirus have been developed and commercialized11,60). EIA and IC kits are useful at clinical laboratory and outpatient clinics. The sensitivity is similar to 1st round PCR. For the detection of norovirus with less than 104 or 105 genome copies/g of stool, 2nd PCR or real-time PCR are more reliable tool for norovirus diagnosis.
Detection from food and environment: There are several methods to extract norovirus genome from food and environmental sources61). Noroviruses from contaminated surface and environmental water are recently extracted by using the filter method62). Norovirus from contaminated food are extracted by anti-norovirus antibody coated beads or staphylococcus to extract virus61,63). After that, RNA extraction kits are used. Details are shown in 3–5.
2–10. TreatmentThere are no specific anti-norovirus drugs and vaccine at this moment. Treatment focuses on supportive care, especially preventing and treating dehydration. For dehydration, oral fluid or intravenous fluid for replacement therapy is used depending on the severity of the patients7,14). Anti-vomiting drugs are also used. For other complications, individual treatments for each disease are used. Probiotics have shown to be an effective treatment for viral infections in intestinal surface and antibody activation64). High titer human IgG or IgA against human norovirus is useful for immunoglobulin deficient patients. Bone marrow transplantation or organ transplant are useful to elevate humoral and cellular immune responses in immunodeficient patients65). According to the norovirus lifecycle, several candidate drugs have been developed to target the actions of polymerase and protease enzymes66).
2–11. VaccinesIn comparison with rotavirus vaccine, development of norovirus vaccine has difficulties and limitation. Human norovirus infects only human hosts, with the exception of gnotobiotic pigs. Symptoms are varied in individuals even when the same dose is inoculated. There is no animal model for human norovirus infection. Because of the inability to cultivate human norovirus, the development of a live vaccine is limited. Neutralizing antibody assays is difficult. Cross protection may not be obtained even in the same genotype, since humans infected with one subgenotype may still be infected with another subgenotype. Moreover, new variants of norovirus occurred frequently year by year67). Until today, norovirus vaccines have been developed under the following concepts. Recombinant VLPs (rVLPs) are used as immunogens. Volunteer experiments are needed to evaluate for phase I and II.
Because the production of large scale of rVLP is difficult, adjuvants are needed for enhancing and directing the adaptive immune response to rVLP vaccine antigen. Enhancement of cross reactive antibodies between norovirus GI and II is relatively difficult. The component of the vaccine is GII only or combination of GI and GII. Routes of vaccination are also important and need to be considered for norovirus vaccine challenging, either per oral, nasal, intramuscular, or subcutaneous.
A candidate vaccine finished a phase II clinical trial. The vaccine composes with whole capsid of GI.1 and GII.4 and is injected intramuscally with adjuvant68). Another vaccine also finished phase II. The vaccine is comprised of whole capsid GI.3 and GII.4, and is injected intramuscally without adjuvant. The vaccine is also combined with human rotavirus G133). The other vaccine has just started. This vaccine is composed of GII.4 P particle administrated intranasally with adjuvant. The vaccine also contains rotavirus recombinant VP669). A norovirus vaccine is now planning to be evaluated by a phase III clinical trial.
2–12. Infection ControlRecent outbreaks of norovirus gastroenteritis show that infection control is only the way to stop or prevent the disease. The three ways to stop norovirus from spreading are: (1) do not bring norovirus from outside the environment (target area); (2) do not grow norovirus inside the area; and (3) do not carry out from the area. There are guidelines for the prevention and control of norovirus gastroenteritis outbreaks70,71). At the time of foodborne infection, rapid identification of the source and route of the viral spreading are important. The contaminated foods are collected and discarded. The industry or the shop is closed and cleaned. Infected food handlers are prohibited to work until the disease is under controlled. Norovirus is generally resistant to detergent or ethanol substrates. However, the neutral and alkaline conditions of ethanol derivatives are more effective against murine norovirus than the acidic state in vitro72). Hypochlorite is an effective substance to disinfect norovirus at different concentrations for cleaning2,3).
There are many sources of foodstuff that are possible to be contaminated with noroviruses. Shellfish such as oyster is the most common. Although the shellfish-borne infection has been well-controlled, most recently there has been a slight increase of oyster-borne norovirus infection in some reports (personal communication from Tokyo Metropolitan Food Safety Information Evaluation Committee). There are outbreaks from hands of apparent and latent norovirus-infected patients.
Sporadic infection occurs in early winter time in infants (Fig 6)12,17). For these cases, the spreading of norovirus may not occur via foodborne contamination. The reason of the infection is still unknown.

Monthly distribution of norovirus genotypes in Japan.
Outbreaks of foodborne norovirus infection have increased recently. There are several reasons: (1) Original food is contaminated with norovirus. For example, oysters and other shellfish are contaminated at the production area. Norovirus contaminated water comes from drainage canal to the river and finally goes to the sea. The viruses contaminate shellfish directly through water or through contaminated plankton. The patients get infections by consuming the contaminated shellfish. (2) Food is not originally contaminated. However the viruses from contaminated food handler’s hands are transferred to other foods. Here the foodborne disease with norovirus is described from several points of view.
3–2. Source of Foodstuff and Food of Norovirus ContaminationThe following foods have been reported in Food Poisoning Statistics by Department of Food Safety, Ministry of Health, Labor and Welfare, Japan3). Oysters, raw oysters, raw oysters in vinegar, oyster gratin (Oysters are contaminated with infectious norovirus): Other shellfish; soy-marinated freshwater clams, short-neck clams marinated in aged wine, shellfish with salad (Shellfish are contaminated with infectious norovirus. Shellfish-related norovirus infection was reported in Asia, especially in Japan73).): Ready to eat foods; bread roll sandwich with croquette filling, pork cutlet lunch, vegetable salad, boiled spinach, chicken and pork cutlet, spaghetti and salad, spinach with whitebait, cabbage role, bean-starch vermicelli salad, sautéed carrot, asparagus bacon, radish namul, crab in vinegar (They are contaminated by washing water, food handlers’ and cooks’ hands):Cake and confectionaries; bread with soybean flour, bread, cake, butter-enriched roll, Japanese sweet, rice cake, crepe, almond jelly (They are contaminated with food handlers’ hands): Drinking water from wells and small-scale water supply system. Norovirus foodborne diseases have also been reported to be associated not only with shellfish but also with berries and leafy greens74,75).
3–3. Food Poisoning StatisticsThe following figures were reported in Food Poisoning Statistics by Department of Food Safety, Ministry of Health, Labor and Welfare, Japan3).
(1) Annual variation in the number of incidents from food poisoning by major bacterial and viral pathogenic substances was shown (Fig.1). Salmonella and Vibrio parahaemolyticus infections were major foodborne infections until 2000. However, control measures taken afterward for prevention of the bacterial contamination and growth in fish, shellfish, and chicken eggs resulted in decreases in outbreaks of these infections. After that, Campylobacter and norovirus became the major pathogens. Chicken meat is contaminated with Campylobacter, especially C. jejuni. Reports of enterohemorrhagic E. coli infection and Staphylococcus aureus infection are low but are still important diseases.
Incidents of foodborne disease due to norovirus increased from November. The peak of the incidents was noted in January, after which they gradually decreased3). Facilities involved were restaurants (73%), hotels (9%), catering operations (6%), offices (5%), factories (2%) and schools (2%) (Fig 7)3).

Norovirus foodborne disease in different facilities from 2008 to 2012. Left side shows difference in incidents and right side shows difference in patients.
Reference: http://www.mhlw.go.jp/topics/syokuchu/04.html and http://www.mhlw.go.jp/topics/syokuchu/03.html
(2) Annual variations in the number of patients from food poisoning by major bacterial and viral pathogenic substances were shown in Fig 2. The number of patients infected with norovirus is extremely high in comparison with the other five pathogens. The peak incidence was in January3). Patients were reported to be infected at restaurants (43%), catering operations (29%), hotels (13%), factories (6%), offices (4%) and schools (2%) (Fig 7)3).
(3) Incidents and genogroups of norovirus in Tokyo from 2010 to 2012 are as follows76). Outbreaks caused by norovirus GII was accounting for about 75–80% of total norovirus outbreaks, while norovirus GI outbreak was around 8%. Outbreaks of co-infection of norovirus GI and GII were 7–10%. Sapovirus outbreak and group A rotavirus outbreak were 2% in each. Incidents of norovirus GI infection outbreaks occurred more frequently than those of sporadic infection (Detail data not shown). The genotypes of norovirus infection in the outbreak and the sporadic infection were similar.
(4) Annual variations in the number of incidents from food poisoning by major bacteria, viruses, and other pathogenic substances in schools are shown (Table 3). The number of norovirus incidents in schools is not so high as those in restaurants and catering operations (Fig 7). Salmonellosis and Campylobacter infections were also found.
| Pathogens | Year | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 03 | 04 | 05 | 06 | 07 | 08 | 09 | 10 | 11 | 12 | Total | |
| Norovirus (SRSV) | 3 | 2 | 3 | 5 | 4 | 2 | 1 | 0 | 1 | 5 | 26 |
| S. enteritidis | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 3 |
| Salmonella spp. | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Campylobacter spp. | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 2 |
| Bacillus cereus | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Histamine | 0 | 0 | 0 | 1 | 0 | 3 | 0 | 0 | 1 | 0 | 5 |
| Pathogenic E. coli O 44 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
| Not Determined | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Total | 5 | 3 | 4 | 7 | 5 | 6 | 1 | 2 | 2 | 5 | 40 |
A large outbreak of foodborne norovirus infection by contamination of the virus in bread occurred in Hamamatsu City in Japan, during January 2014. More than 1,000 children were infected in 15 elementary schools. An investigation conducted by the city government suggested that bread slices were contaminated through handling by contaminated gloves at a bread plant. Because the handlers were asymptomatic norovirus patients, they probably pulled on their plastic gloves with contaminated hands (IASR 35: 164–165, 2014, http://www.nih.go.jp/niid/ja/iasr-sp/2297-related-articles/related-articles-413/). Norovirus outbreaks at several institutions (schools, kindergartens, and nursery schools, etc.) are now an important problem in Japan.
3–5. Detection Method of Norovirus from Foodstuffs and FoodThere are many sources of food contaminated with norovirus; i.e. salad, soft fruits, shellfish, bread, and water. When food contamination is suspected to cause outbreaks, the stool, hands, and clothing from patients and workers must be examined. Facilities and environment at the factory are also examined. If contamination in shellfish is suspected to cause the outbreaks, midgut gland of the shellfish is the specimen of choice for the virus detection because the viruses accumulate in this organ.
There are several methods to extract viruses from shellfish61). Shellfish are crushed and emulsified. Then, the emulsion is centrifuged. The supernatant is collected and centrifuged again and used for RNA extraction. For example, in the Pansorbin Trap method, formaldehyde fixed Staphylococcus aureus and norovirus specific antibody (or human IgG which contains norovirus antibody) are added to the supernatant. After mixing and centrifugating, the precipitation is dissolved with a small volume of buffer63). The solution is used for RNA extraction, then (RT-) PCR is performed. Amorphous calcium phosphate is also used instead of the Pansorbin Trap method77).
Contaminated water (sewage) is another source. The water is first centrifuged to remove impurities. Then, the supernatant is used in the next step. In the polyethylene glycol (PEG) method, PEG 6,000 is added to the supernatant and mixed together. After centrifugation, the supernatant is discarded. The pellet is dissolved in small volume of phosphate-buffered saline and used for further RNA extraction78).
The negative charge membrane concentration method is another way to collect norovirus from water. After starting by adjustment pH to 3.5 with magnesium chloride, the supernatant is absorbed to negative charge membrane and filtrated with diluted beef extract62).A wiping sheet or paper is used to examine the virus from the surface of the materials78). The wiped sheet is then immersed in a buffer. Then, the PEG method or the Pansorbin Trap method is used for the next step63). The freezing and thawing method is another simple way79).
Each country has its own health policies to manage foodborne diseases in different levels. In Japan, when an outbreak of foodborne disease occurs, physicians, patients, or anyone at all can inform the local health center of the outbreak. The center contacts the prefectural and municipal institutes of health to examine the causative agents for the outbreak, the number of patients, the contamination of the environmental source and so on. The outbreak is reported to the local government, then to the Ministry of Health, Labor and Welfare and National Institute of Infectious Disease, according to the scale of outbreak. The reporting network is similar to the system of NIH (National Institutes of Health) and CDC (Center of Disease Control and Prevention) in the United States. There are food safety committees in different levels such as local (city, prefecture, and so on) government and central government (Food Safety Commission of Japan). The WHO (World Health Organization) and FAO (Food and Agriculture Organization) and OIE (Office of International Epizootics) are supported by the specialists and institutes in each country. Codex Alimentarius (International Food Standard Committee) is a joint office of WHO and FAO. Several research institutes were established to formulate working programs and policies on food safety; i.e. FDA (Food Drug Administration) in United States, NIHS (National Institute of Health Sciences) in Japan, NIPHE (National Institute for Public Health and the Environment) in the Netherlands, EFSA (European Food Safety Authority) in Europe, FSANZ (Food Standards Australia and New Zealand) and so on.
Laws and regulations: Codex standard is the international standard; Food Safety Basic Act, Food Sanitation Act, Infectious Diseases Control Law, Abattoirs Act, Poultry Slaughtering Business Control and Poultry Meat Inspection Act exist in Japan; As guidelines, Codex guidelines, FAO/WHO guidelines, Food factory hygiene management manual, and school lunch hygiene management can be accessed through the internet.
4–2. Principle for Prevention of Foodborne Norovirus DiseaseThe key principle for controlling foodborne norovirus outbreak is “do not bring norovirus from outside,” “do not proliferate norovirus at the place,” and “do not bring norovirus to outside.” The guidelines for prevention of foodborne norovirus infections are as follows;
1. Proper food preparation
Some foodstuffs, such as shellfish, are contaminated not only on the surface but also in the midgut gland. Therefore, washing outside only is not enough. Shellfish, dishes, clothes, and anything else that might be contaminated should be heated over 85 or 90 °C for at least 90 seconds.
2. Washing hands and others
Diarrheal stools and vomitus are mostly in liquid form. They are easily and stickily attached to the surfaces of hands, toilet, knobs, etc. The attached viruses are not so easy to remove from the surface. The contaminated surfaces of vegetables and fruits must be washed carefully and repeatedly by clean water. A low concentration of hypochlorite is also useful.
3. Treatment of stools and vomitus
Stools and vomitus should be treated as early as possible. If the treatment is delayed, the infection will spread broadly. When treating stools and vomitus, one must wear masks and gloves. Sodium hypochlorite solution is used at 1,000 ppm and then at 200 ppm with white paper towels. (Details are not described) *White is important for sodium hypochlorite being used for only disinfectant.
4. Disinfection of cookware and floor
Sodium hypochlorite is used at 200 ppm for washing.
5. Improvement of equipment
Automatic switch or handle of faucet is recommended. Automatic doors for toilets are recommended. Separate toilets between workers and customers (visitors) are recommended. Hand washing should be done inside a toilet room.
6. Regular checks of the hands of asymptomatic food handlers are recommended.
7. Food plants, restaurants, and facilities for providing meals are regularly inspected by food safety staffs in local area. Cooking equipment must also be inspected.
8. Management at a hospital
As management of patients and patients’ rooms, patients are separated from healthy persons. Equipment for hand-washing should be kept inside of the room. Patients wash hands with disinfectant frequently when they go outside. Tables, telephones, handrails, door knobs, and other places where the patients touch or vomit are washed by sodium hypochlorite or hypochlorous acid water. Patients should use disposable dishes. Health care providers and caregivers use disposable masks, globes and clothes. The toilet should be separated from the healthy persons.
Norovirus is a major cause of foodborne disease. The infectivity is very high and the virus is stable in the environment. Norovirus evolution is quick and new variants of norovirus, especially GII.4 variants occurred year by year. Sensitive and rapid detection methods for norovirus have been developed, such as RT-PCR including real time RT-PCR and IC. Most recently, several norovirus vaccine candidates have been developed. Norovirus vaccine is expected to have success in near future, which will help to prevent and control norovirus infection effectively. Moreover, there are several candidate anti-norovirus drugs theoretically, but none prove to be effective at this moment.
Foodborne diseases related to norovirus infection remain responsible for the high levels of morbidity and mortality in the general population, especially in at-risk groups, such as infants, young children, the elderly, and immunocompromised patients. To detect norovirus from foodstuffs, there are several methods to extract norovirus genome by using techniques of genetic analysis. Heated water, washing and cleaning with soap, alcohol derivatives and sodium hypochlorite are used in appropriate way for disinfection. Careful handling of patients is also needed to decrease outbreaks in hospitals and families.
We thank Drs. Ariful Hoque at Dhaka University, Pattara Khamrin at Chiang Mai University, Aksara Thongprachum and Nguyen Dinh Tran at the University of Tokyo, and Shoko Okitsu at Nihon University for their comments and help with the manuscript. We also thank the Food Safety Committee of Japan for its support in writing the manuscript.