2015 年 3 巻 1 号 p. 16-29
2015 年 3 巻 1 号 p. 16-29
A novel type of encephalopathy associated with the ingestion of Sugihiratake mushroom (Pleurocybella porrigens) occurred in patients with chronic renal failure treated on hemodialysis in fall, 2004 in Japan. To clarify the mechanism of encephalopathy onset, we, for the first time, purified the cyanogen glycoside fraction (CG) from Sugihiratake mushroom using reversed phase high-performance liquid chromatography and hydrophilic interaction chromatography. Furthermore, we investigated single dose toxicity of the CG in an adenine-induced rat model of chronic renal damage (CRD). Pathological examination of kidneys indicates the development of CRD. Oral administration of the CG induces the accumulation of thiocyanate in the hemolyzed blood and brain in CRD rats, although no morphological changes were found in the brain. No further enhancement of kidney damage is observed after the oral administration of the CG in CRD rats. This is the first experimental report to suggest that acute encephalopathy, induced by Sugihiratake mushroom intake in the patients with chronic renal failure, is associated with intoxication of cyanide and thiocyanate, presumably produced metabolically produced after the ingestion of Sugihiratake mushroom.
Sugihiratake (Pleurocybella porrigens) is a thin, tongue-shaped mushroom that grows on cedar and pine trees in autumn and is widely distributed in Japan1). It has a characteristic flavor and is popularly consumed in Japan with processed foods such as miso soup (fermented bean paste soup) and deep-fried as tempura. In fall, 2004, an outbreak of encephalopathy exclusive to patients with chronic renal failure treated on hemodialysis, occurred after Sugihiratake intake in northern areas of Japan including Akita, Yamagata, and Niigata Prefectures.
To date, several clinical reports are available on the encephalopathy induced after ingestion of Sugihiratake. The reported symptoms are tremor, difficulty in walking, consciousness disturbance, and then followed by status epileptics. Diffuse lesions in the basal ganglia and multiple ringed lesions in the cerebral cortex are also observed by magnetic resonance imaging (MRI). The exact etiology of encephalopathy associated with Sugihiratake intake remains unclear.
Suzuki and Kawagishi et al2,3,4) isolated to characterize a novel lectin and several amino acids from Sugihiratake, and proposed that the lectin might be related to the acute encephalopathy. Takano et al5) reported that intraperitoneal injection of a P. porrigens extract resulted in shock and acute toxic death with severe hemolysis in mice. They also noted a possible association with unidentified thermolabile high molecular weight constituents. Most of aqueous polysaccharides or high molecular weight constituents are negligibly absorbed and transported as intact to the brain. The role of polysaccharide on instigating encephalopathy thus remains uncertain.
We have determined cyanide contents of Sugihiratake samples collected from various regions of Japan where experienced incidents of encephalopathy in fall, 2004. High levels of cyanide were detected in some of the samples collected in 2004. In addition, some reports suggested sublethal doses of cyanide to induce encephalopathy6,7,8,9,10,11). These data suggest a possible association between cyanide intake and encephalopathy onset. No experimental evidence to support the hypothesis for the association is, however, available between cyanide intake and the onset of encephalopathy.
In the present study, we have, for the first time, purified the cyanogen glycoside fraction (CG) from wild Sugihiratake using reversed phase high-performance liquid chromatography (RP-HPLC) and hydrophilic interaction liquid chromatography (HILIC) and investigated its single-dose toxicity in an adenine-induced rat model of chronic renal damage (CRD). The present study suggests the possible association of acute encephalopathy induced by Sugihiratake mushroom intake in the patients having renal damage with cyanide and thiocyanate intoxication. The cyanide and thiocyanate are possibly produced metabolically through the digestion of cyanogen glycoside contained in Sugihiratake mushroom.
Wild Sugihiratake samples (approximately 3 kg) were collected in Higashihouden, Mogamimachi, Yamagata prefecture in October 2009 and extracted as follows. Freeze-dried samples were ground to a fine powder using a grinder (Retsch GmbH, Haan, Germany), and then samples (107.2 g) were extracted three times with 1072 mL each of methanol-water (70:30, v/v) in a sonication bath using 2 L flask. And then, the obtained clear supernatant was collected by centrifugation (2330 × g) at 4ºC for 30 minutes and evaporated under reduced pressure at 40°C. The obtained dried extract (approximately 5 g) was resuspended in 70% methanol.
The CG was consecutively purified from the 70% methanol extract using RP-HPLC, dialysis and HILIC as follows. The 70% methanol extract (total 500 mg, 50 mg for each fractionation) was consecutively fractionated using RP-HPLC, a dialysis membrane, and HILIC. A series of the fractionation was repeated ten times (for each 50 mg). RP-HPLC was performed using a Unison US-C18 column (5 µm ID, 250 × 10 mm; Imtakt Corporation, Kyoto, Japan) at a flow rate of 1.2 mL/min on an apparatus composed of a HITACHI L-2130 pump and HITACHI UV detector L-2400 (Hitachi, Ltd., Tokyo, Japan). The column was maintained at 40ºC using a water bath. The mobile phases were (A) H2O and (B) 95% acetonitrile under the following gradient conditions: 0–20 min (B: 0%), 20–30 min (B: 0%→100%), 30–50 min (B: 100%), 50–60 min (B: 100%→0%), 60–80 min (B: 0%). The content of cyanogen glycoside in each fraction was determined as below. Fractions containing large molecular weight (MW) molecules were subjected to dialysis with a MW cutoff (MWCO) of 3500 (Spectra/Por® Dialysis Membrane MWCO: 3500; Spectrum® Laboratories, Inc., Shiga, Japan). Fractions containing small MW molecules (MW < 3500) were concentrated and subsequently subjected to dialysis with a MWCO of 1000 (Spectra/Por® Dialysis Membrane MWCO: 1000; Spectrum® Laboratories, Inc.).
The fraction containing small MW molecules (MW < 1000) was fractionated by HILIC using an XBridgeTM Amide column (3.5 µm ID, 4.6 × 250 mm; Nihon Waters K.K., Tokyo, Japan) at a flow rate of 0.5 mL/min and column temperature of 30ºC. The mobile phase consisted of (A) 95% acetonitrile and (B) H2O under the following gradient conditions: 0–70 min (B: 0%→77%), 70–80 min (B: 77%→0%), 80–100 min (B: 0%). Fractions were collected every one minute using a MODEL 2110 fraction collector (Bio-Rad Laboratories, Inc., CA, USA), and determination of cyanogen glycoside contents in each fraction was carried out as below.
2-2. Determination of Cyanogen Glycoside Contents by Fluorometric DetectionDetermination of cyanogen glycosides using HPLC with fluorometric detection was performed according to the method of Akiyama et al12) with minor modifications. Briefly, the reaction mixture (1 mL) containing 50 mM sodium citrate (pH 5.2), 52 mU of β-glucosidase from almonds (Sigma-Aldrich Japan K.K., Tokyo, Japan), and 10 µL of sample was placed in the outer well of a Conway cell (Shibata Scientific Technology Ltd., Saitama, Japan). After incubation at 37ºC for three hours, 1 mL of 10% sulfuric acid was added and mixed with the reaction mixture. The liberated hydrogen cyanide was recovered with 1 mL of 0.1N sodium hydroxide in the center chamber for one hour.
2-3. Establishment of Adenine-induced CRD RatsTen male, 9-week-old Crl:CD (Sprague Dawley (SD)) specific pathogen free (SPF) rats (the body weights: 288–315 g) were purchased from Charles River Laboratories Japan, Inc. (Tokyo, Japan). According to the procedure of Nagano et al13), SD rats with CRD were generated by feeding diets containing 0.75% or 0.5% (w/v) of adenine for four weeks. In brief, the animals were maintained in an experimental facility under temperature- and light-controlled conditions, and were allowed free access to diet and ultraviolet-exposed tap water. One week prior to the study start, rats were fed a CE-2 diet (CLEA Japan, Inc., Tokyo, Japan). During the study period, they were allowed free access to diet containing 0.75% adenine (Sigma; minimum 99%, Cat. No. A8626) for the first two weeks. For the next two weeks, they were allowed free access to 0.5% adenine diet. Feeding of the adenine-containing diets for a total of four weeks (28 days) established a rat model of CRD. The adenine-containing diets were composed of CE-2 diet supplemented with 0.75 or 0.5% adenine. The adenine-containing diets were purchased from CLEA Japan, Inc. (Tokyo, Japan). One rat died on the eighth day of adenine diet feeding, presumably due to the renal damage and decrease of feed intake, although the reason of the death remains unclear. The remaining nine 14-week-old CRD rats (the body weights: 217–294 g) were divided into three groups: control, low-dose CG and high-dose CG groups.
2-4. Single Dose Toxicity Test of CG Extracted from Sugihiratake in CRD RatsThe CGs were dissolved in distilled water and orally administered by gavage to CRD rats according to the following groups: control group, only distilled water; low-dose group, 360 mg CG/kg body weight (corresponding to 0.31 mg sodium cyanide/kg body weight); high-dose group, 2840 mg CG/kg body weight (corresponding to 2.44 mg sodium cyanide/kg body weight). All test samples were orally administered to rats at 20 mL/kg body weight. The administration levels were determined in consideration of the amount extracted from Sugihiratake and the toxicity information of the Registry of Toxic Effects of Chemical Substances, i.e., LD50 of sodium cyanide in rats is 4.7 mg/kg or 6.4 mg/kg. The rats were allowed free access to CE-2 diet for 24 hours before administration of the test samples. Blood was collected from rats via the jugular vein without anesthesia before administration, and 8 hours and 24 hours after administration. The collected blood (0.5 mL per rat) was added to 0.5 mL of 0.1M sodium hydroxide, stirred well and hemolyzed. The hemolyzed blood was stored at −30ºC, and then subjected to determinations of cyanide or thiocyanate content according to the method of Akiyama et al12).
The rats were euthanized under deep anesthesia by inhalation of isoflurane 24 hours after administration, and the brain, heart, lungs (including bronchial tubes), liver, pancreas and kidneys were excised. Brains were cut in two sagittal sections. The left parts of the brain were fixed in 10% neutral buffered formalin solution. The right parts of the brain were weighed and then added to an equal amount of 0.1M sodium hydroxide. The brains were homogenized, stored at −20ºC, and analyzed for cyanide and thiocyanate contents. The heart, lungs (including bronchial tubes), liver, pancreas and kidneys were also fixed in 10% neutral buffered formalin and routinely processed for histopathological analysis. Sections at frontoparietal cortex, parietal cortex/hippocampus, and cerebellum pons of each brain were stained with hematoxylin-eosin (HE).
Statistical Analysis All values are expressed as the mean ± standard deviation (SD). A p value of less than 0.05 was considered to be statistically significant. Differences between values were evaluated using Bonferroni’s method after analysis of variance (ANOVA).
The CG was consecutively purified using RP-HPLC, dialysis and HILIC. The cyanogen glycosides were collected using a fraction collector and monitored using HPLC with fluorometric detection. When samples were subjected to RP-HPLC, 92.5% of cyanogen glycosides were found in fractions eluted from 1 to 20 minutes (data not shown). With dialysis, 44.7% of cyanogen glycosides were found in fractions containing small MW molecules (MW < 1000) (data not shown). When HILIC was used to separate small MW molecules, the fractions eluted between 35 and 43 minutes showed a high amount of cyanogen glycoside (Fig. 1). The fractions collected from 35 to 43 minutes were freeze-dried and dissolved in distilled water to be the CG. The CG was then evaluated in the single dose toxicity test using CRD rats.

Cyanide (%) content of fractions obtained from hydrophilic interaction chromatography.
The vertical axis represents the ratio of cyanide content detected in each fraction to total cyanide content detected in the sample before the injection to HPLC.
To prepare an adenine-induced rat model of CRD13), adenine (0.75 and 0.5%)-containing diet was administered to rats for four weeks. Body weight changes during the four-week feeding of adenine-containing diet and single dose treatment with CG are shown in Table 1. Body weight of rats showed minimal changes throughout the study. Features of adenine-induced CRD are described in the pathological findings. Necropsy at the end of the study revealed swollen yellowish kidneys. Histopathological examination of kidneys revealed crystal nephropathy characterized by severe accumulation of 2,8-dihydroxyadenine (DHA) crystals in dilated tubules with foreign body giant cells, inflammatory cells and interstitial fibrosis, indicating successful generation of an adenine-induced CRD rat model.
| IndividualNo. | Animal*No. | Quarantine and acclimation periods | Adenine-component feed feeding period | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ---- 0.75% --- | -------- 0.5% -------- | |||||||||
| Day | 1 | 5 | 9 | 1 | 8 | 15 | 22 | 28 | ||
| 1 | 1302 | 298 | 334 | 355 | 362 | 281 | 270 | 270 | 259 | |
| 2 | exc. | 308 | 355 | 381 | 384 | 267 | - | - | - | |
| 3 | 1203 | 295 | 344 | 366 | 368 | 268 | 282 | 285 | 282 | |
| 4 | 1103 | 288 | 317 | 332 | 334 | 257 | 220 | 219 | 220 | |
| 5 | 1301 | 304 | 337 | 368 | 378 | 270 | 282 | 285 | 256 | |
| 6 | 1303 | 297 | 343 | 366 | 375 | 321 | 303 | 298 | 282 | |
| 7 | 1101 | 315 | 354 | 385 | 388 | 293 | 258 | 260 | 262 | |
| 8 | 1201 | 306 | 348 | 368 | 378 | 301 | 279 | 279 | 261 | |
| 9 | 1102 | 303 | 340 | 361 | 362 | 275 | 271 | 270 | 273 | |
| 10 | 1202 | 305 | 345 | 363 | 369 | 266 | 268 | 264 | 254 | |
| Mean | 302 | 342 | 365 | 370 | 280 | 270 | 270 | 261 | ||
| S.E. | 2 | 3 | 5 | 5 | 6 | 8 | 8 | 6 | ||
Unit: g.
No.2: died on day 8 of adenine-component feed feeding period (266 g body weight).
* Identification numbers after allocation to treatment groups (exc.: excluded from this study).
Quarantine period: 5 days.
Acclimation period: 9 days (with quarantine period).
Rats were clinically evaluated after CG administration. One of three rats in the high-dose group appeared weak and lay down in a prone position one hour after administration. Two hours later, the animal had recovered. No other rats showed any abnormalities.
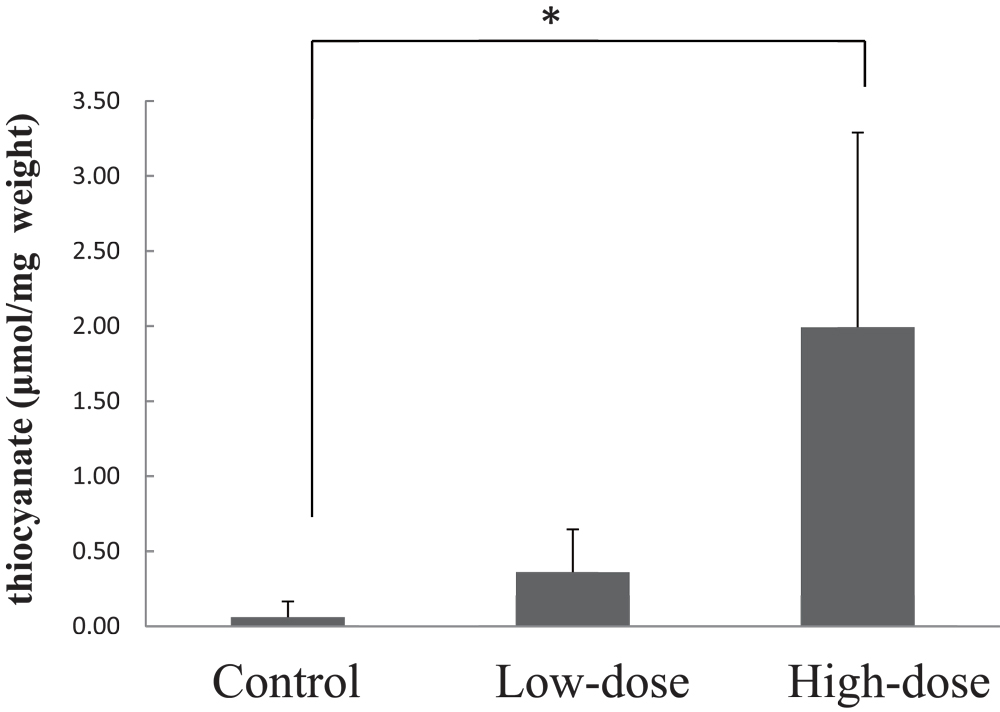
3-2-2. Changes of Cyanide and Thiocyanate Levels in the Hemolyzed BloodAfter administration of CG to CRD rats, cyanide and thiocyanate levels in the hemolyzed blood were determined. As shown in Fig.2, slight increases in the cyanide levels between 0–8 hours were observed in both the low-dose and high-dose groups, although the increase between 0–24 hours was minimal. On the other hand, as shown in Fig.3, the increase of the thiocyanate levels in the hemolyzed blood between 0–8 hours and 0–24 hours in both the low-dose and high-dose groups, were significantly higher than those of the control. In addition, the increases of thiocyanate levels between between 0–8 hours and 0–24 hours in the high-dose group were significantly higher than those of the low-dose group.
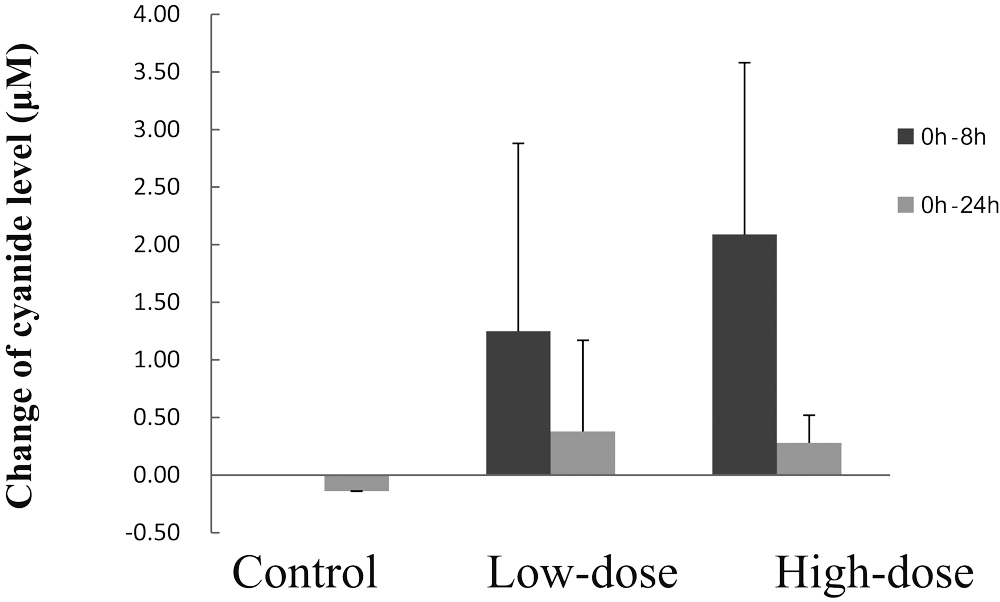
Temporal changes of cyanide levels in the hemolyzed blood in an adenine-induced rat model of CRD.
Control represents the control group administered distilled water alone, Low-dose represents the low-dose group administered 360 mg cyanogen glycoside fraction (CG)/kg body weight (corresponding to 0.31 mg sodium cyanide/kg body weight), and High-dose represents the high-dose group administered 2840 mg CG/kg body weight (corresponding to 2.44 mg sodium cyanide/kg body weight). Bars represent means ± standard deviation (3 rats/group).
 The bar represents the change of cyanide level in the hemolyzed blood from 0 h to 8 h
after administration of CG
The bar represents the change of cyanide level in the hemolyzed blood from 0 h to 8 h
after administration of CG
 The bar represents the change of cyanide level in the hemolyzed blood from 0 h to
24 h after administration of CG
The bar represents the change of cyanide level in the hemolyzed blood from 0 h to
24 h after administration of CG

Temporal changes of thiocyanate levels in the hemolyzed blood in an adenine-induced rat model of CRD.
Control represents the control group administered distilled water alone, Low-dose represents the low-dose group administered 360 mg CG/kg body weight (corresponding to 0.31 mg sodium cyanide/kg body weight), and High-dose represents the high-dose group administered 2840 mg CG/kg body weight (corresponding to 2.44 mg sodium cyanide/kg body weight). Bars represent means ± standard deviation (3 rats/group). Asterisks indicate significant differences from control values (**p < 0.01).
 The bar represents the change of thiocyanate level in the hemolyzed blood from 0 h to
8 h after administration of CG
The bar represents the change of thiocyanate level in the hemolyzed blood from 0 h to
8 h after administration of CG
 The bar represents the change of thiocyanate level in the hemolyzed blood from 0 h to
24 h after administration of CG
The bar represents the change of thiocyanate level in the hemolyzed blood from 0 h to
24 h after administration of CG
After CG administration to CRD rats, brain levels of cyanide and thiocyanate were determined. Brain cyanide levels in the brain were very low and not detectable, only thiocyanate levels are thus shown in Fig. 4. The mean thiocyanate level in the low-dose group appeared to be higher than that of the control. The high-dose group showed significant increase in thiocyanate levels compared to the control group.
Thiocyanate content in the brains of adenine-induced CRD rats.
Control represents the control group administered distilled water only, Low-dose represents the low-dose group administered 360 mg CG/kg body weight (corresponding to 0.31 mg sodium cyanide/kg body weight), and High-dose represents the high-dose group administered 2840 mg CG/kg body weight (corresponding to 2.44 mg sodium cyanide/kg body weight). Bars represent means ± standard deviation (3 rats/group). Asterisks indicate significant differences from control values (*p < 0.05).
The bar represents brain thiocyanate levels after administration of CG
Macroscopically, no abnormalities were detected in the brain of CRD rats administered CG. The kidneys of CRD rats were swollen and yellowish in color, and were comparable in appearance in the control, low- and high-dose groups (Fig. 5). The thymus appeared shrunken in all groups, the arcus aortae of one control rat was whitish-gray in color, and the glandular stomach/colon in the control and high-dose groups was grayish in color.

Macroscopy of the normal kidney (left), and Adenine represents the adenine-induced crystal nephropathy observed in CRD rats (right).
Microscopic observation did not reveal any treatment-related findings or degenerative/necrotic lesions in any parts of the cerebrum or the cerebellum in all treatment groups (Fig. 6). Histopathological changes corresponding to the macroscopic abnormalities in the kidneys were confirmed (Fig. 7). The kidneys of CRD rats showed similar morphological features to chemically-induced obstructive nephritis, an accepted model of CRD13). Briefly, the normal kidney structure was disrupted and replaced with an abnormal one (Fig. 7 A and B). Many distal convoluted tubules were obstructed with DHA crystals, observed as brown to black dots (Fig. 7 D), and showed basophilic changes. Dilated distal renal tubules and inflammatory reactions including neutrophils and giant cells surrounding the obstructed tubules were noted (Fig. 7 D). Cellular infiltration of neutrophils and mononuclear cells as well as fibrosis were also detected in the interstitium. Histopathological findings are summarized in Table 2. The pathological findings in kidney indicate the development of CRD in the present study. In addition, augmentation of CRD treatment-related changes were not observed in the kidneys of CRD rats administered CG. Systemic mineralization in the artery or interstitium of various tissues, known as secondary hyperparathyroidism, which is associated with CRD, was noted in the aorta, heart, lung and glandular stomach in some animals. However, the extent and location of the mineralization was not different in the CG treated rats. All groups showed atrophy of the small thymus, suggesting systemic deterioration as a result of CRD (Table 3).
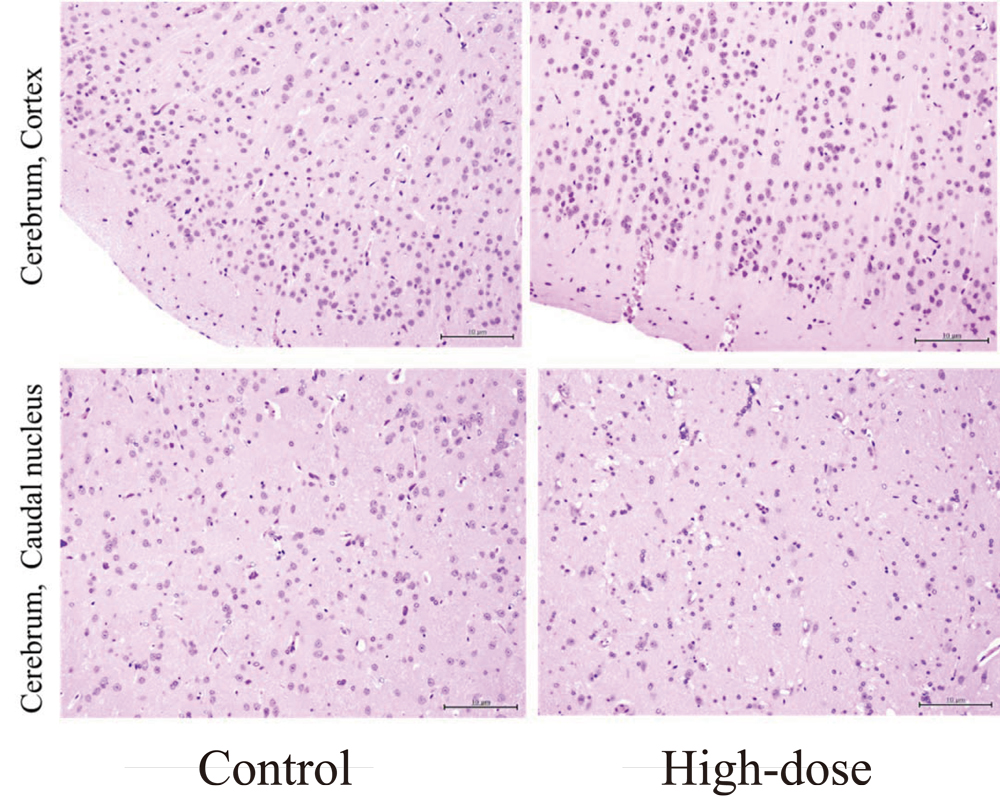
Histopathology of the cerebrum cortex and cerebrum caudal nucleus of CRD rats after administration of Sugihiratake extract.
Control represents the control group administered distilled water alone, and High-dose represents the high-dose group administered 2840 mg CG/kg body weight (corresponding to 2.44 mg sodium cyanide/kg body weight. Microscopically no treatment related abnormalities were observed in neurons and glial cells. x100. Hematoxylin-eosin staining.
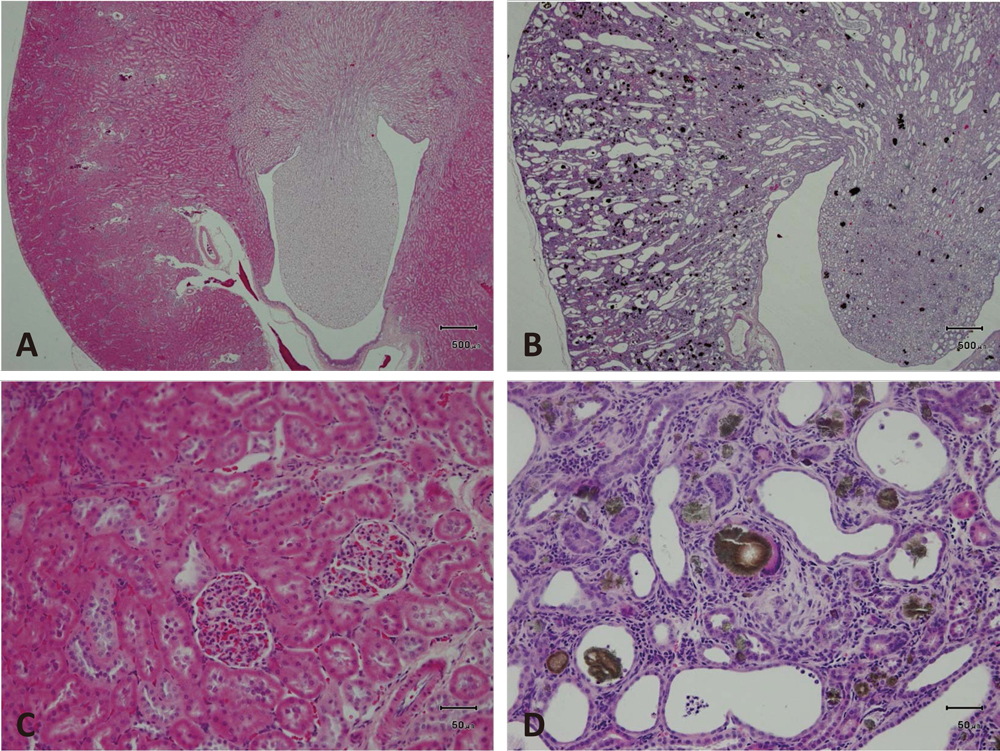
Histopathological images of the kidneys of CRD rats.
A-D, Histopathological changes in the kideny. A and C, Normal structure obtained from a normal adult rat kidney. B and D, Adenine-induced chronic renal damage. The normal structure was destroyed, and brown to black dots, adenine crystals, were diffusely distributed throughout the kidney at low magnification (x10). C and D, Higher magnification (x100) of A and B. C. Normal structure. D, Various sizes of crystals are accumulated in the dilated lumen of renal tubules or deposited in the tubules. Inflammatory cells infiltrate the interstitium. Hematoxylin-eosin staining.
| Test substance | Control | Sugihiratake extract | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 360 mg/kg | 2840 mg/kg | |||||||||||
| Animal No. | 1101 | 1102 | 1103 | 1201 | 1202 | 1203 | 1301 | 1302 | 1303 | |||
| Heart | ||||||||||||
| Myocardium | ||||||||||||
| Focal calcification | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | |||
| Coronary artery | ||||||||||||
| Cellular infiltration, mononuclear cell | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | |||
| Calcification | 0 | 0 | 3 | 3 | 0 | 0 | 3 | 2 | 1 | |||
| Arcus aorta | ||||||||||||
| Tunica media, calcification | 2 | 0 | 3 | 3 | 1 | 0 | 3 | 2 | 1 | |||
| Tunica intima, cellular
infiltration, neutrophil / mononuclear cell |
1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | |||
| Lung | ||||||||||||
| Alveolar septa, calcification | 0 | 0 | 3 | 0 | 0 | 0 | 2 | 0 | 0 | |||
| Bronchial smooth muscle, calcification | 0 | 0 | 2 | 0 | 0 | 0 | 1 | 0 | 0 | |||
| Kidney | ||||||||||||
| Artery | ||||||||||||
| Calcification | 1 | 0 | 2 | 2 | 1 | 0 | 2 | 2 | 1 | |||
| Renal tubule | ||||||||||||
| 2,8-dihydroxyadenine (DHA) crystals | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | |||
| Tubular dilatation | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | |||
| Tubular basophilic change | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | |||
| Cellular infiltration, neutrophil | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | |||
| Giant cells | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |||
| Interstitium | ||||||||||||
| Cellular infiltration,
neutrophil / mononuclear cell |
1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |||
| Fibrosis | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | |||
0: No change, 1: Slight, 2: Moderate, 3: Marked.
No significant changes were detected: Spleen, Liver.
| Test substance | Control | Sugihiratake extract | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 360 mg/kg | 2840 mg/kg | |||||||||||
| Findings | Animal No. | 1101 | 1102 | 1103 | 1201 | 1202 | 1203 | 1301 | 1302 | 1303 | ||
| Kidney | ||||||||||||
| Enlargement, pale yellow | + | + | + | + | + | + | + | + | + | |||
| Thymus | ||||||||||||
| Small size | + | + | + | + | + | + | + | + | + | |||
| Arcus aorte | ||||||||||||
| Grayish white | - | - | + | - | - | - | - | - | - | |||
| Coronary artery | ||||||||||||
| Grayish white | - | - | + | - | - | - | - | - | - | |||
| Glandular stomach | ||||||||||||
| Grayish white | - | - | + | - | - | - | + | - | - | |||
| Colon | ||||||||||||
| Grayish white | - | - | + | - | - | - | + | - | - | |||
-: No change, +: Change.
We, for the first time, purified the CG from the autumn mushroom Sugihiratake using RP-HPLC and HILIC. We further investigated the single dose toxicity of the CG in a rat model of adenine-induced CRD. The cyanide and thiocyanate levels in the hemolyzed blood were increased in CRD rats administered CG; a substantial increase of thiocyanate was observed in both the low-dose and high-dose CG groups compared to control. In the preliminary experiments using normal SD rats orally administrated with potassium cyanate, increases of blood cyanide and thiocyanate levels are not observed at eight hours after the administration (data not shown). The study suggests that cyanide from cyanogen glycoside is metabolized to thiocyanate until eight hours after administration probably by rhodanese in the liver and kidneys. Also, the thiocyanate levels in the hemolyzed blood continued to increase for one day after administration in CRD rats. In addition, the study suggests that the thiocyanate produced from cyanogen glycoside was transported to and distributed in the brain because of the increase of thiocyanate levels in brain after administration of CG. Therefore, these findings suggest that cyanogen glycoside contained in Sugihiratake was absorbed and metabolized by β-glucosidase distributed in the organs or tissue, and then transported to the blood and brain. It is known that cyanide is metabolized to thiocyanate as a final metabolite by rhodanese expressed in various organs such as liver and kidney. Accordingly, we presume that patients with renal dysfunction who consumed Sugihiratake mushroom exhibit accumulation of cyanide and thiocyanate because of inhibited thiocyanate urine excretion14).
One of the three rats in the high-dose group was weak and lay down in a prone position one hour after CG administration; two hours later, the animal recovered. This symptom may be attributed to the high cyanide level due to intake of cyanogen glycoside in the CG; however, any treatment-related morphological changes were not observed in the brain. Histopathological examination confirmed the successful establishment of a rat model of adenine-induced CRD using the protocol in the present study. In addition, CG extracted from Sugihiratake did not appear to enhance CRD or induce any treatment-related changes in the kidney in the present study.
A number of clinical case studies focused on the outbreak of acute encephalopathy in fall of 2004 in Japan15,16,17,18,19). All cases involved patients with varying degrees of renal dysfunction who had consumed Sugihiratake. Common clinical symptoms at onset included weakness and tremor or dysarthria and subsequent intractable focal motor seizures, resulting in the generalized status epilepticus or a comatose state. Magnetic resonance imaging (MRI) examination of the brain revealed diffuse lesions in the basal ganglia and multiple ringed lesions in the cerebral cortex. Nomoto et al18) reported a case of encephalopathy associated with Sugihiratake ingestion in a patient with diabetic nephropathy. Results of brain MRI and cerebrospinal fluid examination led to the conclusion that the observed demyelination was likely associated with Sugihiratake intake.
These clinical case studies discussed similarities in the symptoms and characteristics of Sugihiratake intake-induced encephalopathy with those induced by exposure to respiratory toxins such as manganese, methanol, 3-nitro propionic acid (3-NPA) and cyanide. However, there are no reports that manganese and 3-NPA were detected in high levels in Sugihiratake, and these compounds were not detected in our investigation (data not shown). Although symptoms of methanol intoxication are mainly characterized by visual disturbance and abnormalities in the optic nerve, there are no reported clinical case studies dealing with these symptoms. Therefore, this led us to consider that Sugihiratake-induced encephalopathy is unlikely to associate with intoxication of manganese, 3-NPA and methanol.
We quantified the cyanide content of Sugihiratake samples collected from specific areas of Japan during the fall of 2004; the samples contained cyanide in the range of N.D. (not detected)-114.0 µg/g12). The findings suggested that the cyanide in samples is present in sodium or potassium salt form, and that the cyanide contained in the Sugihiratake might be associated with the onset of encephalopathy in patients with chronic renal failure treated on hemodialysis, documented in fall, 2004 in Japan.
Many edible plants, including agriculturally important species such as cassava, flax, sorghum, alfalfa, peaches, almonds, and beans, are cyanogenic20). The precursor of cyanide production is cyanogen glycoside. Cassava flour from central Africa contains large quantities of linamarin, a cyanogen glycoside. In diets where cassava is a staple, the daily human consumption is equivalent to about one-half the lethal dose, and is thought to be the reason for the widespread incidence of chronic neurological disorders, called “konzo”, frequently found in this area21). In addition, cyanide production has been observed in a wide range of fungal species such as Phaeolepiota aurea, Rozites caperatus, and Leucopaxillus giganteus, in addition to P. porriggens (Sugihiratake) 22). In the present study, we have purified the cyanogen glycoside as a source of cyanide from P. porriggens, the Sugihiratake mushroom. The reason why these fungal species produce cyanide and the identity of the precursor of cyanide remain unknown, although some reports have suggested that fungal cyanide production is associated with snow mold disease and fairy ring disease in some plants22).
While the studies dealing with cyanide-induced encephalopathy have been reported6,7,8,9,10,11), Smith et al6) demonstrated that long-term low-dose cyanide ingestion caused histological changes in the central nervous system of rats. In a clinical study of patients that attempted suicide with cyanide, Rachinger et al9) showed that intermittent exposure produced histological changes in the central nervous system, causing cerebral damage primarily to the basal ganglia. These symptoms are consistent with the cases reported in Akita Prefecture of Japan.
Furthermore, a recent study has shown that cyanide and thiocyanate are accumulated in the blood of hemodialysis patients due to cigarette smoking23). Cyanide is metabolized to thiocyanate by rhodanese and excreted in urine. The detoxification of cyanide is an essential defense mechanism, and thiocyanate synthesis can be accelerated under cyanide-loaded conditions such as cigarette smoking and eating certain plants including cyanogen glycoside or cyanide24).
Recently, the sodium iodide symporter, mediating active iodide transport into the thyroid, the lactating mammary gland and placenta, was found to be competitively inhibited by thiocyanate from maternal smoking for the intoxication of thiocyanate25). In addition, a recent study has also shown that the risk of cerebral infarction is significantly increased in individuals with high thiocyanate concentrations in the hemolyzed blood26).
Thiocyanate enhances the action of glutamate for a subclass of neuronal glutamate receptors involved in neurodegenerative disorders27). Some evidence exists that thiocyanate modulates (RS)-α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor function in rodent brain tissue in vitro and in vivo28,29,30). Several receptor-binding studies have shown that potassium thiocyanate enhances the affinity of [3H] AMPA for its receptor and increased [3H] glutamate binding to the AMPA receptor without altering the number of binding sites31,32,33,34,35,36). Quantitative autoradiography shows that potassium thiocyanate similarly increases the number of high-affinity [3H] AMPA-binding site without altering the binding affinity36). These findings are consistent with the idea that cyanide-induced elevated thiocyanate provides a coalition, in which synaptic glutamate increases its potency selectively at AMPA receptors, thereby resulting in selective damage to AMPA-enriched neurons in the motor cortex.
Cyanide and thiocyanate intoxication has been known to occur with medications such as the hypotensive drug nitroprusside (NP), which consists of an iron molecule bound to five cyanide molecules and one nitric oxide molecule. After NP is administered, nitric oxide is rapidly liberated in blood during infusion, whereas the cyanide molecules are released gradually. Therefore, the use of NP can potentially cause cyanide and thiocyanate intoxication as side effects. Nessim et al37) reported a case of thiocyanate toxicity secondary to NP infusion in which the patient was successfully treated with continuous venovenous hemodiafiltration. It was suggested that thiocyanate can lead to toxicity, especially in the context of impaired renal function, although it is less toxic than cyanide. Symptoms of thiocyanate toxicity include confusion, hallucination, delirium, seizures, fatigue, weakness, miosis, tinnitus and rash, and are closely similar to the symptoms of Sugihiratake-induced acute encephalopathy. These reports imply that cyanogen glycoside intake from foods may cause the accumulation of thiocyanate in the blood and brain of patients with chronic renal failure treated on hemodialysis.
In the findings of present study considering the context of previous reports, we suggest the possibility of association of acute encephalopathy induced by Sugihiratake mushroom intake in the patients having renal damage with cyanide and thiocyanate intoxication. In addition, we suggest that the cyanide and thiocyanate are possibly produced metabolically through the digestion of cyanogen glycoside contained in Sugihiratake mushroom. However, the human capacity for cyanide detoxification, and for cyanide and thiocyanate toxicities from food exposure in hemodialysis patients, has not been fully investigated36). Therefore, the further research is necessary to elucidate the structure of cyanogen glycosides contained in Sugihiratake and to clarify the further mechanism of encephalopathy onset by cyanogen glycoside intake.
We thank Dr. Taku Nagao, Dr. Yukihiro Goda and Dr. Satoshi Kitajima for their useful suggestions. This study was supported by a grant from the Food Safety Commission, Cabinet Office, Government of Japan (Research Program for Risk Assessment Study on Food Safety, No 0903).