2015 年 3 巻 1 号 p. 1-15
2015 年 3 巻 1 号 p. 1-15
Evidence has been accumulating that environmental chemicals contained in foods can cause neurodevelopmental disabilities. However, regular developmental neurotoxicity (DNT) studies require large numbers of animals for detection of subtle dose-response changes, and it is urgent concern to establish a rapid and efficient evaluation system of DNT. Evidence from our recent studies points to the notion that adult neurogenesis in the hippocampus may represent vulnerable endpoint to cause DNT. Adult neurogenesis is the postnatal process of continued production of new neurons through the adult stage in the brain. Monitoring of granule cell lineage in the subgranular zone (SGZ) and γ-aminobutyric acid (GABA)-ergic interneurons in the dentate hilus is effective for detection of target cell populations of DNT as manifested by disruption of neurogenesis. Especially, reelin-expressing GABAergic interneurons are a useful marker to detect disruption of the migration and correct positioning of developing neurons following impairment of neurogenesis. Because axon terminal toxicants target granule cell lineage population growing dendritic processes, there may be common target mechanisms between the DNT and adult-type neurotoxicity affecting mature nervous system. Adult neurogenesis may also be a suitable endpoint for detection of DNT in a scheme of standard regular 28-day toxicity study. In other words, adult-type neurotoxicity could be detected by measuring the cellular responses in adult neurogenesis. Moreover, it should be stressed that there may be epigenome toxicity mechanisms to affect the process of hippocampal neurogenesis involving both neuronal stem cells and interneuron subpopulations, with continued disruption through the adult stage. These findings suggest that hippocampal neurogenesis is considered to be a critical target of environmental neurotoxicants contained in foods.
It is reported that around 13 percent of children were reported to have ever had a developmental disability and in most cases these disabilities affect the nervous system1). Such neurodevelopmental disorders involving inherent neural dysfunctions include pervasive developmental disorder (autism spectrum disorder), learning disability, cerebral palsy, seizures, attention-deficit hyperactivity disorder (ADHD) and mental retardation. Their causes are mostly unknown. Among them, there is an increasing trend in prevalence of ADHD and learning disability in a survey conducted in the United States1). The disabilities in these disorders are permanent in nature, and there are no effective therapeutic choices; they become a heavy burden to families and to society.
There is a growing concern on infantile health effects by exposure to environmental contaminants through ingestion of foods, and developmental neurotoxicity (DNT) by ingested chemicals is one of the urgent global concerns because of lifelong disability. Neurodevelopmental disabilities caused by environmental chemicals are mostly subclinical ones2). Environmental chemicals, such as lead, methylmercury, arsenicals, polychlorinated biphenyls (PCBs), and toluene, can cause developmental neurotoxicities in man2). An expert committee from the US National Research Council concluded that around 3% of developmental neurotoxicities are considered to be the direct effect due to exposure to such environmental chemicals, and another 25% are regarded to be the outcome of interactions between individual genetic susceptibility and environmental factors3). These estimates were based on scarce information about neurotoxicity and could therefore underestimate the true prevalence of chemically-induced abnormalities2).
The developing human brain is inherently much more susceptible to injury caused by toxic agents than is the brain of an adult4). If a developmental process of the brain is inhibited or disrupted, there is little potential for later repair, and the consequences can therefore be permanent5). Generally, during embryonic and fetal period, organogenesis stage is most sensitive for induction of serious morphological defects against exposure to exogenous agents. On the other hand, brain development continues even after organogenesis period, and a considerable amount of cellular and physiological events occur during postnatal development, such as neurite outgrowth, neuronal guidance, synaptogenesis, myelination and maturation in the central nervous system6). Of note, the human brain continues to develop postnatally, and the period of heightened vulnerability therefore extends over many months, through infancy and into early childhood. Although most neurons are formed by the time of birth, growth of glial cells and myelination of axons continues for several years5,7). It is also noted that reorganization of synaptic plasticity of neuronal networks in the brain continues during postnatal period in children8). Therefore, all of these neurodevelopmental events are suggested to be vulnerable endpoints to cause DNT.
During development, neonates and infants have particularity in the variety and content of foods daily ingested as well as in the behavior under the living environment, such as crawling and hand-to-mouth behavior at the living floor9). Therefore, these immature populations have inherent exposure risk to environmental contaminants different from those of adult populations. For example, infants have limited variety in foods, and they are fed initially and solely breast milk and then baby foods made largely of fruits and dairy products. Breast milk may be contaminated with persistent lipophilic chemicals, including specific pesticides and halogenated industrial compounds, and limited variety of daily foods may increase the exposure risk to chemicals contaminated in particular foods. Persistent lipophilic chemicals, such as PCBs, accumulate in maternal adipose tissue and are passed on to the infant via breast milk, resulting in infant exposure that exceeds the mother’s own exposure by 100-fold on the basis of body weight10). The susceptibility of infants and children to chemicals is further enhanced by their increased exposures and absorption rates, and diminished ability to detoxify many exogenous compounds, relative to that of adults11). Moreover, infants intake higher amount of foods per body weight basis reflecting higher body burden on exposed chemicals than adults.
There are hundreds of chemicals that have been proved to cause neurotoxic insults in adults2). There also are many other chemicals that have been shown to be neurotoxic experimentally. However, the neurotoxic effects of such chemicals in the developing humans are largely unknown and they are not subjected to regulatory control to protect from exposure of humans including children. There are two major impediments to prevention of DNT by chemical exposure. One is the lack of available mechanism-based models in testing chemicals for DNT and another being the high level of proof required for regulation. Novel and precision approaches that capture the unique vulnerability of the developing brain are necessary for testing and control of chemicals.
The hippocampus is belonging to the limbic system and plays essential roles in the memory formation for short and long term, as well as consolidation of information, and spatial navigation. The hippocampal formation comprises a group of cortical tissues including the cornu ammonis and dentate gyrus (Fig. 1). The dentate gyrus is the primary input into the hippocampal formation receiving connections from the entorhinal cortex. The subgranular zone (SGZ) of the dentate gyrus, a subregion of the hippocampus located at the base of granule cell layer (GCL), uniquely continues to generate new neurons during postnatal life12). This process is called as “adult neurogenesis”. Adult neurogenesis in the dentate gyrus is critical for some forms of learning and memory and is modulated by pathological conditions13). and some neurological and psychiatric disorders are associated with malformation and dysfunction of the dentate gyrus14). Of note, hippocampal neurogenesis is prominent in adult rodents as well as primates and humans15,16,17,18).
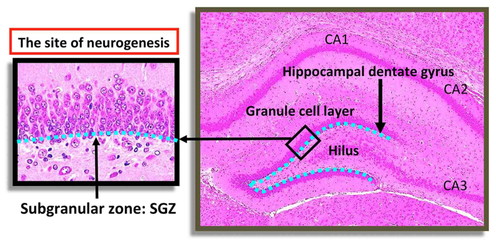
Neurogenesis in the dentate gyrus of the rat hippocampus.
Current DNT studies require large numbers of animals for detection of subtle dose-response changes. For screening purposes of many new chemicals, smaller scale studies, preferably with short-term experiments, employing suitable and sensitive neurodevelopmental endpoints focusing on histopathological parameters need to be established. To establish a rapid screening system for developmental neurotoxicants, it is reasonable to focus on cellular response in the hippocampal adult neurogenesis and its regulatory system using rodent models19,20,21,22,23,24,25,26). Because adult neurogenesis includes all processes from neuron production to their maturation, it can be hypothesized that monitoring of the dentate gyrus may provide a valuable tool for the detection of developmental neurotoxicants. The aim of this review is to overview our recent study findings regarding the availability of hippocampal neurogenesis to monitor as a critical target of environmental neurotoxicants contained in foods. At first, this review provides the information regarding the availability of monitoring the γ-aminobutyric acid (GABA)-ergic interneurons distributed in the hilus of the dentate gyrus for detection of chemicals that can affect neurogenesis and migration using a rat model of developmental hypothyroidism. The next topic is regarding the presence of vulnerable targets of DNTs in the process of neurogenesis by neurotoxicants that can injure mature nerve tissue using acrylamide and glycidol as examples in rats. The third topic is regarding the availability of adult neurogenesis for detection of DNT in the framework of 28-day regular toxicity study using rats. As a last topic, an example of epigenome toxicity in the process of neurogenesis is provided in a mouse model of manganese (Mn)-induced neurogenesis toxicity.
In mammals, adult neurogenesis consists of multiple processes, including a number of developmental phases such as the self-renewal of stem cells, facilitation of cell division of precursor cells to produce newborn granule cells, and the subsequent differentiation and migration of new granule cells to the correct position within the GCL (Figs. 2 and 3)12). Stem cells (type-1 cells) exist in the SGZ of the dentate gyrus and divide slowly to produce intermediate progenitor cells, a type of transient amplifying cells27,28,29). Undifferentiated intermediate progenitor cells (type-2a and type-2b) divide rapidly to produce neuronally committed intermediate progenitor cells (type-3) and react to stimuli that influence neuronal generation in the hippocampal dentate gyrus28). Type-3 intermediate progenitor cells produce postmitotic immature neurons, and those neurons that come through regulatory period integrate into the GCL as granule cells27). By monitoring of cellular subpopulations in the SGZ and GCL as well as cell proliferation activity and apoptosis in the SGZ, it is possible to detect changes in entire processes of the granule cell lineages (Fig. 4). For this purpose, immunohistochemical analysis could be a powerful tool for detection of target cells using primary antibodies against proliferating cell nuclear antigen (PCNA), a cell proliferation marker, brain lipid binding protein (BLBP), expressed in radial glia as type-1 cells in the brain30), paired box 6 (Pax6), expressed in type-1 stem cells and type-2a progenitor cells31), SRY (sex determining region Y)-box 2 (Sox2), expressed in type-1 stem cells and type-2a and type-2b progenitor cells31), T box brain 2 (Tbr2), expressed in type-2b progenitor cells31), dihydropyrimidinase-like 3 (Dpysl3), also known as Tuc4, an early postmitotic cell marker of immature granule cells32), NeuroD1 (Nd1), expressed in type-3 progenitor cells and immature granule cells33), and doublecortin (Dcx), expressed in type-3 progenitor cells and immature granule cells27), as well as measurement of apoptosis utilizing terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end-labeling assay.
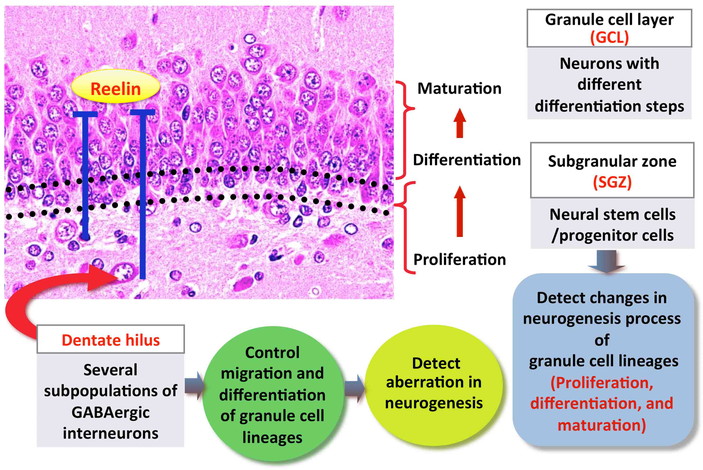
Schematic presentation of adult neurogenesis in the hippocampal dentate gyrus.
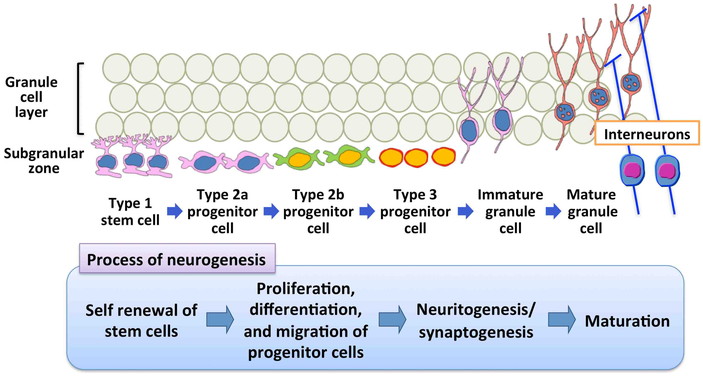
Generation of granule cell lineages in the hippocampal dentate gyrus.

Detection of aberrations in the hippocampal neurogenesis of rodent animals. Distribution of granule cell lineages can be immunohistochemically analyzed employing cellular markers, such as paired box 6 (Pax6), T box brain 2 (Tbr2), NeuroD1 (Nd1), doublecortin (Dcx), dihydropyrimidinase-like 3 (Dpysl3) and neuron-specific nuclear protein (NeuN) in the subgranular zone (SGZ) and granule cell layer (GCL). Apoptosis and cell proliferation activity in the SGZ can also be measured. Distribution of neuronal inputs from the outside of the SGZ, such as reelin-producing cells and GABAergic interneurons can be immunohistochemically detected in the hilus of the dentate gyrus.
In the hilar region of the dentate gyrus, GABAergic interneurons are known to make synaptic connections with adult-born dentate granule cells and play an important role in regulating adult neurogenesis (Figs. 2 and 3)34). In particular, subpopulation GABAergic interneurons secrete reelin, an extracellular matrix glycoprotein that modulates progenitor cell migration to maintain normal integration of granule cells in the neonatal and adult dentate gyrus35). Moreover, there are unique populations of GABAergic interneurons expressing calcium-binding proteins, such as calbindin D-28k (Calb1), parvalbumin (Pvalb), and calbindin D-29k (Calb2, also known as calretinin), that form a family of proteins containing the EF-hand Ca-binding motif, and potentially serve critical roles in the development and functioning of the brain36). It is known that interneurons innervate progenitor cells in the SGZ resulting in promotion of progenitor cell differentiation36,37). Because the number of GABAergic interneurons in the dentate hilus changes in relation with the disruption of neurogenesis, immunohistochemical approaches for analysis of these cellular populations also provide valuable information with regard to the type and severity of aberration in neurogenesis (Fig. 4).
Various neurons outside the hippocampus also make synaptic connection with neurons in the dentate gyrus to function on progenitor cell proliferation and differentiation. For example, cholinergic neurons originated from the septal nucleus and nucleus of the diagonal band of Broca innervates neurons in the dentate hilus. Noradrenergic neurons in the locus ceruleus innervate neurons in the SGZ38). Several studies indicated that acetylcholine can act directly on neuronal progenitors in the adult brain39,40), and cholinergic denervation is reported to cause cell death and decrease the numbers of new neurons in the hippocampal SGZ41). Therefore, dysfunction of cholinergic inputs, such as that evidenced by the presence of histopathological changes in the hippocampal fimbria may directly induce the reduction of intermediate progenitor cells in the hippocampal dentate gyrus. Of note, synaptic connections from the outside the hippocampus are seen with SGZ/GCL cells and/or GABAergic interneurons42). By monitoring neuronal inputs from the outside of the dentate gyrus or their postsynaptic receptors distributed within the dentate gyrus, it is also possible to detect aberrations in adult neurogenesis. For this purpose, immunohistochemical analysis and/or real-time reverse transcription-polymerase chain reaction technique with regard to related molecules, such as ligands, receptors and transporters, may be applied, as well as histopathological analysis of nerve tract changes.
Thyroid hormones (THs) play essential roles for normal brain development in mammals. It is well known that THs regulate neuronal proliferation, migration, and differentiation in discrete brain regions during development43). In an experimental model of developmental hypothyroidism, exposure to anti-thyroid agents (ATAs) leads to growth retardation, neurological abnormalities and impaired performance in a variety of behavioral learning actions44,45). Rat offspring exposed maternally to ATA show impaired brain growth involving neuronal mismigration as well as white matter hypoplasia46), due to limited axonal myelination and reduction in oligodendroglial population47,48). The outcome of this type of neural deficit is permanent accompanied by apparent structural and functional abnormalities.
There are environmental chemicals that are thought to have a TH-disrupting potential to cause abnormal brain development49). Particularly, brominated flame retardants (BFRs), some of which are environmental contaminants used in electronic circuitry, plastics, textiles and other materials to prevent fires, are known to be weak TH-disruptors. Therefore, there is a growing concern with regard to the DNT by BFRs50,51). Decabromodiphenyl ether (DBDE), tetrabromobisphenol A (TBBPA) and 1,2,5,6,9,10-hexabromocyclododecane (HBCD) are three major and widely used BFRs52). In experimental studies using developmental exposure models in rats and mice, these BFRs have been reported to cause neurobehavioral and auditory response effects51,53,54,55,56,57,58). These BFRs have shown weak anti-thyroid activities in some studies51,58,59,60,61). In contrast, there are experimental studies suggestive of direct effect of these BFRs on brain development62,63,64,65).
In a study to detect cellular evidence of the disruption of hippocampal neurogenesis due to developmental hypothyroidism19), we performed immunohistochemical analysis in the hippocampal dentate gyrus in rat offspring after developmental exposure to ATAs, 6-propyl-2-thiouracil and methimazole (MMI). Effects on neurogenesis were examined in terms of the detection of target cells in the granule cell lineages and interneuron subpopulations. For this purpose, distribution of reelin-producing interneurons was analyzed in the hippocampal dentate hilus. Obtained results indicate persistent increases in GABAergic interneurons producing reelin in an immature population through the adult stage in the dentate hilus after developmental hypothyroidism (Fig. 5). Interestingly, we observed sustained increases of immature GABAergic interneurons synthesizing reelin in the hilus, and this phenomenon was considered to be a signature of compensatory regulation for persistent impairment of neurogenesis and following migration during the neuronal development as a hypothyroidism-related brain effect.

Summary of developmental exposure effects of anti-thyroid agents (ATAs) and brominated flame retardants (BFRs) in rats.
In a study to investigate the impact and reversibility of developmental exposure to BFRs, DBDE, TBBPA or HBCD, we immunohistochemically examined reelin-producing interneuron population and cell proliferation and apoptosis in the dentate SGZ using rats66). Obtained results indicate that all the BFRs examined had developmental exposure effect on neurogenesis (Fig. 5). Our results suggested that a direct effect of DBDE and TBBPA on hippocampal neurogenesis. However, effect of hypothyroidism may also be operated at least at the highest dose of DBDE as well as that of HBCD. Because of the reversibility at the adult stage, it was considered that the effect of these BFRs on neurogenesis and following neuronal migration to the GCL occur during the exposure period. We also observed increase in neuron-specific nuclear protein (NeuN)+ mature neuronal populations at the adult stages; however, the its biological significance should be further assessed.
There are many environmental chemicals having a potential to interfere with TH signaling in the developing brain. The above-mentioned study results of ATAs and BFRs suggest that reelin-expressing GABAergic interneurons in the dentate hilus may be a useful marker for assessment of the effects of developmental neurotoxicants that can disrupt the migration and positioning of developing neurons following disruption of neurogenesis.
In the hippocampal dentate gyrus, all of the cell populations and their inherent phenomena involved in the process of adult neurogenesis may be sensitive target of DNT. Especially, self-renewal of stem cells, proliferation and migration of progenitor cells, neuritogenesis, synaptogenesis and myelinogenesis may be the vulnerable developmental processes against chemical toxicity. Because the molecular mechanisms to control neuronal development processes have many similarities with those described for mechanisms for neuronal maintenance after maturation, neurogenesis process may be vulnerable to “adult-type neurotoxicants” to show affection of mature nervous tissue (Fig. 6). Of note, among adult-type neurotoxicants, myelin toxicants may affect myelination of interneuron populations and neuronal inputs from outside the SGZ, and granule cells may not directly be affected because they are consisted of non-myelinated fibers.
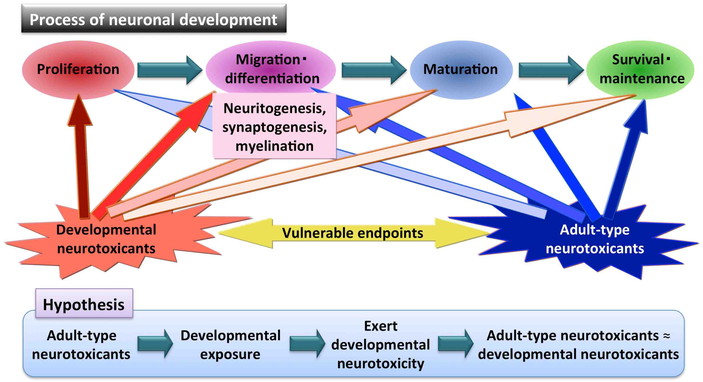
Hypothetical relationship between developmental neurotoxicants and adult-type neurotoxicants on toxicity targets during the process of neuronal development.
In this review, DNT of acrylamide in the rat hippocampal neurogenesis is presented, and similarity in the target mechanisms between developmental and adult-stage neurotoxicity is discussed. Acrylamide, a widely used chemical in many industries, is known to be a neuronal and reproductive toxicant, and to act as a carcinogen in animals67). Because it was recently found that acrylamide is generated during heating of foods containing carbohydrate and asparagine, risk assessment studies of acrylamide in foodstuffs have been conducted globally68,69). Acrylamide is a well-known axon terminal toxicant in both the central and peripheral nervous systems in adult animals70).
Axon guidance during development and after axonal injury is an important process in maintaining neuronal plasticity at the axon terminals71). It is well established that the molecular mechanisms controlling neuronal migration during development have many similarities with those necessary for axon guidance72). Therefore, both migrating neuronal progenitor cells and immature axon terminals may have sensitivity to acrylamide.
In a study of maternal exposure study of acrylamide using rats20), we found that acrylamide impairs hippocampal neurogenesis in rat offspring as evident by the increase in GABAergic interneurons producing reelin in the dentate hilus (Fig. 7). We then examined the cellular target of acrylamide on hippocampal neurogenesis and its reversibility after maternal exposure using the similar developmental exposure model21), and found reversible affection of neurogenesis targeting the proliferation of type-3 progenitor cells resulting in a reduction of immature granule cells (Fig. 7). In the SGZ, it is known that immature granule cells already have both dendritic growth cones and recurrent basal dendrites, suggesting an entry into the process of synaptogenesis73). Because acrylamide targets nerve terminals due to impairment of neurotransmission by affecting diverse nerve terminal processes74), it was suggested that acrylamide directly injure immature granule cells by affecting the newly generating nerve terminals. Importantly, we also found that acrylamide suppressed progenitor cell proliferation without accompanying facilitation of their apoptosis. Because immature granule cells can no longer proliferate, acrylamide may rather target earlier type-3 progenitor cells to suppress their proliferation causing a reduction in immature granule cells.
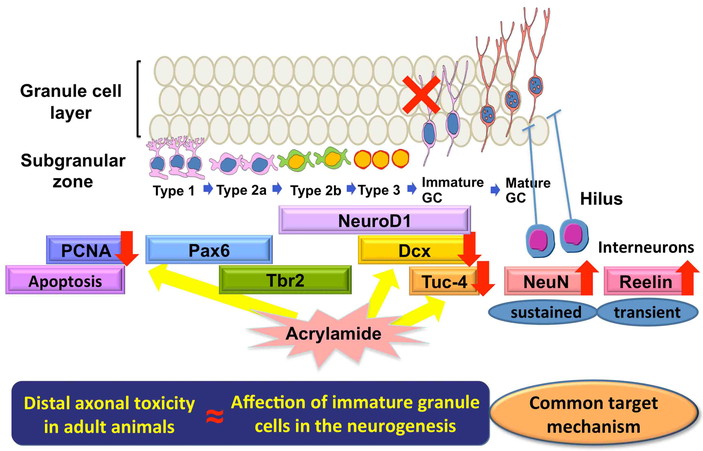
Developmental neurotoxic target of acrylamide in the process of hippocampal neurogenesis in rats.
We found another case of axon terminal toxicant to show similar target cell population at the late-stage differentiation in the hippocampal neurogenesis after developmental exposure. In a developmental exposure study of glycidol25), we found induction of distal axonopathy in dams and reduction of immature granule cells in the SGZ of offspring (Fig. 8). Glycidol is a carcinogen that has been classified by the International Agency for Research on Cancer (IARC) as a group 2A carcinogen, which is “probably carcinogenic to humans”75). Exposure to glycidol through food has recently become a worldwide concern based on possible release of glycidol by hydrolysis of glycidol fatty acid esters in the gastrointestinal tract. Glycidol fatty acid esters can be found in refined edible oils and fats, including infant formulas, especially in diacylglycerol oil at high concentrations76,77). In a repeated-oral-dose toxicity study for 13 weeks in rats and mice, neurotoxicity involving cerebellar necrosis was reported78). Our study results in the developmental exposure study of glycidol suggest that glycidol targets the newly generating nerve terminals of immature granule cells, resulting in the suppression of late-stage hippocampal neurogenesis, similar to acrylamide (Fig. 8).
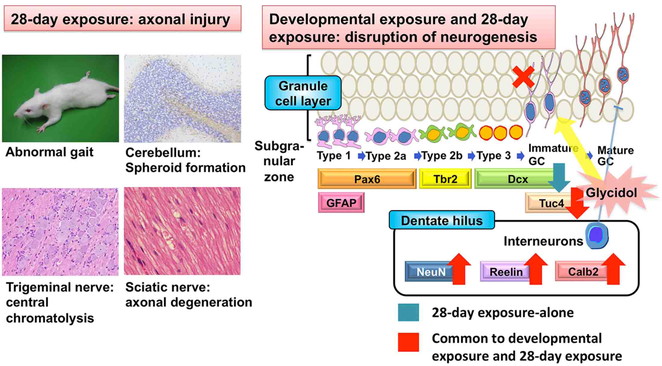
Comparison of the effect on hippocampal neurogenesis between developmental exposure study and 28-day exposure study of glycidol in rats.
Our study results of developmental exposure to acrylamide or glycidol suggest that there may be common target mechanisms between the DNT and adult-type neurotoxicity (Fig. 6).
Because postnatal adult neurogenesis continues through the adult stage, it is reasonable to capture the toxicity in the process of neurogenesis in a framework of regular toxicity studies, such as 28-day repeated oral dose toxicity study. To clarify this possibility, we compared the effects on neurogenesis in the DNT study and repeated oral dose toxicity study using MMI as an ATA and glycidol as an axon terminal toxicant25,79,80).
In the study of MMI, pregnant rats were exposed to MMI from gestational day (GD) 10 until weaning on day 21 after delivery (developmental hypothyroidism)79). Adult male rats were also exposed to MMI by setting an identical exposure period from postnatal day (PND) 46 through to PND 77 (adult-stage hypothyroidism). As a result, a sustained reduction of Pax6+ stem or early progenitor cells and a transient reduction of Dcx+ late-stage progenitor cells were observed after developmental hypothyroidism (Fig. 9). These cells were unchanged by adult-stage hypothyroidism. On the other hand, the number of Pvalb+ GABAergic was reduced and the number of Calb2+ interneurons was increased in the dentate hilus after both developmental and adult-stage hypothyroidism with MMI (Fig. 9). Our results indicate that fluctuations in GABAergic interneuron subpopulations are the common feature in both developmental and adult-stage hypothyroidism. Considering their role in neurogenesis, fluctuations in GABAergic interneuron subpopulations may provide a sensitive tool for detection of aberrant neurogenesis even after the adult-stage exposure. Our study also suggests that analysis of interneuron subpopulations may provide a tool for detection of subtle changes in neurogenesis, particularly for regular toxicity tests such as the 28-day repeated-oral dose-toxicity test.
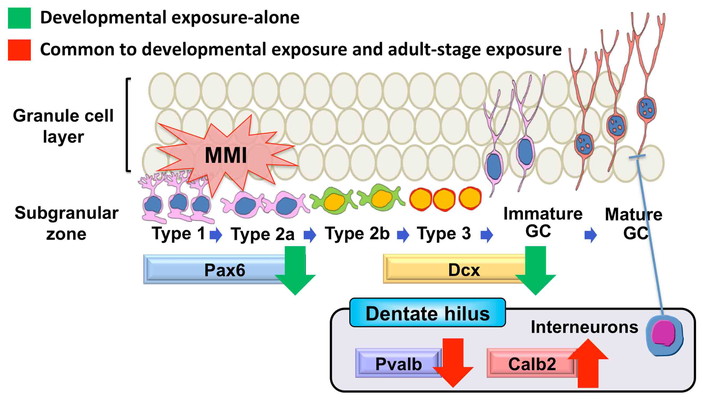
Comparison of the effect on hippocampal neurogenesis between developmental exposure study and adult-stage exposure study of methimazole (MMI) in rats.
In a study of glycidol, glycidol was exposed to pregnant rats from GD 6 until weaning on day 21 after delivery (developmental exposure study) and male young adult rats by gavage for 28 days (adult-stage exposure study)25,80). In the developmental exposure study, offspring reduced Dpysl3+ immature granule cells in the SGZ and increased immature reelin+ or Calb2+ GABAergic interneurons and NeuN+ mature neurons in the dentate hilus on weaning (Fig. 8). Hilar changes remained until adult stage, with the increases in reelin+ interneurons and NeuN+ mature neurons being present, although the SGZ change disappeared. In the adult-stage exposure study, animals revealed aberrations in neurogenesis at the late-stage differentiation as evidenced by reductions of both Dcx+ and Dpysl 3+ cells in the SGZ and increases of reelin+ and Calb2+ GABAergic interneurons and NeuN+ mature neurons in the dentate hilus (Fig. 8). These results suggest that glycidol targets the newly generating nerve terminals of immature granule cells, resulting in the suppression of late-stage hippocampal neurogenesis in both developmental and adult-stage exposure studies.
These results suggest that adult neurogenesis in the SGZ may provide a suitable endpoint for detection of DNT in a standard regular 28-day toxicity study. In other words, both developmental and adult-type neurotoxicity may be detected by analysis of the cellular responses in the adult neurogenesis using adult animals. From this point of view, a rapid screening system for developmental neurotoxicants may be constructed by measurement of the effect on adult neurogenesis in a framework of 28-day toxicity study. After initial screening in the 28-day toxicity study, DNT may be definitively detected in a framework of DNT studies by incorporating the analysis of neurogenesis-related parameters like those presented here in the histopathological endpoint, in combination with functional and behavioral observation battery. It is necessary to collect information on adult-type neurotoxicants regarding relationship between the toxicity target on adult nervous system and that on neurogenesis.
Recent studies indicate that various epigenetic mechanisms, including DNA methylation of gene promoter region, histone deacetylation and microRNAs, are involved in regulating multiple processes of adult mammalian neurogenesis81), and environmental factors can influence this regulation82). The understanding of epigenetic gene regulation as a long-lasting cellular memory necessary to maintain or evolve a cellular phenotype has recently been challenged by discoveries of its dynamic nature82). In general, gene expression is related inversely to DNA methylation83). This relationship is particularly evident in CpG islands (CGIs) at gene promoter regions, where DNA methylation may directly interfere with transcription factor binding to DNA and/or indirectly suppress transcription through methylated DNA binding proteins that recruit histone deacetylases, leading to chromatin condensation and subsequent gene silencing84). Although the results of epigenetic regulation changes on neurogenesis have remained unexplored, environmentally induced disruption of DNA methylation clearly deserves further study85) given the clear importance of DNA methylation to processes of neuronal development.
Mn is a trace essential element important for protein and energy metabolism, bone mineralization, metabolic regulation, and cellular protection from reactive oxygen species86). Excess Mn exposure causes serious neurotoxicity, such as manganism, which shares multiple features with Parkinson’s disease87). We have recently found that maternal developmental Mn exposure in mice affects neurogenesis targeting immature granule cells in the SGZ of offspring even at the adult stage, accompanied with a sustained increase in the immature population of reelin-synthesizing GABAergic interneurons88). These changes may represent continued aberrations in neurogenesis and subsequent migration to cause an excessive response to overproduce immature granule cells through the adult stage. To clarify the effects of maternal Mn exposure on epigenetic gene regulation contributing to this sustained disruption, we searched epigenetically downregulated genes by global promoter methylation analysis in the hippocampal dentate gyrus of Mn-exposed offspring at the end of maternal exposure on weaning and further examined to confirm methylation status as well as transcript expression levels of candidate genes on both weaning and adult stage89).
By global promoter methylation analysis using CpG promoter microarrays, we have found that maternal Mn exposure induced CGI hypermethylation of the promoter region and transcript downregulation of Pvalb, Mid1 (midline 1), Atp1a3 (ATPase, Na+/K+ transporting, alpha 3 polypeptide) and Nr2f1 (nuclear receptor subfamily 2, group F, member 1; also known as COUP-TF1) in the hippocampal dentate gyrus of offspring89). Sustained promoter hypermethylation and transcript downregulation through adult stage were confirmed with Mid1, Atp1a3 and Nr2f1, whereas Pvalb showed a transient hypermethylation only on weaning (Fig. 10). By immunohistochemical analysis, we also observed reductions of Pvalb+ and ATP1a3+ neurons suggestive of GABAergic interneurons in the hilus, Mid1+ cells suggestive of late-stage progenitors or postmitotic granule cells in the SGZ and interneurons in the hilus and COUP-TF1+ cells suggestive of stem cells and early-stage progenitors in the SGZ. All of these reductions except for those of Pvalb+ cells were sustained through the adult stage. These observations suggested continued disruption of neurogenesis and subsequent neuronal differentiation by Mn exposure.
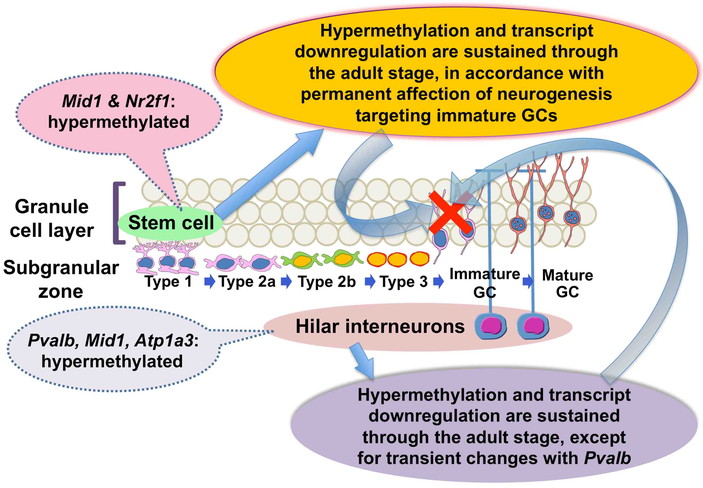
Epigenome toxicity in the hippocampal neurogenesis after developmental exposure to manganese (Mn) in mice.
Evidence suggests that Pvalb+ cells, a subpopulation of GABAergic interneurons, play a central role in the inhibition of granule cell activity90). With regard to ATP1a3, an ion pump component that maintains sodium and potassium gradients across the plasma membrane, a subpopulation of GABAergic interneurons in the hippocampus were reported to express this protein91). Because GABAergic interneurons provide direct connection to type-2 progenitor cells in the SGZ to promote progenitor cell differentiation37), reduction of Pvalb+ cells or ATP1a3+ cells may lead to inhibition of neuronal differentiation after Mn exposure.
Mid1 is a gene encoding a ubiquitin ligase92) and affects brain development by regulating expression of Gli393), a Wnt target gene involved in hippocampal development94). Loss of Mid1 function by mutation is involved in X-linked Opitz G/BBB syndrome characterized by defective midline development95). Therefore, promoter hypermethylation and subsequent Mid1 downregulation may be linked to Mn-induced disruption of neurogenesis. Interestingly, it is reported that Mid1 is expressed at the right side of Hensen’s node of chick embryos to play a key role in gene cascade downregulation during the early stages of left-right determination through sonic hedgehog pathway inhibition96). We have also found right-side predominance of Mid1+ cells and its abolishment by Mn exposure by promoter hypermethylation of this side, suggestive of the disruption of higher brain functions specialized in the left or right side of the brain89).
COUP-TF1 is one of the most characterized orphan receptors of the steroid/thyroid hormone receptor superfamily and is an important regulatory component of neurogenesis and following differentiation during development97). COUP-TF1 is expressed in both neuronal progenitors and postmitotic neurons98). Therefore, our result suggests a permanent effect on epigenetic gene control of Nr2f1 involving stem cells by developmental Mn exposure to trigger disruption of neurogenesis and subsequent neuronal differentiation because epigenetic gene changes can be inherited to daughter cells.
These findings point to epigenetic mechanisms as mediators, through which developmentally administered neurotoxicants modulate postnatal adult neurogenesis with long-lasting and stable repercussions.
Monitoring of granule cell lineage in the SGZ and GABAergic interneurons in the dentate hilus is effective for detection of target cell populations of DNT in the process of adult neurogenesis. Especially, monitoring of reelin-expressing GABAergic interneurons is useful for detection of neuronal mismigration following impairment of neurogenesis. Because axon terminal toxicants target granule cell lineage population growing dendritic processes, there may be common target mechanisms between the DNT and adult-type neurotoxicity. Adult neurogenesis may also serve as a suitable endpoint for detection of DNT in a scheme of standard regular 28-day toxicity study. In other words, it is suggested that both developmental and adult-type neurotoxicity could be predicted by the cellular responses in the adult neurogenesis. Moreover, epigenome toxicity mechanisms may be operated in the process of hippocampal neurogenesis involving both neuronal stem cells and interneuron subpopulations, with continued disruption through the adult stage. These findings suggest that hippocampal neurogenesis is considered to be a critical target of environmental neurotoxicants contained in foods. Considering pluripotent nature of neural stem cells in the SGZ to differentiate to both neuronal and glial cell lineages, further studies are necessary on the late-stage onset of stem cell toxicity in the neurogenesis.
The authors declare that no conflicts of interest exist.
Our studies covered by this review were supported in part by Health and Labour Sciences Research Grants (Research on Risk of Chemical Substances) from the Ministry of Health, Labour and Welfare of Japan, by a Grant-in-Aid for Scientific Research (B) from the Ministry of Education, Culture, Sports, Science and Technology of Japan (Grant No. 25292170) or by a grant from the Ministry of Economy, Trade and Industry (METI), Japan.