2017 年 5 巻 1 号 p. 14-23
2017 年 5 巻 1 号 p. 14-23
In the early 90s’, Europe was shaken by the fear that the prions from “mad cow disease” (bovine spongiform encephalopathy) would transmit the disease to humans via beef products. In 1996, the first variant Creutzfeldt-Jakob (vCJD) patients were described, and the same year our Bovine Spongiform Encephalopathy (BSE) transmission studies to cynomolgus macaques demonstrated that the BSE prion was highly infectious for primates, inducing brain lesions identical to those observed in vCJD patients. These studies provided the first experimental evidence that vCJD was BSE in humans. Subsequent studies established the BSE/vCJD-infected cynomolgus macaque as a robust model to study the pathogenesis of vCJD. We showed rapid adaptation of BSE prions to primates upon subsequent passage, and their distribution in peripheral tissues and blood. Some key studies are summarized in the present paper.
In 1987, a new prion disease affecting dairy cattle was described in the United Kindom1). Affected cows presented signs of aggressiveness, anxiety, ataxia and were finally found recumbent. The disease was rapidly classified in the group of “transmissible spongiform encephalopathies”, or TSEs, due to the transmissibility of the disease2), as well as the similarities of the neuropathological lesions and molecular hallmark with those found in sheep scrapie and human CJD: neuronal death, spongiform changes, and accumulation of misfolded and aggregated prion protein (termed PrPsc)3). PrPsc is the infectious form of the host prion protein PrP. It is also called a prion (for “proteinaceaous infectious particle4)) or TSE agent. The number of affected cows increased rapidly to top at a 37,280 diagnosed animals in the year of 1992 (OIE data). Thankfully, British epidemiologists recognized that BSE was due to the consumption of prion-tainted meat and bone meal (MBM)5), and the first feed-ban was implemented in 1988, prohibiting the feeding of ruminants with ruminant-derived MBM.
1.2. vCJD in Humans and Transmission of BSE to Non-human PrimatesIn 1991, BSE was reported in a domestic cat that presumably was contaminated via pet food6). Transmission of scrapie from small ruminants to cats had never been described, raising concern that BSE might be more pathogenic than scrapie not only for cats, but also for humans. In order to probe the cow-to-primates species barrier of the BSE agent, we inoculated cynomolgus macaques (Macaca fascicularis) with BSE-infected cow brains at the French Atomic Energy Commision (CEA).
In 1996, 10 young individuals were described in the UK and one in France, harboring an unusual form of CJD that was coined variant CJD (vCJD)7,8). Besides patients being exceptionally young (adolescents and young adults, while sporadic CJD (sCJD) affects people over the age of 60), they exhibited unusual symptoms. Early symptoms were dysaesthesia, behavioral symptoms, depression, ataxia, with myoclonus appearing later on, contrasting with the cognitive course of the disease (memory impairment, dementia) preceding motor impairment, which is most frequently observed in sCJD. Moreover, vCJD patients presented specific neuropathological features with spongiosis and neuronal loss most evident in the basal ganglia and thalamus, and the presence of PrP amyloid plaques (abundant in the cerebral cortex and cerebellum) that were surrounded by vacuoles, giving them a flower-like appearance. These peculiar plaques were called florid plaques7).
At the same time as the first vCJD patients were being described, we were examining the brains of our 3 macaques that had all come down with disease 3 years after intracerebral (IC) inoculation with BSE-infected cow brain. Clinical signs were characterized by behavioral signs such as depression or edginess, as well as truncal ataxia (broad-based gait, tremors) and myoclonus. Neuropathological examination of the brains of the BSE-macaques revealed the presence of florid plaques and other neuropathological features similar to those observed in vCJD patients (Fig. 1). Florid plaques were not present in the brains of macaques inoculated with Kuru or sCJD, and thus were considered specific for infection by the BSE prion. Moreover, PrPsc in BSE-infected macaques and vCJD patients exhibited a similar electrophoretic pattern by western blot (Fig. 1).
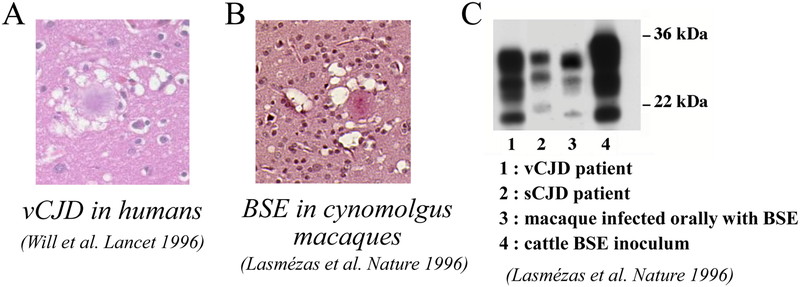
BSE in cynomolgus macaques reproduces neuropathological and biochemical features of human vCJD. A: Florid plaque in the brain of a vCJD patient, (reproduced from ref.7). B: Florid plaque from the brain of a cynomolgus macaque infected with cattle BSE (reproduced from ref.9). C: Western blot showing a similar electrophoretic pattern of disease-associated, proteinase-K (PK) resistant PrP in a macaque infected with BSE and a vCJD patient, differing from the pattern observed in a sCJD patient (reproduced from ref.9).
In summary, macaques infected by BSE reproduced the behavioral and motor symptoms, the neuropathology and the biochemical signature of vCJD in humans. This study provided the first experimental evidence supporting that vCJD was due to human infection by the BSE agent9), and an experimental model to study the new disease.
1.3. Determination of the Minimal Infectious BSE Dose in Non-human PrimatesIn a concerted European effort involving 5 laboratories including ours, the BSE-macaque model was then used to evaluate the minimal amount of BSE-infected material necessary to induce vCJD in primates. Results so far show that 5g of infectious BSE cattle brain is sufficient to induce the disease in all recipient animals by the oral route, with 500 mg yielding an incomplete attack rate10,11). The ID50 of BSE cattle brain is 200 mg for cattle12). These results suggest a low species barrier between cattle and non-human primates.
1.4. Adaptation of the BSE Agent to Non-human Primates: Consequences for Human HealthThe macaque BSE model provided an opportunity to evaluate the possible risk for humans of secondary inter-human transmission of the BSE/vCJD prion. Accidental human-to-human transmissions of sCJD, resulting in iatrogenic CJD (iCJD) has occurred in several unfortunate circumstances (described in ref.13). One of them was the infection of children with CJD-contaminated human growth hormone (hGH) extracted from cadaveric hypophyses. These iCJD patients had been treated for short stature by injection of hGH in childhood, and 226 of them died of iCJD as young adults, mainly in France and the USA13). Other dramatic iCJD cases had been linked to the surgical implantation of dura-mater grafts, resulting in 228 deaths13). A few cases were also due to corneal grafts and intracranial electrodes. Although all known iCJD cases prior to 2004 had been linked to contamination with central nervous system (CNS) tissue, the possibility existed that the BSE agent would harbor a different distribution in primates than the sCJD agent, thus representing a higher risk of transmission via organ/tissue grafts, contamination of surgical instruments or even blood transfusion.
As a first step for risk assessment, we transmitted the BSE prion from macaque to macaque via different routes. We also established a dose-response (incubation time) for the IC route to provide a baseline for subsequent infectivity measurement studies. This experiment showed that the BSE agent adapts rapidly to primates, as incubation periods shortened from 3 to 1.5 years upon secondary passage at the highest dose14). It also showed that, for a given amount of BSE material (40 mg BSE brain homogenate), the incubation period was the same whether inoculation was done by the IC or the intravenous (IV) route.
We compared second passage macaques inoculated with BSE prions by the oral or IV routes15). PrPsc was detected by immunohistochemistry and by ELISA after “scrapie associated fibril” (SAF) purification15). In addition to the brain, we detected PrPsc in spleen, tonsils, intestine and sciatic nerve in amounts that did not depend on the inoculation route, with the exceptions of the spleen where PrPsc amounts were up to 4% the amounts found in the brain after IV inoculation, and up to 0.2% those of the brain after oral dosing15).
2. 2. Distribution of Prions in Tissues and Organs of vCJD, Sporadic and Iatrogenic CJD Infected MacaquesWe also infected macaques with vCJD, sCJD, iCJD16). As determined earlier, BSE and vCJD prions correspond to the same prion strain, and one or the other denomination is used depending on the species of origin for the brain tissue used as inoculum. All prion strains were inoculated in the same manner (intracerebral and intratonsillar combined), in order to be able to directly compare tissue distribution of PrPsc between strains.
Disease-associated PrP deposits were detected by immunocytochemistry in various organs. They were found in the Peyer’s patches of the gut and other lymphoreticular system (LRS) tissue of BSE/vCJD infected animals (Fig. 2). By PET-blot, we showed that these deposits corresponded to proteinase K-resistant PrP, a biochemical subset of PrPsc. Interestingly, not all Peyer’s patches of a single animal were PrPsc positive (Fig. 2), showing that PrPsc-negative LRS tissue biopsies may lead to false negative diagnostic results.

Pathological PrP deposits in Peyer’s patches of BSE infected macaques. Disease-associated PrP accumulates in germinal centers of the Peyer’s patches in macaques infected with BSE by the oral or intravenous routes. Of note, not all Peyer’s patches are positive in a single animal, as shown in the right panel depicting a Peyer’s patch negative for disease-associated PrP despite oral inoculation of this animal. PrP detected by immunohistochemistry corresponds to PK-resistant PrP, as shown on the PET-blot. Reproduced with modification from ref.15.
Pathological PrP deposits were also detected in the enteric nervous system in macaques infected with all prion strains. Fig. 3 shows the localization of pathological PrP in the pericarya of neurons of the myenteric plexus, as well as in small nerve fibers of the inner muscular layer of the intestine.
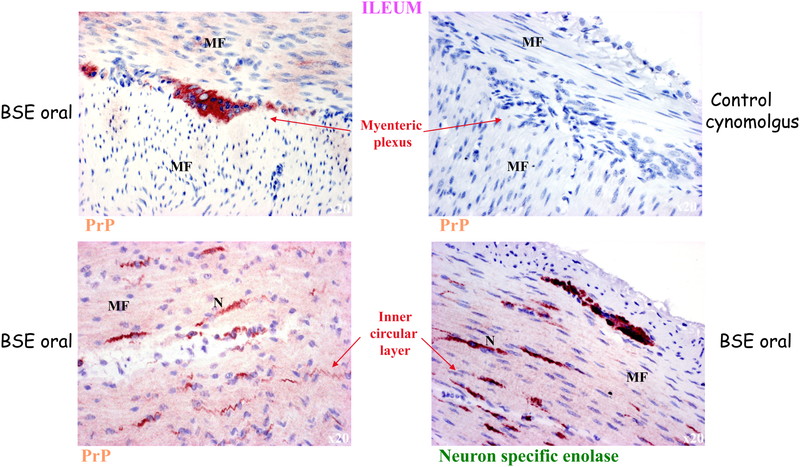
Pathological PrP in the enteric nervous system in BSE infected macaques. Disease-associated PrP is also found in the enteric nervous system and in nerve fibers of the inner circular muscle layer of the intestine. Neuron specific enolase staining shows the specificity of the staining for nervous structures. MF: muscle fibers; N: nerve. Reproduced with modification from ref.15.
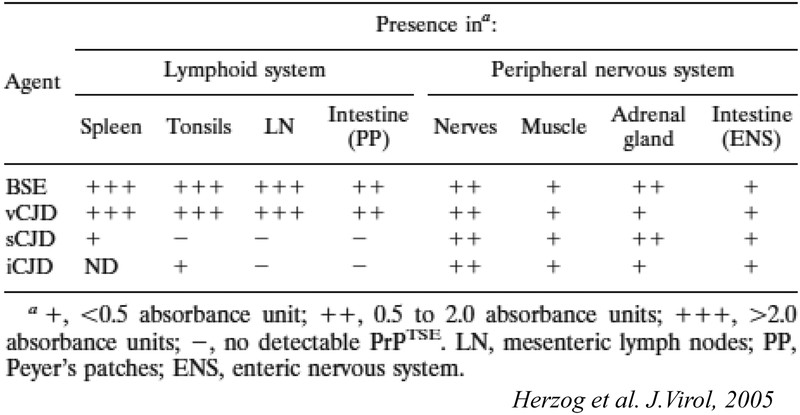
Pathological PrP deposits were also found in peripheral nerves and in muscle for all CJD strains (Table 1). In the peripheral nerves, they were found mostly at the surface of Schwann cells (Fig. 4). In muscle, they were localized to specific foci in the vicinity of nerve fibers (Fig. 5). Our results suggest that the heterogeneous, patchy distribution of pathological PrP deposits in muscles corresponds to the distribution zones of motor end plates. This study provides a possible explanation for the variably positive detection of pathological PrP in muscle samples of sCJD patients17).

Pathological PrP in nerves of BSE infected macaques. Disease-associated PrP labeling in the sciatic nerve of a cynomolgus macaque infected (A) by the intravenous route and (B) by the oral route. (Inset) PrP labeling in the sciatic nerve of an uninfected control animal. C: sympathetic nerve fibre along the carotid artery, animal infected orally with BSE. PrP labeling (D) and GFAP labeling (E) in the sciatic nerve of a macaque infected intravenously. F shows the merge. Reproduced from ref.15.

Pathological PrP in muscles of BSE infected macaques. Disease-associated PrP in the muscles of BSE-infected macaques is showing a heterogenous distribution compatible with that of the distribution zones of motor end plates. Reproduced from ref.16.
PrPsc amounts were also measured semi-quantitatively using a sensitive biochemical detection method including phosphotungstic acid precipitation as a concentration method, and western blot or ELISA detection16). This method revealed the presence of PrPsc in the spleen of the sCJD infected macaque and tonsils of the iCJD infected macaque, but no PrPsc could be detected in lymph nodes and Peyer’s patches of these animals, a result most likely due to the presence of PrPsc amounts at the threshold of detection in the LRS of sCJD and iCJD macaques, and sampling variations. These results are summarized in Table 1.
In summary, PrPsc was detectable at high levels in organs and tissues of the LRS only in BSE/vCJD infected animals (0.1% to 10% of the amounts found in the brains of the same animals). We interpreted these results as the BSE prion being highly lymphotropic in primates. These findings correlated indeed with the tonsils, spleens and appendices of vCJD patients being found positive for PrPsc18,19,20). We therefore proposed that LRS tissues be considered ‘high-risk’ in vCJD patients only.
However, lower amounts of PrPsc were detected in adrenals, muscles and intestinal tissue of macaques infected with BSE/vCJD as well as sCJD and iCJD, associated with peripheral nerves. Levels were less than 10,000 times lower than brain PrPres levels (<0.001%). We therefore proposed that these tissues be considered “low-risk” for all CJD patients.
Our results expanded upon observations made in vCJD patients that PrPsc is detectable in tonsils, emphasizing that BSE prions are largely lymphotropic in primates, and may replicate in lymph notes, tonsils, spleen and Peyer’s patches before the symptomatic phase. Our subsequent studies confirmed that lymph node biopsies of BSE-inoculated macaques were positive for PrPsc prior to the onset of clinical signs (see below). In another study, gut-associated lymphoid tissue and gut-draining lymph nodes were found positive for PrPsc within one year of oral infection of macaques with cattle BSE21). On the other hand, distribution of PrPsc in muscle of macaques inoculated with vCJD, sCJD and iCJD suggests a centrifugal spread of prions from the CNS to muscle motor plates via motor nerves, occurring after CNS invasion by prions. In addition, it is probable that centripetal spread of prions via peripheral nerves also occurs in earlier stages of infection stochastically from various points of entry, even in the absence of prior LRS replication. We demonstrated this paradigm earlier in severely immunodeficient (SCID) mice infected with mouse-adapted scrapie22). Moreover, spread from the gut to the CNS via autonomic nerve fibers has been shown in experimental scrapie and BSE23,24,25). Fig. 6 illustrates the distribution and proposed propagation of prions in our non-human primate model.
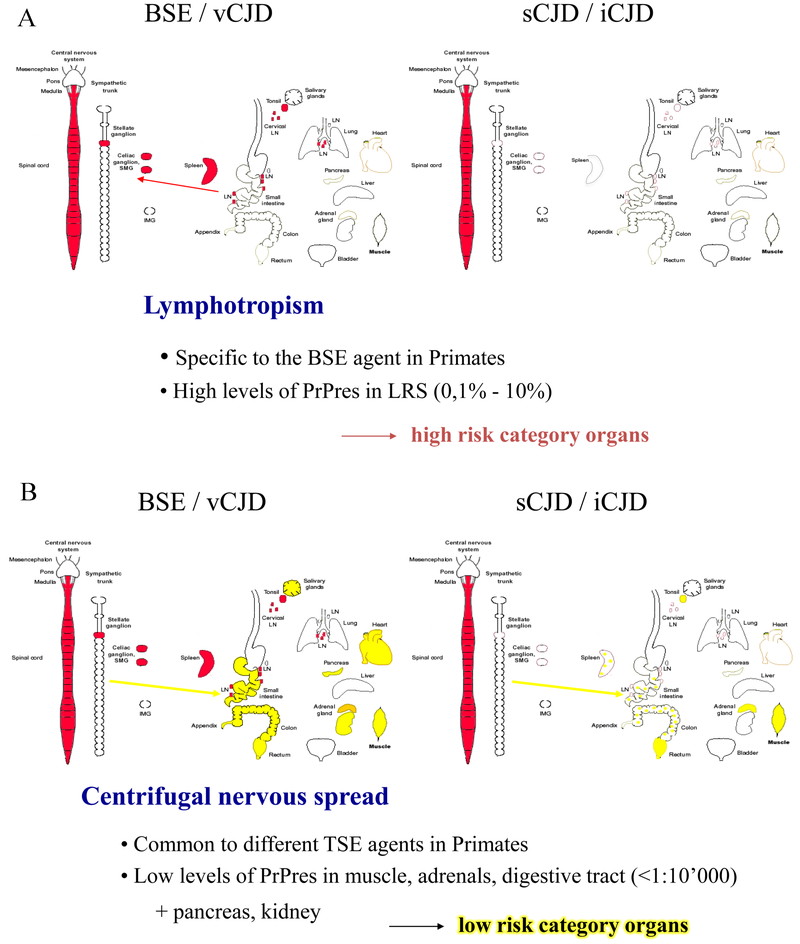
Proposed propagation routes of BSE/vCJD versus sCJD and iCJD prions in primates. A: organs and tissues of the LRS (red) are infected preferentially in BSE/vCJD and correspond to high doses of infectious prions. B: non-LRS organs and tissues are infected by low levels of prions (yellow) in BSE as well as in sCJD and iCJD.
The lymphotropic properties of BSE prions raised the important question of the presence of infectivity in blood of vCJD patients.
We initiated a large blood transfusion study where whole blood, white blood cells or plasma from either vCJD patients or BSE/vCJD macaques was injected by IV or IC to recipient macaques. This experiment led to most macaques surviving over prolonged periods of time (>10 years), and few coming down with BSE/vCJD or intercurrent illnesses. These studies continued after the author of this manuscript left the CEA. Interim transmission results are shown in Fig. 7, and some important observations were as follows. Blood depleted for red blood cells (RBC) from a vCJD patient (7.5 mL) injected intravenously did not result in any clinical disease in the recipient macaque after 10 years, yet this animal harbored positive IHC staining in the inguinal lymph nodes (LNs). Another macaque, who had received 25 mL of RBC-depleted blood intravenously from another vCJD patient, died suddenly at 42 months after inoculation, and harbored PrPsc positive inguinal LNs. Two other animals, that received 500 µL of buffy coat (BC) from vCJD patients by IC, were still alive 10 years after inoculation (with PrPsc positive LNs, Fig. 7A). A whole blood transfusion of 40 mL from a vCJD macaque (who died 3 years after intracerebral + intratonsillar inoculation of human vCJD brain homogenate) induced clinical signs of vCJD in the recipient macaque 66 months after the transfusion. Inguinal lymph nodes biopsies had been positive since 45 months, i.e. 75% of the incubation period (Fig. 7B). Other macaques transfused with blood from BSE-macaques survived more than 10 years, but some had positive LNs (Fig. 7C). Another notable result was the transmission of BSE infection by the plasma from a macaque that had been dosed orally with cattle BSE 27.5 months earlier. The donor macaque died with a behavioral syndrome of self-injury 117 months after challenge with a diagnosis of probable BSE, hence infectivity was present in its blood at a quarter of the incubation period (Fig. 7D).
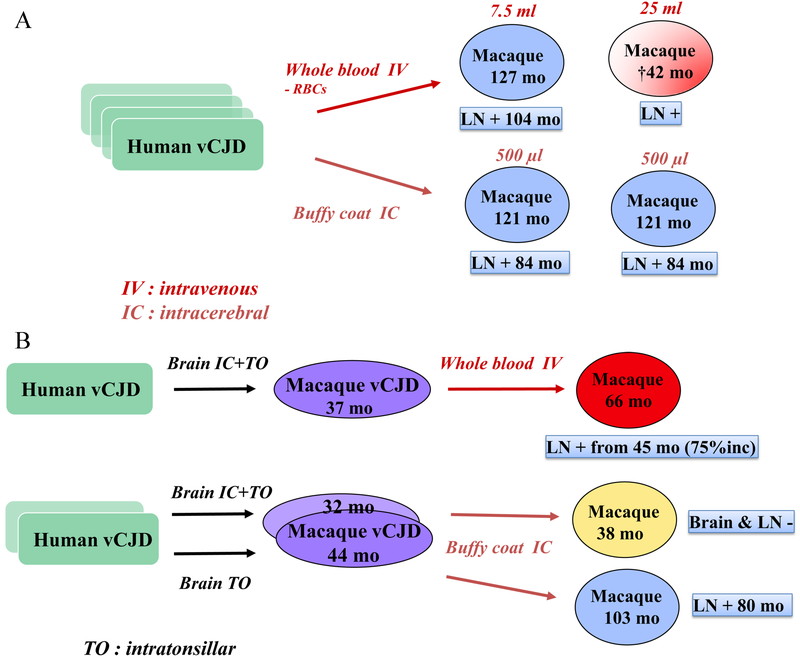
BSE/vCJD blood transmission experiments. Each individual rectangle represents a different vCJD patient (7 A&B), or a pool of BSE cattle (7 C&D). Each individual oval represents a different cynomolgus macaque. The color code is as follows: green: original donor, clinically affected; purple: experimental animal, clinically affected and histopathologically confirmed; shaded purple: experimental animal, euthanized with probable BSE; red: experimental animal, clinically affected after receiving blood-derived material; shaded red: sudden death of an animal that received blood-derived material, probably due to vCJD; yellow: intercurrent death; blue: healthy animal. RBCs: red blood cells. LN: lymph nodes. Lymph nodes positivity was assessed by PrPsc immunohistochemistry. mo: months post-inoculation. “+”: PrPsc detected by IHC; “-“: PrPsc not detected by IHC. Volumes indicated correspond to the volumes of inoculated materials.

BSE/vCJD blood transmission experiments. Each individual rectangle represents a different vCJD patient (7 A&B), or a pool of BSE cattle (7 C&D). Each individual oval represents a different cynomolgus macaque. The color code is as follows: green: original donor, clinically affected; purple: experimental animal, clinically affected and histopathologically confirmed; shaded purple: experimental animal, euthanized with probable BSE; red: experimental animal, clinically affected after receiving blood-derived material; shaded red: sudden death of an animal that received blood-derived material, probably due to vCJD; yellow: intercurrent death; blue: healthy animal. RBCs: red blood cells. LN: lymph nodes. Lymph nodes positivity was assessed by PrPsc immunohistochemistry. mo: months post-inoculation. “+”: PrPsc detected by IHC; “-“: PrPsc not detected by IHC. Volumes indicated correspond to the volumes of inoculated materials.
In 2004, the first transfusion-related case of vCJD was described in a patient who had been transfused with non-leucoreduced red blood cells from a donor who developed vCJD 3.5 years after the donation26). A total of four transfusion-related vCJD transmissions have been reported to date27).
I thank the many people who supported and participated in this work at the CEA: Dominique Dormont, Jean-Philippe Deslys, Christian Herzog, Nathalie Lescoutra, Nicole Salès, Emmanuel Comoy, René Rioux. I also thank Ray Bradley and Michael Dawson for providing BSE-infected cattle brain homogenates. I am thankful to Robert Will and Nicolas Kopp for providing vCJD samples, and to James Ironside for his collaboration on the neuropathology of BSE-infected macaques. I am grateful to my European collaborators Maurizio Pocchiari, Gerhard Hunsmann, Johannes Löwer, Pär Bierke, Loredana Ingrosso, Uwe Hahmann, Dirk Motzkus, Edgar Holznagel, for their friendship and for embarking on this challenging project.