2018 年 6 巻 1 号 p. 33-43
2018 年 6 巻 1 号 p. 33-43
Monitoring and management programs for marine toxins in seafood depend on efficient detection tools for their success in protecting public health. Here we review current methods of detection for neurotoxic shellfish poisoning (NSP) toxins, and current knowledge in brevetoxin metabolism in shellfish. In addition, we discuss a novel approach to developing monitoring tools for NSP toxins in molluscan shellfish. NSP is a seafood-borne disease caused by the consumption of brevetoxin-contaminated shellfish. Brevetoxins are a suite of cyclic polyether compounds found in blooms of the marine dinoflagellate Karenia brevis (K. brevis) and are potent neurotoxins. Preventive controls for NSP in the U.S. currently rely upon environmental monitoring of K. brevis blooms and assessment of their shellfish toxicity by mouse bioassay. The mouse bioassay for NSP approved by National Shellfish Sanitation Program was developed in the 1960s when very little information on the structural and toxicological properties of brevetoxins in algae and shellfish was available. Alternative methods to mouse bioassay based on current scientific knowledge in the area are needed for monitoring NSP toxins. It is now established that brevetoxins are metabolized extensively in shellfish. Algal brevetoxins undergo oxidation and reduction, as well as conjugation with fatty acids and amino acids in shellfish. Recently, three metabolites have been identified as biomarkers of brevetoxin exposure and toxicity in Eastern oyster (Crassostrea virginica) and hard clam (Mercenaria sp.). The role of these biomarkers in monitoring NSP toxins in K. brevis exposed molluscan shellfish is reviewed. Comparisons of biomarker levels by liquid chromatography-mass spectrometry (LC-MS) with composite toxin as measured by enzyme linked immunosorbent assay (ELISA), and shellfish toxicity by mouse bioassay, support the application of these biomarkers as a dynamic and powerful approach for monitoring brevetoxins in shellfish and prevention of NSP.
Marine toxins are naturally occurring chemicals produced by certain marine and fresh water algae that can contaminate seafood on exposure1,2). Various marine species such as crustaceans, fish and molluscan shellfish (i.e., clams, mussels, scallops, and oysters) can accumulate many of these toxins with no significant adverse effects3,4,5). However, consumption of toxin contaminated seafood can cause intoxication in humans. Azaspiracid Shellfish Poisoning (AZP), Diarrhetic Shellfish Poisoning (DSP), Neurotoxic Shellfish Poisoning (NSP), Paralytic Shellfish Poisoning (PSP), Amnesic Shellfish Poisoning (ASP), and Ciguatera Fish Poisoning (CFP) are some of the major syndromes associated with seafood toxin poisoning6,7). In order to protect seafood consumer health and prevent economic loss, monitoring programs have been established in many countries around the world to detect algal toxins and the causative harmful algal blooms8,9,10,11,12). Unfortunately, detection and characterization of marine toxins is difficult due to their low concentrations and the diversity of congeners that may be present in algal blooms and seafood13). Several marine toxins are metabolized by marine animals used for food and present a more complex detection challenge14,15,16). Mouse bioassays are still used in regulatory monitoring of several marine toxins in many parts of the world. However, measures are being taken globally to replace mouse bioassays due to their lack of specificity and sensitivity, labor intensiveness and ethical concerns. Therefore, efficient and effective analytical methods are needed for successful monitoring and management programs.
In the United States, the mouse bioassay is used in regulatory monitoring of NSP, one of the syndromes associated with shellfish toxin poisoning12). Control of NSP in the US is achieved largely through environmental monitoring of the causative algal blooms of Karenia brevis (K. brevis) and toxicity assessment of shellfish by mouse bioassay. The management of NSP consists of shellfish harvest area closures when K. brevis density is >5000 cells/L. Harvest areas are re-opened when K. brevis concentrations fall below this threshold and the toxicity of shellfish exposed to the bloom are ≤20MU/100g as assessed by mouse bioassay12). Although this strategy has been very successful in the protection of public health from NSP, alternative methods are needed for monitoring and management programs.
Potent neurotoxins known as brevetoxins have been isolated from the marine dinoflagellate K. brevis17). Blooms of K. brevis occur regularly in the Gulf of Mexico. Brevetoxins are accumulated in many filter-feeding molluscan shellfish on exposure to blooms of K. brevis and cause NSP in humans when contaminated shellfish are consumed. Brevetoxins are lipophilic, cyclic polyether compounds18) that are colorless, tasteless, and heat stable. Brevetoxins are grouped into A-type and B-type based on the two distinct backbone structures recognized in brevetoxins isolated from K. brevis19,20). Brevetoxin (BTX)-1 (A-type)20) and BTX-2 (B-type)19) are the principal algal toxins (Fig. 1) with BTX-2 produced in abundance by K. brevis.
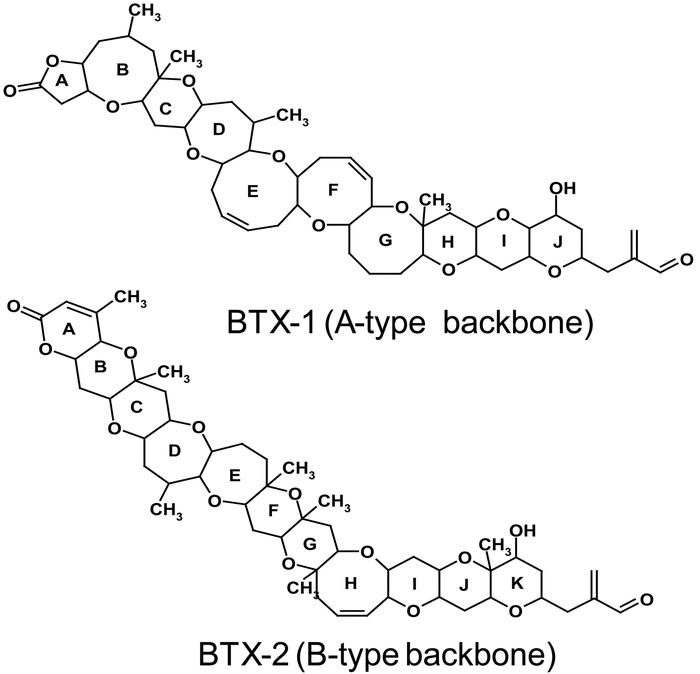
Structure of principal algal brevetoxins BTX-1 (A-type) and BTX-2 (B-type)
Even though BTX-1 and BTX-2 differ in the backbone structure, they have similar functional groups in the head and tail region of the molecule. Both possess a lactone moiety in the A-ring and terminate in an α, β-unsaturated aldehyde side chain which leads to similar chemical reactions in both types of these algal toxins. Bloom water has been found to contain a suite of derivatives of the parent algal toxins, including products of reduction, oxidation, and hydrolysis21,22). Thus, during K. brevis blooms, filter feeding molluscan shellfish are exposed to a complex suite of brevetoxins with varying toxicities.
2-2. Brevetoxins in Molluscan ShellfishBrevetoxins accumulate rapidly in molluscan shellfish through ingestion of K. brevis cells and absorption from the surrounding water23). Extensive metabolism of brevetoxins in shellfish has been reported by several investigators24,25,26). The first report of brevetoxin metabolism was following an NSP outbreak in New Zealand in 1992–199327). Since then, several brevetoxin metabolites have been well characterized in K. brevis exposed shellfish. Metabolites include products of oxidation, reduction, hydrolysis, and amino acid/fatty acid conjugation26,28,29,30) to the algal toxins. Metabolites of the B-type backbone structure are present in abundance compared to those with the A-type backbone. Metabolism of brevetoxins in shellfish generates products of varying chemical properties; some are more polar and hydrophobic than the parent algal toxins. For example, amino acid/peptide conjugation of the algal toxins generates products with increased polarity while conjugation to fatty acids generates products of greater hydrophobicity in shellfish. Major metabolites identified in shellfish are presented in Fig. 2 together with the parent algal toxin BTX-2. Metabolism of BTX has been determined to be shellfish species specific; a few of the metabolites found in clams were not found in oysters. BTX-B1, the N-taurine conjugate of BTX-2, and its corresponding A-type taurine conjugate are found in hard clams (Mercenaria sp.) and not in oysters (Crassostrea virginica)31). Elimination rates of individual metabolites have been found to vary32) and thus, composite toxicity is a reflection of the metabolite profile in the shellfish. Therefore, species-specific metabolites may contribute to variation in shellfish toxicity. Cysteine conjugates, S-desoxy BTX-B2 and BTX-B2, were the most abundant25) and persistent of all the metabolites examined in Eastern oyster. They were detectable up to six months after exposure in a controlled laboratory K. brevis exposure study of Eastern oyster and found to highly influence the composite toxins in the oyster as measured by enzyme linked immunosorbent assay (ELISA)32). In addition to S-desoxy BTX-B2 and BTX-B2, BTX-1 was found to significantly influence the composite toxins in K. brevis exposed clams33).

BTX-2 and major metabolites in molluscan shellfish
The complex metabolism of brevetoxins leading to numerous metabolites of varying polarity and hydrophobicity has been the major hurdle in developing methods for monitoring NSP toxins in shellfish. It is very challenging to prepare analytical standards of numerous complex metabolites present in the shellfish. Currently, several methods such as cytotoxicity, receptor binding assay, immunoassay, and LC-MS are found to be useful in detecting and quantifying brevetoxins. Based on the elements of toxin recognition (at site 5 of voltage gated sodium channels), cytotoxicity and receptor binding are pharmacology-based methods, whereas, immunoassay and LC-MS are structure-based methods34). liquid chromatography-mass spectrometry (LC-MS) and ELISA methods correlated better with mouse bioassay than cytotoxicity and receptor binding assays in a multi-laboratory study of five methods for the determination of brevetoxins in shellfish tissue extracts35). Bottein et al tested in vitro methods such as ELISA, radioimmunoassay, receptor binding assay and N2a cytotoxicity and in vivo mouse bioassay for the detection of BTX-B4, the N-palmitoyl conjugate of BTX-B236). Even though receptor binding assay of BTX-B4 showed comparable sensitivity to that for BTX-3, N2a cytotoxicity assay was 12 times more sensitive to BTX-B4 than BTX-3. The study showed that the relative sensitivity of the N2a cytotoxicity assay for BTX-B4, BTX-3 and BTX-B2 paralleled that of the mouse bioassay (relative LD50 values 1:20:30 for BTX-B4: BTX-3: BTX-B2). Method correlations and comparability becomes difficult with the complex metabolism of brevetoxins in shellfish.
2-3-1 Mouse bioassayToxicity of shellfish from K. brevis affected areas is currently assessed by mouse bioassay37) and this approach has been successful in managing NSP. In the United States, a value of 20MU/100g is the guidance level12), at or above which the shellfish are prohibited from harvest and unsafe for human consumption. The guidance level expressed in BTX-2 equivalents is approximately 0.8 mg/kg shellfish tissue. However, application of this equivalency value is not very practical due to the extensive metabolism of BTX-2 to metabolites of various toxic potencies. The NSP mouse bioassay developed in the 1960s continues to be the only approved regulatory method for NSP toxins even with the current information of the structural and toxicological properties of brevetoxins in K. brevis blooms and shellfish. Evaluation of the method based on the current knowledge of the algal toxins and their shellfish metabolites clearly reveals the need for new methods for monitoring brevetoxins in K. brevis exposed shellfish. Use of the mouse bioassay has several drawbacks as a monitoring tool. The method is laborious, time-consuming and extremely low-throughput, resulting in delayed regulatory decisions. It is also evident from the metabolite chemistry that the extraction protocol is not ideal for the polar metabolites and thus may fail to include the major metabolites present in the shellfish. It has been demonstrated that polar metabolites very likely play a major role in NSP38). Methods of high specificity, sensitivity, and sample throughput and providing equal public health protection as the mouse bioassay are needed as alternatives for monitoring and management of brevetoxins in shellfish.
2-3-2. Cytotoxicity assayThe cytotoxicity assay is based on the actions of brevetoxins on voltage-gated sodium channels of cultured neuronal cells39). It is a highly sensitive and useful screening assay for sodium channel activity of compounds present in shellfish extracts. It measures the composite toxicity, which reflects the toxic potencies of the individual metabolites present in the extract. Cytotoxicity of individual brevetoxin metabolites and their difference in toxic potencies were demonstrated in Eastern oyster25,40). The difference in toxicities of the metabolites was also demonstrated with semi-synthetic metabolites and metabolites isolated from shellfish41,42). Some of the disadvantages of the cytotoxicity assay are low sample throughput, low specificity, and interference of co-extracted shellfish compounds with the assay response; nevertheless, the assay serves as a very useful screening tool.
2-3-3. Receptor binding assayThe receptor binding assay is also a pharmacology-based method based on brevetoxin affinity for the sodium channel receptors, and is described using whole cell preparations and in high throughput microplate format40,43,44). Brevetoxin metabolites demonstrate differential affinities for the receptor and the assay measures composite brevetoxins in the extracts that is deduced from the metabolite affinities for the receptors. The assay is simple, rapid, and sensitive enough to use as a monitoring tool. However, its use of radiolabeled compounds is a major disadvantage, requiring specially trained operators and a license for radiation work. Recently, a binding assay with synaptosomes was developed using a fluorescent ligand, avoiding the use of radiolabeled ligands45). The receptor binding assay responds to all sodium channel receptor binding toxins and thus lacks a high level of specificity. The assay is also influenced by the chemistry and binding affinity of numerous brevetoxin metabolites present in the extract.
2-3-4. ImmunoassaysImmunoassays are structure-based methods for assessing the levels of brevetoxins based on epitope specificity of an incorporated antibody. Currently, most immunoassays for NSP toxins use an antibody of high specificity for the B-type brevetoxin backbone structure with little or no cross-reactivity with the A-type brevetoxins or other polyether marine toxins. A competitive ELISA described by Naar et al. has been shown to measure brevetoxins in wide variety of matrices such as shellfish, seawater, and clinical samples with minimum sample clean-up and an excellent sensitivity of 0.025 mg/kg46). Since the antibody is directed against the H-K ring region of B-type backbone structure, most of the B-type brevetoxin metabolites present in the shellfish are recognized and identical antibody affinities have been demonstrated for major metabolites41). Immunoassays provide high sensitivity and specificity and are available in high sample throughput format. ELISA kits are commercially available and provide rapid measurement of composite B-type brevetoxins in the extract with excellent precision.
2-3-5. Liquid chromatography-mass spectrometryLC-MS methods are structure-based methods that have been in use for measuring the level of individual brevetoxin congeners in shellfish extracts. LC-MS provides unambiguous identification and confirms the presence of NSP toxins in various matrices. Mass spectrometry has been used successfully in characterizing various brevetoxin derivatives in K. brevis blooms, metabolites in shellfish, clinical samples, and epidemiological outbreak investigations22). Extraction of brevetoxins from shellfish samples is challenged by matrix complexity and various chemical natures of the numerous metabolites present in shellfish. Selection of extraction solvents and clean-up procedures for optimal recovery of numerous polar and non-polar metabolites is also challenging. Quantification of individual brevetoxins by LC-MS requires analytical standards for each due to the chemical complexity of the shellfish metabolites. Unfortunately, only few brevetoxin standards are currently available commercially and as a result, utilization of LC-MS in determining the composite toxins level in the extract is not possible. LC-MS offers superior specificity and adequate sample throughput for the detection of individual brevetoxins in various matrices, but the unavailability of standards hinders the application of LC-MS as a monitoring tool in its current format.
2-4. A Novel Approach to Developing Methods for Monitoring NSP Toxins 2-4-1. Brevetoxin biomarkers in shellfishWith advancing analytical techniques, brevetoxin metabolism and elimination have been characterized in several species of molluscan shellfish. Review of the methods for brevetoxins analysis points out that the complex metabolism of brevetoxin in shellfish is the primary hindrance to developing new methodologies for monitoring NSP toxins in shellfish. The presence of numerous metabolites and lack of analytical standards makes it difficult to determine the toxicity equivalency factor for each metabolite for deriving the composite toxicity of the shellfish sample, which is currently assessed by mouse bioassay. Species differences are also evident in metabolism and may be reflected in rates of elimination of brevetoxins in shellfish. Major metabolites present in Pacific oyster, cockle, greenshell mussel, Eastern oyster, and hard clams have been identified and the profile consists of metabolites of various toxic potencies28). Information on metabolism and elimination of brevetoxins in molluscan shellfish has led to the development of a novel monitoring approach focused on addressing brevetoxin metabolites in shellfish rather than parent algal brevetoxins. In the Eastern oyster, S-desoxy BTX-B2 and BTX-B2 were found to be the most abundant and persistent metabolites as shown by controlled laboratory exposure studies and K. brevis bloom exposure studies24,32) and the summed level of S-desoxy BTX-B2 and BTX-B2 as measured by LC-MS showed excellent correlation (R2=0.9) to composite B-type brevetoxins as measured by ELISA24). Brevetoxin uptake and elimination were examined in Eastern oysters exposed to recurring blooms of K. brevis in Sarasota, FL over a 3 year period32) by LC-MS, ELISA, and mouse bioassay. LC-MS measured the levels of individual metabolites, ELISA measured composite B-type brevetoxins and mouse bioassay assessed shellfish toxicity. The summed levels of S-desoxy BTX-B2, and BTX-B2 in oysters by LC-MS was highly correlated with composite toxin as measured by ELISA (R2=0.9); biomarkers (LC-MS) and composite toxin level (ELISA) also correlated well, R2=0.73 and R2=0.74 respectively, with mouse bioassay32). The study identified composite B-type toxins by ELISA and level of S-desoxy BTX-B2, and BTX-B2 measured by LC-MS as potential screening and confirmatory methods, respectively, for monitoring brevetoxins in Eastern oyster.
Metabolism studies in the hard clam (Mercenaria sp.) found several metabolites contributing to the composite brevetoxin response by ELISA. Five metabolites, S-desoxy BTX-B2, BTX-B2, BTX-B5, open A-ring BTX-B5, and BTX-B1, were identified as major contributors when the clams were highly toxic. During a two-and-a-half-year study period, hard clams were examined from a single site in Sarasota, FL where multiple K. brevis blooms occurred. Composite B-type toxins by ELISA and individual metabolite concentrations by LC-MS were determined in the clams collected throughout the study period. Study results showed that three of the major metabolites, S-desoxy BTX-B2, BTX-B2, and BTX-B1 were the most persistent and detected metabolites in hard clam for several months after dissipation of the blooms33). Composite B-type brevetoxin response by ELISA was significantly influenced by S-desoxy BTX-B2, BTX-B2, and BTX-B1during and post-bloom (Fig.3). Summed values of these metabolites were highly correlated with composite B-type brevetoxins as measured by ELISA (R2=0.9). ELISA and LC-MS also correlated moderately with toxicity assessed by mouse bioassay, R2=0.6 and R2=0.5, respectively. The study identified S-desoxy BTX-B2, BTX-B2, and BTX-B1 as major components of the composite toxin levels in hard clams exposed to K. brevis blooms. These summed metabolites accurately reflected the composite B-type toxins by ELISA, intensity of K. brevis bloom and toxicity of shellfish by mouse bioassay (Fig. 4).
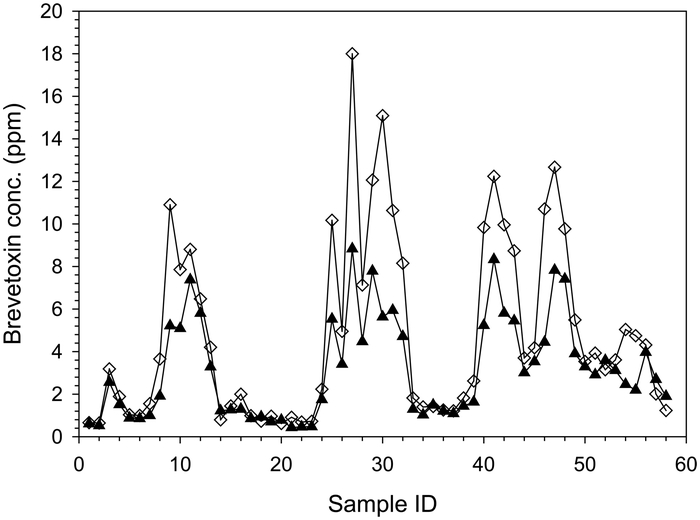
Composite B-type metabolites by ELISA ( ) and sum of S-desoxy BTX-B2, BTX-B2, and BTX-B1 by LC-MS (
) and sum of S-desoxy BTX-B2, BTX-B2, and BTX-B1 by LC-MS ( ) in hard clams (Mercenaria sp.).
Sample IDs represent sequential collections from a single site over time.
) in hard clams (Mercenaria sp.).
Sample IDs represent sequential collections from a single site over time.

Sum of BTX-B1, BTX-B2, and S-desoxy BTX-B2 by LC-MS, composite B-type brevetoxins by ELISA and toxicity by mouse bioassay of hard clams (Mercenaria sp.) collected during and after K. brevis blooms. Sample IDs represent sequential collections from a single site over time. Arrows indicate the bloom peaks.
Brevetoxin metabolism and elimination studies in oyster and clam have led to the identification of S-desoxy BTX-B2, BTX-B2, and BTX-B1 as biomarkers of exposure and toxicity in these species (Fig. 5). One of the common features of these selected biomarkers is that they are the products of conjugation of BTX-2 to amino acid derivatives, making all three products of comparable polarity, thus enabling similar conditions for sample extraction and analysis by LC-MS. The analytes are eluted from the LC column very closely using a gradient and the mass spectrometer parameters are similar for all three biomarkers33), thus making the analytical method appropriate for its purpose when limited availability of analytical standards of metabolites is a major obstacle for the development and application of an LC-MS method for monitoring NSP toxins in molluscan shellfish.
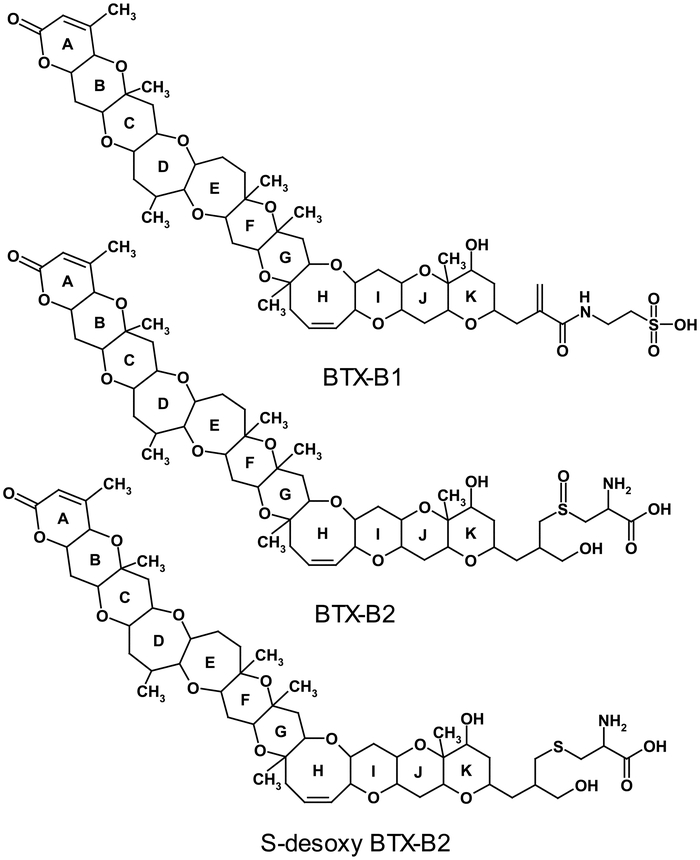
Brevetoxin biomarkers in Eastern oyster and hard clams exposed to blooms of K. brevis.
In the Gulf of Mexico, the most important commercial shellfish species are Eastern oysters and hard clams. Sunray venus clams (Macrocallista nimbosa), are a relatively new aquaculture shellfish species in Florida, and has been found to metabolize brevetoxins similarly to hard clams. Aquacultured clams are an important product for the seafood industry in the Gulf of Mexico region and thus were also examined to assess the utility of the three biomarkers in monitoring NSP toxins. Shellfish sampling and testing is routinely conducted by state authorities in the Gulf of Mexico region where K. brevis blooms frequently occur. A long-term project designed to examine these species (Eastern oyster, hard clam, and sunray venus clam) is ongoing to gauge the application of brevetoxin biomarkers for monitoring NSP toxins in shellfish and to potentially update the guidance level used in conjunction with the method. The toxicity of the appropriate samples had been assessed by mouse bioassay for opening shellfish harvest areas affected by K. brevis blooms. There is a distinct difference between the mouse bioassay and LC-MS sample preparation procedures. The extraction conditions used for ELISA and LC-MS use mild conditions and thus the structural integrity of metabolites is preserved as present in the shellfish. Brevetoxins in the shellfish homogenates were extracted with 80% methanol for ELISA and LC-MS analysis33,46). Methanol extract was further defatted with hexane and cleaned-up using C18 solid phase extraction (SPE) column for LC-MS analysis. For mouse bioassay, shellfish homogenates were boiled with concentrated HCl and NaCl for 5 minutes before extraction with diethyl ether37). The mouse bioassay extraction conditions are harsh and may generate compounds of varying toxic potencies that are different than those present in the shellfish. The examination of 138 of these shellfish samples, of which 36 were above the action limit by mouse bioassay (>20MU/100g), showed good correlation of biomarker levels by LC-MS and composite B-type brevetoxins measurement by ELISA (Fig. 6). The sum of the concentrations of the biomarkers by LC-MS determined in the shellfish samples ranged from 0.09 ppm to 15.71 ppm with a composite B-type brevetoxin value of 0.2 ppm to 37.3 ppm. The biomarker concentrations in all three matrices showed high correlation with composite B-type toxins by ELISA, correlation coefficient, R2=0.93, confirming the agreement observed in the earlier studies examining the in vitro methods24,28,33). Correlation in each matrix were, R2=0.96, R2=0.93, and R2=0.96 for hard clam, sunray venus clam, and oyster, respectively. Comparison of biomarkers by LC-MS and shellfish toxicity by mouse bioassay showed a correlation of R2=0.97, and R2=0.81for hard clam and sunray venus clam, respectively (Fig. 7). Samples passing by mouse bioassay are not expressed in numerical mouse units, but are reported as <20MU/100g). This semi-quantitative nature of the mouse bioassay restricts data use for the correlation of methods and for use in deriving a 20 mouse unit equivalent value for in vitro methods to only those samples that are above the action limit (≥20MU/100g). To date, quantitative mouse bioassay data for oysters is not adequate for deriving information on correlation of biomarker concentration with shellfish toxicity by mouse bioassay. Efforts are ongoing in collecting shellfish as K. brevis blooms occur in the Gulf of Mexico region for generating additional data for comparing the utility of biomarkers in monitoring NSP toxins with the mouse bioassay. As examination of shellfish exposed to K. brevis blooms continues, additional samples above the action limit will be available and will ultimately generate sufficient data for a final assessment report for utilizing biomarkers by LC-MS for monitoring NSP toxins in shellfish.

Correlation of biomarkers by LC-MS with composite B-type toxins by ELISA in farmed clams impacted by K. brevis blooms: (A) Hard clams, (B) Sunray venus clams.
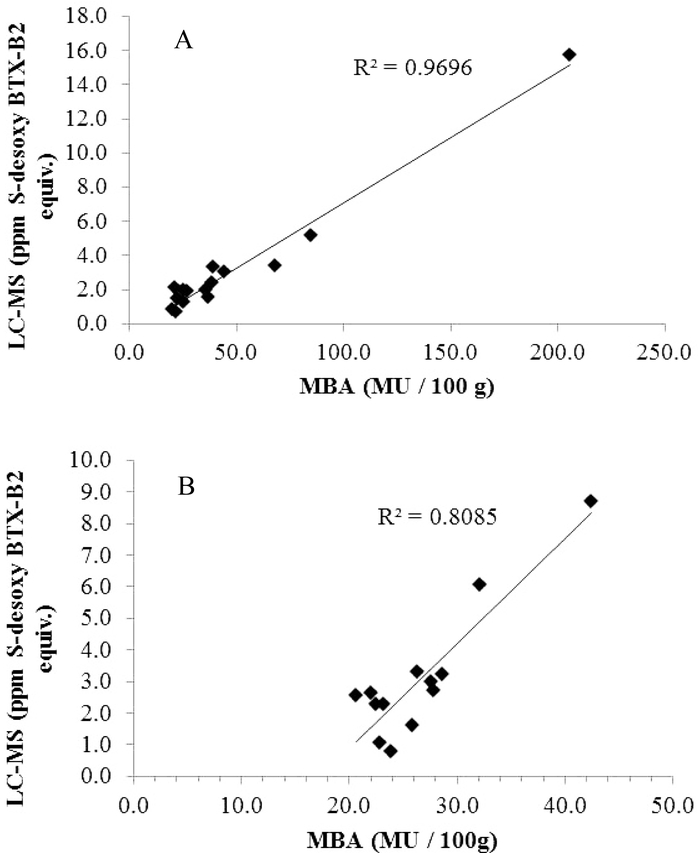
Correlation of biomarkers by LC-MS with shellfish toxicity by mouse bioassay in farmed clams impacted by K. brevis blooms: (A) Hard clams, (B) Sunray venus clams.
The complexity of brevetoxin metabolism in molluscan shellfish has been the major hurdle in developing in vitro methods for the detection of NSP toxins as alternative methods to the mouse bioassay. The presence of numerous metabolites with varying chemical properties and toxicities, a broad range of analytical standards, currently not available, would be required to determine the full composite toxin burden in K. brevis exposed shellfish. It is also very challenging to optimize sample preparation techniques that suit metabolites of varying polarities and chemistries. Mouse bioassay sample preparation is harsh and selective and the resulting extract does not include the full spectrum of brevetoxin shellfish metabolites. The novel approach of utilizing biomarkers to assess the toxin burden of brevetoxin contaminated shellfish will overcome the need for having analytical standards for each of the metabolites and the sample preparation challenges in including all metabolites. Biomarker levels determined by LC-MS correlated with composite toxins by ELISA and toxicity assessment by mouse bioassay in wild and farmed shellfish. Study results support application of biomarkers by LC-MS as an alternative method for monitoring brevetoxins in K. brevis exposed shellfish. Extensive method assessment and additional data are needed for this purpose.
The authors have no conflicts of interest.