論文ID: 2014036
論文ID: 2014036
A novel type of encephalopathy associated with the ingestion of Sugihiratake mushroom (Pleurocybella porrigens) occurred in patients with chronic renal failure treated on hemodialysis in fall, 2004 in Japan. To clarify the mechanism of encephalopathy onset, we, for the first time, purified the cyanogen glycoside fraction (CG) from Sugihiratake mushroom using reversed phase high-performance liquid chromatography and hydrophilic interaction chromatography. Furthermore, we investigated single dose toxicity of the CG in an adenine-induced rat model of chronic renal damage (CRD). Pathological examination of kidneys indicates the development of CRD. Oral administration of the CG induces the accumulation of thiocyanate in the hemolyzed blood and brain in CRD rats, although no morphological changes were found in the brain. No further enhancement of kidney damage is observed after the oral administration of the CG in CRD rats. This is the first experimental report to suggest that acute encephalopathy, induced by Sugihiratake mushroom intake in the patients with chronic renal failure, is associated with intoxication of cyanide and thiocyanate, presumably produced metabolically produced after the ingestion of Sugihiratake mushroom.

Cyanide (%) content of fractions obtained from hydrophilic interaction chromatography.
The vertical axis represents the ratio of cyanide content detected in each fraction to total cyanide content detected in the sample before the injection to HPLC.

Temporal changes of cyanide levels in the hemolyzed blood in an adenine-induced rat model of CRD.
Control represents the control group administered distilled water alone, Low-dose represents the low-dose group administered 360 mg cyanogen glycoside fraction (CG)/kg body weight (corresponding to 0.31 mg sodium cyanide/kg body weight), and High-dose represents the high-dose group administered 2840 mg CG/kg body weight (corresponding to 2.44 mg sodium cyanide/kg body weight). Bars represent means ± standard deviation (3 rats/group).
The bar represents the change of cyanide level in the hemolyzed blood from 0 h to 8 h after administration of CG
The bar represents the change of cyanide level in the hemolyzed blood from 0 h to 24 h after administration of CG

Temporal changes of thiocyanate levels in the hemolyzed blood in an adenine-induced rat model of CRD.
Control represents the control group administered distilled water alone, Low-dose represents the low-dose group administered 360 mg CG/kg body weight (corresponding to 0.31 mg sodium cyanide/kg body weight), and High-dose represents the high-dose group administered 2840 mg CG/kg body weight (corresponding to 2.44 mg sodium cyanide/kg body weight). Bars represent means ± standard deviation (3 rats/group). Asterisks indicate significant differences from control values (**p < 0.01).
The bar represents the change of thiocyanate level in the hemolyzed blood from 0 h to 8 h after administration of CG
The bar represents the change of thiocyanate level in the hemolyzed blood from 0 h to 24 h after administration of CG
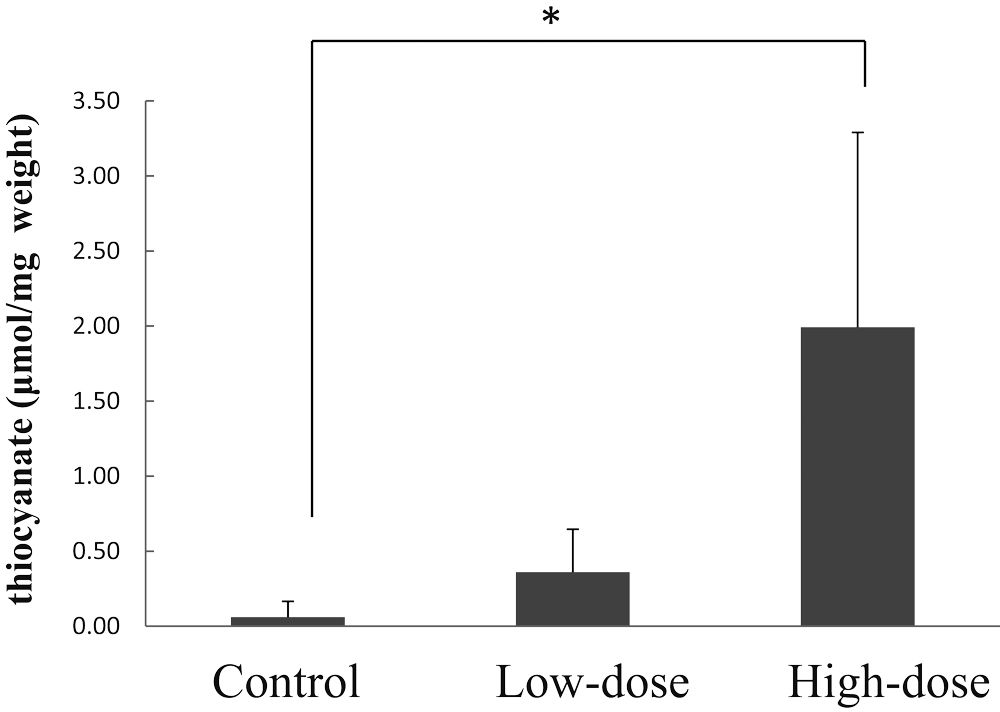
Thiocyanate content in the brains of adenine-induced CRD rats.
Control represents the control group administered distilled water only, Low-dose represents the low-dose group administered 360 mg CG/kg body weight (corresponding to 0.31 mg sodium cyanide/kg body weight), and High-dose represents the high-dose group administered 2840 mg CG/kg body weight (corresponding to 2.44 mg sodium cyanide/kg body weight). Bars represent means ± standard deviation (3 rats/group). Asterisks indicate significant differences from control values (*p < 0.05).
The bar represents brain thiocyanate levels after administration of CG

Macroscopy of the normal kidney (left), and Adenine represents the adenine-induced crystal nephropathy observed in CRD rats (right).

Histopathology of the cerebrum cortex and cerebrum caudal nucleus of CRD rats after administration of Sugihiratake extract.
Control represents the control group administered distilled water alone, and High-dose represents the high-dose group administered 2840 mg CG/kg body weight (corresponding to 2.44 mg sodium cyanide/kg body weight. Microscopically no treatment related abnormalities were observed in neurons and glial cells. x100. Hematoxylin-eosin staining.
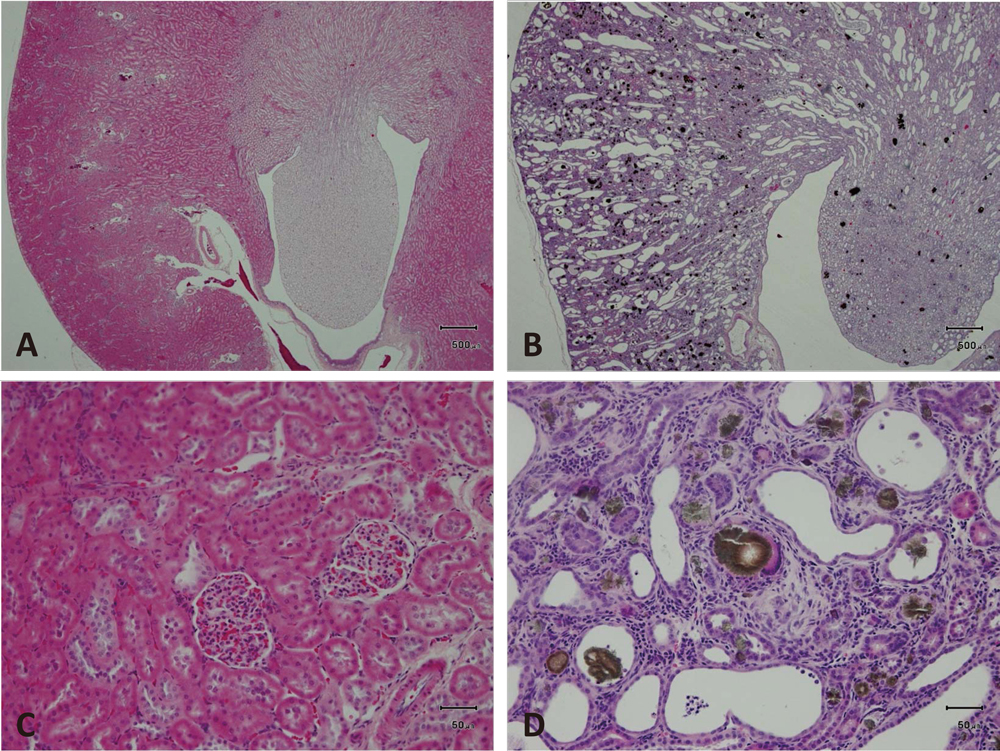
Histopathological images of the kidneys of CRD rats.
A-D, Histopathological changes in the kideny. A and C, Normal structure obtained from a normal adult rat kidney. B and D, Adenine-induced chronic renal damage. The normal structure was destroyed, and brown to black dots, adenine crystals, were diffusely distributed throughout the kidney at low magnification (x10). C and D, Higher magnification (x100) of A and B. C. Normal structure. D, Various sizes of crystals are accumulated in the dilated lumen of renal tubules or deposited in the tubules. Inflammatory cells infiltrate the interstitium. Hematoxylin-eosin staining.
| IndividualNo. | Animal*No. | Quarantine and acclimation periods | Adenine-component feed feeding period | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ---- 0.75% --- | -------- 0.5% -------- | |||||||||
| Day | 1 | 5 | 9 | 1 | 8 | 15 | 22 | 28 | ||
| 1 | 1302 | 298 | 334 | 355 | 362 | 281 | 270 | 270 | 259 | |
| 2 | exc. | 308 | 355 | 381 | 384 | 267 | - | - | - | |
| 3 | 1203 | 295 | 344 | 366 | 368 | 268 | 282 | 285 | 282 | |
| 4 | 1103 | 288 | 317 | 332 | 334 | 257 | 220 | 219 | 220 | |
| 5 | 1301 | 304 | 337 | 368 | 378 | 270 | 282 | 285 | 256 | |
| 6 | 1303 | 297 | 343 | 366 | 375 | 321 | 303 | 298 | 282 | |
| 7 | 1101 | 315 | 354 | 385 | 388 | 293 | 258 | 260 | 262 | |
| 8 | 1201 | 306 | 348 | 368 | 378 | 301 | 279 | 279 | 261 | |
| 9 | 1102 | 303 | 340 | 361 | 362 | 275 | 271 | 270 | 273 | |
| 10 | 1202 | 305 | 345 | 363 | 369 | 266 | 268 | 264 | 254 | |
| Mean | 302 | 342 | 365 | 370 | 280 | 270 | 270 | 261 | ||
| S.E. | 2 | 3 | 5 | 5 | 6 | 8 | 8 | 6 | ||
Unit: g.
No.2: died on day 8 of adenine-component feed feeding period (266 g body weight).
* Identification numbers after allocation to treatment groups (exc.: excluded from this study).
Quarantine period: 5 days.
Acclimation period: 9 days (with quarantine period).
| Test substance | Control | Sugihiratake extract | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 360 mg/kg | 2840 mg/kg | |||||||||||
| Animal No. | 1101 | 1102 | 1103 | 1201 | 1202 | 1203 | 1301 | 1302 | 1303 | |||
| Heart | ||||||||||||
| Myocardium | ||||||||||||
| Focal calcification | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | |||
| Coronary artery | ||||||||||||
| Cellular infiltration, mononuclear cell | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | |||
| Calcification | 0 | 0 | 3 | 3 | 0 | 0 | 3 | 2 | 1 | |||
| Arcus aorta | ||||||||||||
| Tunica media, calcification | 2 | 0 | 3 | 3 | 1 | 0 | 3 | 2 | 1 | |||
| Tunica intima, cellular infiltration, neutrophil / mononuclear cell | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | |||
| Lung | ||||||||||||
| Alveolar septa, calcification | 0 | 0 | 3 | 0 | 0 | 0 | 2 | 0 | 0 | |||
| Bronchial smooth muscle, calcification | 0 | 0 | 2 | 0 | 0 | 0 | 1 | 0 | 0 | |||
| Kidney | ||||||||||||
| Artery | ||||||||||||
| Calcification | 1 | 0 | 2 | 2 | 1 | 0 | 2 | 2 | 1 | |||
| Renal tubule | ||||||||||||
| 2,8-dihydroxyadenine (DHA) crystals | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | |||
| Tubular dilatation | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | |||
| Tubular basophilic change | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | |||
| Cellular infiltration, neutrophil | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | |||
| Giant cells | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |||
| Interstitium | ||||||||||||
| Cellular infiltration, neutrophil / mononuclear cell | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |||
| Fibrosis | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | |||
0: No change, 1: Slight, 2: Moderate, 3: Marked.
No significant changes were detected: Spleen, Liver.
| Test substance | Control | Sugihiratake extract | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 360 mg/kg | 2840 mg/kg | |||||||||||
| Findings | Animal No. | 1101 | 1102 | 1103 | 1201 | 1202 | 1203 | 1301 | 1302 | 1303 | ||
| Kidney | ||||||||||||
| Enlargement, pale yellow | + | + | + | + | + | + | + | + | + | |||
| Thymus | ||||||||||||
| Small size | + | + | + | + | + | + | + | + | + | |||
| Arcus aorte | ||||||||||||
| Grayish white | - | - | + | - | - | - | - | - | - | |||
| Coronary artery | ||||||||||||
| Grayish white | - | - | + | - | - | - | - | - | - | |||
| Glandular stomach | ||||||||||||
| Grayish white | - | - | + | - | - | - | + | - | - | |||
| Colon | ||||||||||||
| Grayish white | - | - | + | - | - | - | + | - | - | |||
-: No change, +: Change.