2023 年 57 巻 2 号 p. 73-84
2023 年 57 巻 2 号 p. 73-84
An optimised and improved process is presented for the sequential separation of Pb, Sr, and light rare earth elements (LREEs) from a single aliquot of digested silicate sample. Cation exchange resin, Sr resin, and Ln resin columns are used to achieve 80–90% recovery yield of each element with high purity. Pb and Sr are recovered using a miniaturised Sr column after the separation of the Pb-Sr fraction from the digested silicate sample using cation exchange resin (AG50W-X8). The rare earth elements (REE) fraction recovered from the same step is used to separate Nd using Ln resin. We have used dilute HNO3 to elute a Ce and Sm free Nd fraction (Ce/Nd < 10–5). The REE fraction was oxidised with >5 mM NaBrO3 solution prior to loading on the Ln resin. The required concentration of NaBrO3 was tested using reference rock JB-2 and 5 mM was found to be sufficient for REE from 20 mg rock powder. The separation process also yields highly pure Ba, La, Ce, and Sm as by-products which can be used for isotopic analyses. The activity of the NaBrO3 solution degrades rapidly, and therefore should be used within 2–3 days of preparation. Pb-Sr-Nd isotopic ratios of nine reference rocks and sediments from GSJ and USGS were measured using TIMS and are found to be accurate and reproducible. We also report the first Pb-Sr-Nd isotopic ratios for JSl-1 (slate from GSJ) and Pb isotopic ratio of SDO-1 (shale from USGS).
Sr, Nd, and Pb are the most analysed elements in isotope geochemistry and have been used to decipher the origin and evolution of geochemical, cosmochemical, environmental, archaeological, and forensic samples from materials such as rock, soil, water, metal, bone etc. (Kenoyer et al., 2013; Molofsky et al., 2014; Font et al., 2015; Sharma et al., 2017; Chatterjee and Ray, 2018). Precise and rapid analyse of multiple samples are a requirement in most of these fields. Rare samples such as extra-terrestrial material, mantle xenoliths, mineral segregates, and archaeological samples require accurate analysis from a small amount of material making it difficult to obtain precise data unless multiple elements are extracted from a single aliquot. Standard procedures used in isotope geochemistry require individual aliquots of digested material, containing 100–200 ng of the target element, for determination of isotopic ratios of Pb, Sr, Nd, Hf etc. (Koide and Nakamura, 1990; Makishima and Nakamura, 1991; Yoshikawa and Nakamura, 1993). Lengthy column chemistry procedures are often involved in producing pure element fractions (e.g., Pin and Gannoun, 2019), increasing time requirement, cost, and blank of analysis. Recent developments (Makishima et al., 2008; Pin et al., 2014; Li et al., 2015a; Kagami and Yokoyama, 2016; Pin and Gannoun, 2017) have overcome these problems to some extent by combining separation methods. However, further development in this field is needed to meet the growing demands in isotope geochemistry.
The costs of ultraclean reagents, ion-exchange resins, extraction resins, and other consumables required for mass spectrometry add to the cost of regular maintenance of laboratories and instruments. Extracting multiple elements simultaneously for isotopic analysis reduces these costs significantly, and efforts have been taken over recent years to combine multiple processes, reducing the time and cost of routine isotope analysis (Makishima et al., 2008; Li et al., 2015a). Makishima et al. (2008) separated Pb, Sr, and Nd from an aliquot of acid-digested sample using a single Sr resin column for Pb and Sr, and two columns (cation exchange column and Ln resin column) for Nd. Although their method produces three element fractions from the same aliquot, Pb-Sr and Nd require two different loading fractions and more amount of sample altogether. Another drawback of this method is the presence of interfering elements such as Ba, La, and Ce in the Nd fraction which reduces ionization efficiency of Nd. 142Ce has an isobaric effect on 142Nd making it difficult to determine 142Nd/144Nd ratios.
Ce-Nd separation using Ln resin, HNO3, and an oxidising reagent (NaBrO3 or KBrO3) have been widely used in recent years (Li et al., 2015b; Kagami and Yokoyama, 2016). These methods were built on liquid-liquid phase exchange techniques (Rehkämper et al., 1996; Caro et al., 2006) involving HDEHP in n-heptane and the oxidised sample solution in concentrated HNO3 (~10 M). Concentrated HNO3 results in the recovery of all REEs except Ce, however, it is difficult to handle and causes severe damage to equipment and containers. Therefore, we have attempted to produce a pure Nd fraction using diluted nitric acid (~0.2 M HNO3), which allows for better separation between Ba, La, Ce, Nd, and Sm in a single step, and is safer to handle.
We aim to establish a combined method of separation for Pb-Sr-Nd in this study. Our use of a cation exchange resin at the first step enables efficient separation between Ba and Sr. Ba, Ce, Sm, and La are also recovered as a by-product during Nd separation and enable simultaneous analysis of radiogenic Pb-Sr-Nd isotope geochemistry, Ba stable isotope geochemistry, and Sm-Nd and La-Ce geochronology. We have developed the current method in order to reduce the time and cost required for sequential separation while producing pure fractions of Pb, Sr, and Nd from geological samples. We have also optimised reagent concentrations in order to minimize blank and maximise the purity of the target elements.
Electronic grade (EL) HCl and HNO3, ultrapure grade HClO4, (Kanto Chemicals, Japan) and TAMAPURE AA-10 grade HF and H2O2 (Tama Chemicals, Japan) were used without further purification. A prototype TAMAPURE H3PO4 (Tama Chemicals, Japan) was used for Pb loading. High purity NaBrO3 (Sigma Aldrich, USA) was used as an oxidising reagent. Ultra-pure Milli-Q water (>18.2 MΩ cm; Millipore System, USA) was used to dilute all reagents to the desired concentration.
AG50W-X8 cation exchange resin (200–400 mesh; Bio-Rad, USA), Sr resin (100–150 μm; Eichrom) and Ln resin (50–100 μm; Eichrom) were thoroughly washed with EL grade 6 M HCl, 6 M HNO3, and Milli Q water before loading onto the columns to reduce blank. Cation exchange resin was used in polypropylene mini-columns (Muromachi Chemicals, Japan) with 1 mL volume (i.d. 5 mm) for the 1st step. Ln resin and Sr resin (Eichrom, USA) were used in the subsequent steps of separation. Teflon columns were prepared inhouse with ~3 mm inner diameter and 4.3 cm length for Ln column (0.3 mL) and 1.5 cm length for Sr resin (0.1 mL). Nine different silicate reference rocks and sediments including granite, granodiorite, basalt, shale, slate, and sediments i.e., JG-1a, JG-2, JG-3, JB-2, JSd-1, JSl-1, and JLk-1 from GSJ; and BCR-2 and SDO-1 from USGS were used to determine Pb-Sr-Nd isotopic ratios using TIMS to confirm applicability of the proposed method to various types of rocks. Eight different aliquots of JB-2 (GSJ) were prepared to test the reproducibility of isotopic data using this method.
Sample digestionSample preparation and column chemistry were carried out in a clean room at Hiroshima University, Japan, on a clean bench on which a particle counter detected few particles larger than 0.3 μm. 20–40 mg of silicate samples were digested in clean, 7 mL PFA vials (Savillex, USA) using HF, HClO4, and HCl and stepwise heating following the methods of Yokoyama et al. (1999) to ensure complete dissolution of the target elements, and finally dissolved with 2.5 M HCl for loading onto the 1st column. Complete dissolution was confirmed by lack of precipitation after centrifuging the solution at 3000 rpm for 5 minutes.
Column chemistryColumn chemistry was performed sequentially using three different columns to separate Pb, Sr, and Nd. Ba, Ce, La, and Sm were also separated in the same process from a 1 mL aliquot of digested silicate sample (Fig. 1; Table 1).
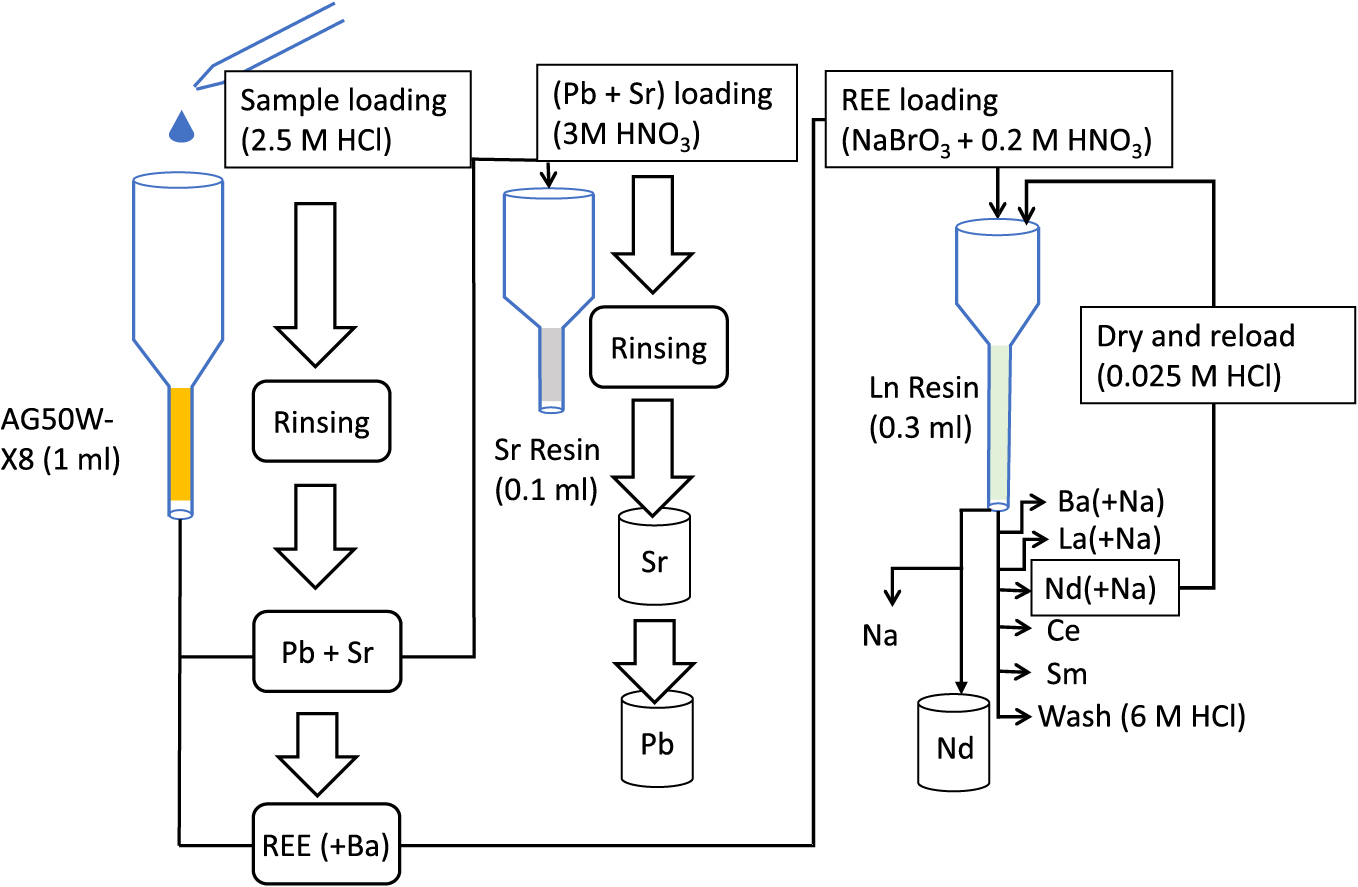
Schematic diagram of the sequential column chemistry procedure
| Function | AG50W-X8 (1 ml) | Sr resin (0.1 ml) | Ln resin (0.3 ml) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Eluent | Amount | Fraction | Eluent | Amount | Fraction | Eluent | Amount | Fraction | |
| Pre-washing | 7 M HF | 1 ml | 7 M HF | 1 ml | 7 M HF | 1 ml | |||
| 6 M HCl | 6 ml | 6 M HCl | 4 ml | 6 M HCl | 4 ml | ||||
| H2O | 6 ml | H2O | 4 ml | H2O | 4 ml | ||||
| Conditioning | 2.5 M HCl | 1 ml | 3 M HNO3 | 0.1 ml | 0.2 M HNO3 + 20 mM NaBrO3 | 0.3 ml | |||
| Loading | 2.5 M HCl | 0.5 ml | Major elements | 3 M HNO3 | 0.6 ml | 0.2 M HNO3 + 20 mM NaBrO3 | 0.1 ml | ||
| 0.5 ml | Pb | ||||||||
| Washing | 2.5 M HCl | 1 ml | Pb | 3 M HNO3 | 1 ml | Remaining major elements | 0.2 M HNO3 + 20 mM NaBrO3 | 0.5 ml | Ba |
| 1.5 ml | Major elements | ||||||||
| Collection | 2.5 M HCl | 5.5 ml | Sr | 0.05 M HNO3 | 1 ml | Sr | 0.2 M HNO3 + 20 mM NaBrO3 | 0.6 ml | La |
| 6 M HCl | 5.5 ml | REE | 6 M HCl | 3 ml | Pb | 2 ml | Nd | ||
| Washing | H2O | 1 ml | Na | ||||||
| 0.025 M HCl | 4 ml | ||||||||
| Collection | H2O2 | 0.1 ml | Ce | ||||||
| 0.25 M HCl | 0.8 ml | ||||||||
| 2 ml | Sm | ||||||||
A column filled with 1 mL of pre-cleaned AG50W-X8 resin was used to separate the Pb-Sr fraction and REE fraction. The column was sequentially washed with 1 mL 7 M HF, ~6 mL 6 M HCl and ~6 mL Milli Q water to clear any potential contamination during manufacturing and storage. HF was used to remove any potential silicate contamination during manufacturing. Before loading, the column was conditioned with 1 mL of 2.5 M HCl. 1 mL sample solution in 2.5 M HCl was loaded on the column in 0.25 mL fractions. Pb-Sr and REE fractions along with Ba were eluted sequentially with 7 mL 2.5 M HCl and 5.5 mL 6 M HCl (Table 1; Fig. 2) and evaporated to dryness in a clean evaporator. The total time required for this column process, including prewashing and conditioning, was ~4 h.

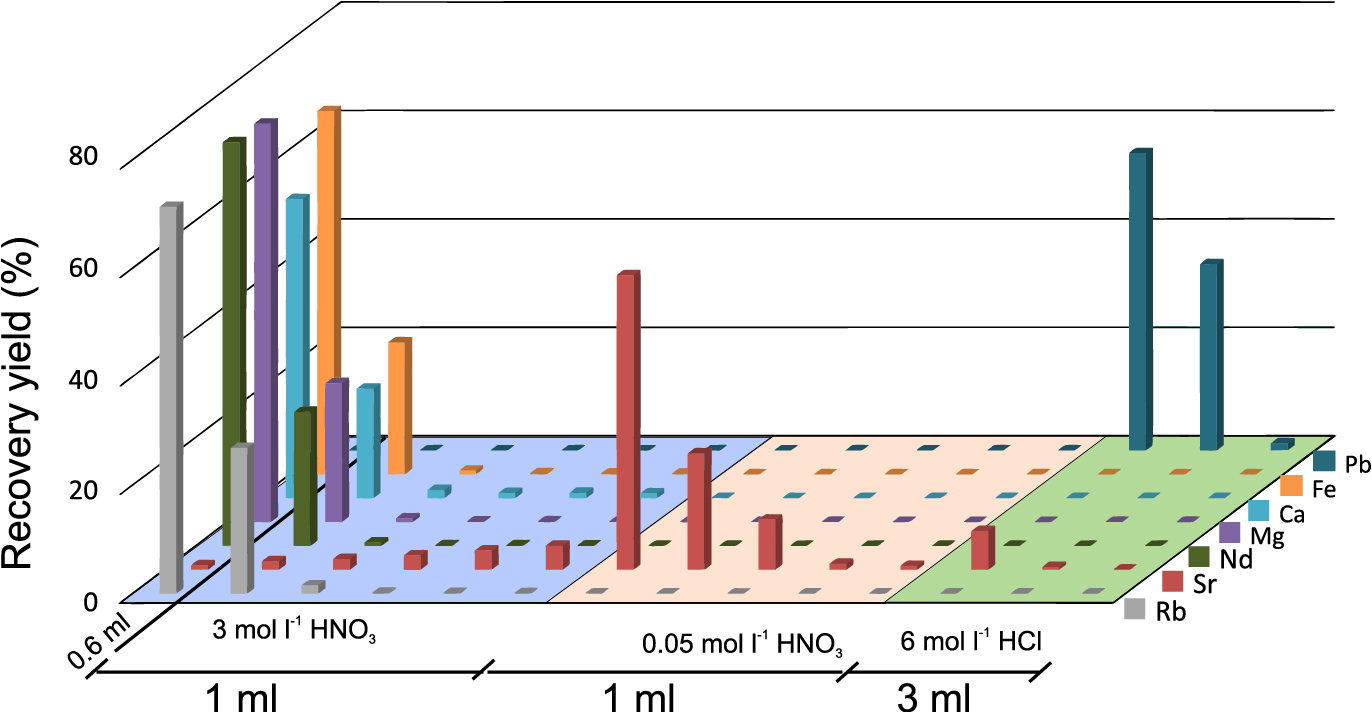
Plot showing elution profile of Pb, Sr, Rb, LREEs and major elements from 1 mL AG50W-X8 resin. Each fraction of 0.5 mL was analysed in ICP-MS for element concentrations. Pb was present in 3 fractions (1.5 mL 2.5 M HCl) while Sr was present in 10 fractions (5 mL 2.5 M HCl). REEs were present 5.5 mL 6 M HCl.
0.1 mL Sr resin was used in the next step to separate Pb and Sr. The column was washed similar to the 1st column and conditioned with 0.1 mL 3 M HNO3. Pb-Sr fraction was dissolved in 0.6 mL 3 M HNO3 and loaded in 0.1 mL fractions before rinsing with 1 mL of 3 M HNO3 to remove the remaining major elements and Rb. Sr was eluted with 1 mL of 0.05 M HNO3 and Pb with 3 mL of 6 M HCl (Fig. 3). The total time required for the Sr column is ~1.5 h.
Plot showing elution profile of Pb, Sr, Nd and major elements from 0.1 mL Sr resin. A loading fraction of 0.6 mL and subsequent rinsing fractions of 0.2 mL HNO3 and 1 mL fractions of 6 M HCl were analysed using ICP-MS. Major elements, Rb and Nd are present in the loading and 1st rinsing fractions. Sr is present in 1 mL of 0.05 M HNO3 while Pb is present in 3 mL of 6 M HCl.
0.3 mL of Ln resin in an inhouse column was used for the separation of Nd from REEs. 0.2 M HNO3 mixed with 20 mM NaBrO3 solution was used as the eluent in this step. The REE fraction obtained from the previous step was dissolved in 0.1 mL of this solution to oxidise Ce3+ to Ce4+ state. The REE fraction was loaded after washing the Ln resin column with HF, HCl and Milli Q water similar to the first column, and conditioning with the 0.2 M HNO3–20 mM NaBrO3 solution. Ba was eluted with 0.5 mL of the same solution and La was eluted with a further 0.6 mL of the HNO3-NaBrO3 solution. Nd (along with Pr) was eluted with a further 2 mL of the same solution. (Fig. 4). The resin column was rinsed with ~1 mL Milli Q water and ~4 mL 0.025 M HCl to remove NaBrO3 before Ce was eluted with 2–3 drops of H2O2 and 0.8 mL of 0.25 M HCl consecutively. Sm is recovered subsequently with 2 mL of 0.25 M HCl (Fig. 4). Distribution coefficient of Na in Ln resin is very low compared to REEs, and both Ce and Sm can be collected Na free from this column.
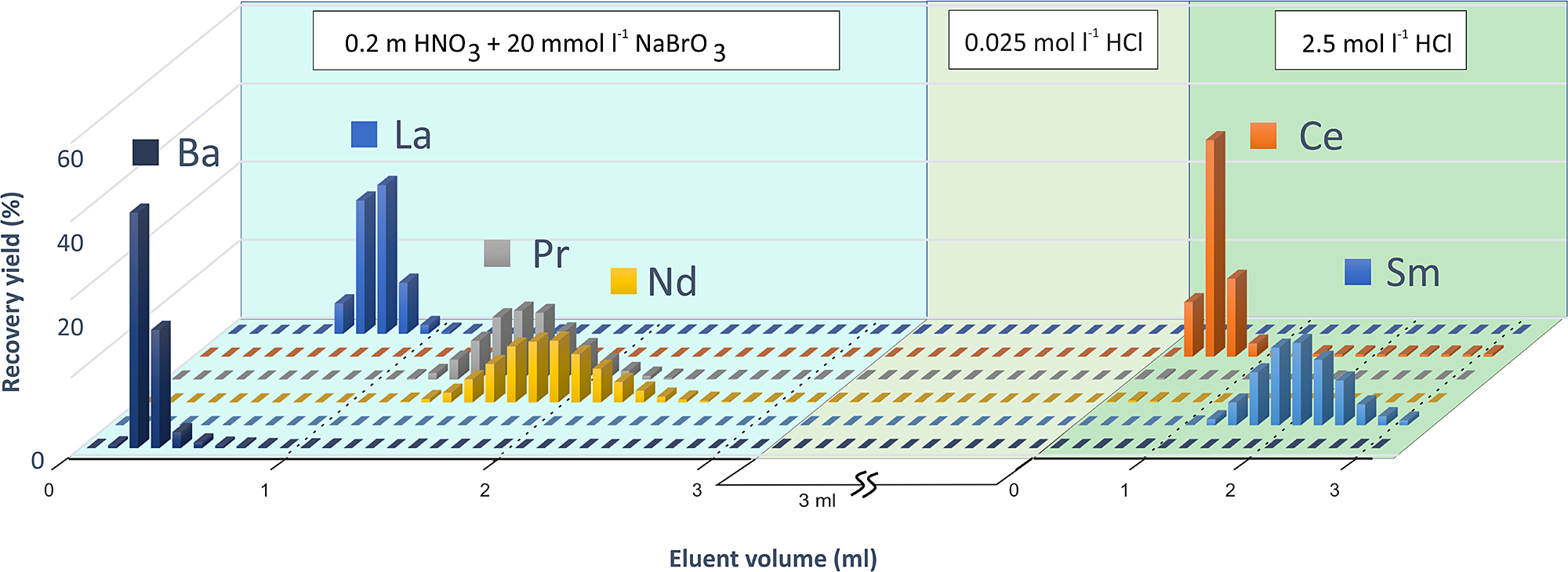
Elution profile of LREEs from Ln resin using multi element standard solution. The column was rinsed with 0.2 M HNO3 mixed with 20 mM NaBrO3 to elute Ba, La and Nd. Ce and Sm was eluted with 0.25 M HCl. Fractions of 0.1 mL were analysed using ICP-MS for elemental abundances.
Na present in the Nd fraction was removed by reusing the Ln resin column after rinsing with ~4 mL each of 6 M HCl and Milli Q water to remove the remaining REEs. The eluted Nd from the previous step was evaporated to dryness in a clean evaporator and treated once with conc. HNO3 and diluted in 0.1 mL of 0.025 M HCl. This fraction was reloaded on the cleaned Ln resin and rinsed with 4 mL 0.025 M HCl to remove residual Na (Fig. S1). Nd was then eluted with 1 mL 2.5 M HCl. Na present in Ba and La fraction can be removed in the same process, however, it was not done in this study as we did not analyse Ba or La isotopic ratios. The recovery of Nd (as well as Ba and La) is quantitative in this step as very dilute HCl is used to remove Na; thus, the final yield of these elements does not reduce due to this step. The total time required for the Ln column procedure for all fractions (without Na removal for Ba and La) is ~10 h, and ~7 h if only Nd is required.
Determination of elution curvesElution curves for each column were determined from eluted fractions collected using a fraction collector (Bio-Rad). concentrations of target elements in the fractions were determined by inductively coupled plasma mass spectrometer (ICP-MS) from Thermo Fisher (X-SERIES-2) situated at the Laboratory for Igneous Geochemistry (LIRG), Department of Earth and Planetary Systems Science, Hiroshima University. Pb, Sr and LREEs from basaltic rock standard JB-2 (GSJ) were used for the quantitative determination of recovery yield by ICP-MS.
Mass spectrometrySr, Nd, and Pb isotopic ratios were determined using TIMS (MAT-262) from Thermo Fisher Scientific situated at the LIRG, Hiroshima University, Japan. The instrument is equipped with eight movable Faraday cups, and one centre Faraday cup with a secondary electron multiplier (SEM). The Faraday cups are equipped with 1011 Ω amplifiers and the SEM with 107 Ω amplifier which can measure 10–14–10–11 and 10–15–10–12 A ion currents, respectively. Amplifier gain was measured each day before analysis of samples to remove any inter-cup bias in the static multi-collection mode. The target elements were analysed as follows. All isotopic measurements were conducted using Faraday cups in static multicollection mode.
Pb: Final elute was collected in round-bottomed PFA vials and divided into two fractions. 12.5 μL 0.075 M H3PO4 was added to both parts before drying in a clean evaporator. 204Pb-207Pb enriched double spike solution was added to one fraction after drying. Un-spiked and double spiked Pb fractions were loaded onto degassed Re single filaments using newly prepared silica gel following the methods of (Miyazaki et al., 2003). Measurement was done in positive ionization mode at >1200°C with filament current ~2.8 A. Source vacuum was kept at <5 × 10–7 mbar during measurement. Data were collected in 5 blocks with 10 scans per block for each sample. The baseline was measured mid-way between peaks, before and after each block of measurement. Run time for each sample was 15 minutes. Instrumental mass fractionation was corrected using the double spike method (Compston and Oversby, 1969) using a 204Pb-207Pb enriched spike.
Sr: The final Sr elute was collected in a flat bottomed PFA vial and dried before loading on a degassed flat single Re filament with Ta activator (Birck, 1986) and 5% m/v HNO3. Measurement conditions were similar to those of Pb, with a ~3.0 A filament current. Data was collected for 10 blocks with 11 measurements per block. The run-time for each sample was 30 min. Instrumental mass fractionation was corrected by assuming 86Sr/88Sr = 0.1194 (Nier, 1938) and applying the Rayleigh fractionation law.
Nd: Final Nd elute was collected in a conical PFA vial and dried after treatment with concentrated HNO3. The sample was loaded onto a degassed flat Re filament with 5% m/v HNO3. Double filament assembly was used for Nd measurements. Analysis was done with the ion source at <5 × 10–7 mbar. Ionization and evaporation filaments were raised to 4.5 A and ~2.5 A, respectively, until the 142Nd beam intensity reached 1.0 V (Shibata and Yoshikawa, 2004). Data was collected for 10 blocks with 10 measurements per block. Run time for each sample was ~30 minutes. Instrumental mass fractionation was corrected by assuming 146Nd/144Nd = 0.7219 and Rayleigh fractionation law. Ce and Sm were not detected during TIMS analysis.
Ba, La, Ce, and Sm: The final yields of Ba, La, Ce, and Sm in their respective fractions were determined quantitatively by ICP-MS. Isotopic ratios were not determined in this work.
The elution profile of the first column (Fig. 2) indicates that Pb, along with some major elements, is eluted early in the process during loading and subsequent rinsing with 2.5 M HCl, in about 1.5 mL of solution, whereas Sr, along with Rb and Ca are eluted afterwards with 5.5 mL of 2.5 M HCl. The two fractions were collected together for the next step as they can be purified using the same column. Recovery yields of both the target elements were quantitative at this step. LREE fraction including Ba is recovered with 5.5 mL of 6 M HCl and collected separately for the next step using Ln column. REEs elute from heavy to light in this step and full recovery of Sm to La and Ba is obtained in 5.5 mL 6 M HCl. Use of 1st column enables efficient separation between Ba and Sr. If the sample solution is loaded directly to the Sr column, Ba is present in significant amounts (~70%) in the Sr fraction and hinders precise Sr isotopic analysis. Ba can also be removed in the Sr column using 7 M HNO3 (e.g., Pin et al., 2014) however, high concentration nitric acid degrades the equipment (e.g., Teflon column) over time.
Pb is collected early in the rinsing process of the first column and a lot of major elements (e.g., Fe, Mg, Ca) end up in the Pb-Sr fraction. Rb and Ca are also present in the Sr fraction which needs to be eliminated for precise isotopic analysis. Sr resin is therefore used to remove residual interfering elements in the next step. The elution curve in Fig. 3 indicates that the use of 3 M HNO3 eliminates Rb and major elements during loading (0.6 mL) and rinsing with 1 mL of acid. 1 mL 0.05 M HNO3 was used to recover Sr before eluting Pb using 3 mL 6 M HCl.
The Ln column was used to separate the Ba and LREEs from each other. Presence of Ce and Sm in the Nd fraction hinders precise determination of 142Nd/144Nd and 143Nd/144Nd isotopic ratios as 144Sm has an isobaric effect on 144Nd and 142Ce on 142Nd. We also found that the beam intensity of Nd was lower and unstable while Ce and Ba were detectable during TIMS measurement. Trace amounts of Ce evaporated on heating the filament and the Nd beam stabilized over time. However, the presence of significant amounts of Ce produces an unstable and weak beam, causing problems in 143Nd/144Nd determination and reducing the precision of measurement. The REE fraction obtained from the first column was dissolved in 0.1 mL of 0.2 M HNO3 and 20 mmol–1 NaBrO3. NaBrO3 is used to oxidise Ce3+ to Ce4+ state, allowing it to create a complex with HDEHP present in the Ln resin in presence of HNO3 (Peppard and Ferraro, 1959). Previous studies have reported Ce/Nd requirement to be in the order of 10–5 for 142Nd/144Nd isotopic studies (Kagami and Yokoyama, 2016; Pin and Gannoun, 2019). The Ce/Nd ratio need not be this low for 143Nd/144Nd isotopic studies as trace amount of Ce evaporates relatively fast during filament heating. We found that a Ce/Nd ratio of the order of 10–3 produces similar precision to pure Nd fraction for 143Nd/144Nd isotopic data.
Sm must be eliminated from the Nd fraction as 144Sm has an isobaric interference on 144Nd. We have also observed that presence of Ba causes imprecise Nd isotopic data due to low Nd beam intensity. So, we have optimised the HNO3 concentration in order to elute all the elements separately. The Sm/Nd ratios in the final Nd fraction is similar to the Ce/Nd ratios (<10–4). No other REEs except Pr is present in the Nd fraction.
Ce4+ needs to be reduced back to Ce3+ state in order to ensure full recovery in the next step, this is achieved by rinsing the resin bed with 0.1 mL dilute H2O2. 0.8 mL 0.25 M HCl is used to elute Ce before recovering Sm with 2 mL of the same solution.
Na introduced through the use of NaBrO3 in the Nd fraction needs to be eliminated for a clean measurement in TIMS (Caro et al., 2006; Kagami and Yokoyama, 2016), therefore, we used the same Ln resin column to eliminate Na and reduce the cost and likelihood of contamination.
Eluent optimizationWe have systematically studied the effect of NaBrO3 concentration and shelf life required for separating Ce from Nd. The Ln column elution process was repeated with 0.2 M HNO3 mixed with NaBrO3 concentrations varying from 0.15 mM to 15 mM, and Ce/Nd ratio was determined in the Nd fractions using ICP-MS. The results (Table S1; Fig. 5) indicate that Nd fraction eluted with NaBrO3 concentrations above 5 mM contain Ce equivalent to blank levels for 20 mg of JB-2. As the detected Ce was below the limit of quantification and similar to the ICP-MS blank level in some cases, we have assumed the limit of detection (1 ppt in analysed solution) as the highest possible level of Ce present in those fractions to determine Ce/Nd ratio. The results show that >5 mM of NaBrO3 can produce Ce/Nd ratios of ~10–4 which is enough for accurate determination of Nd isotopic ratios as no 140Ce was observed during TIMS analysis in this case. Although the calculated upper limit of 10–4 in Nd fraction is higher than the recommended ~10–5 (Kagami and Yokoyama, 2016; Pin and Gannoun, 2019), the true value of Ce/Nd is likely to be lower than 10–4 as Ce was below detection limit in these fractions.
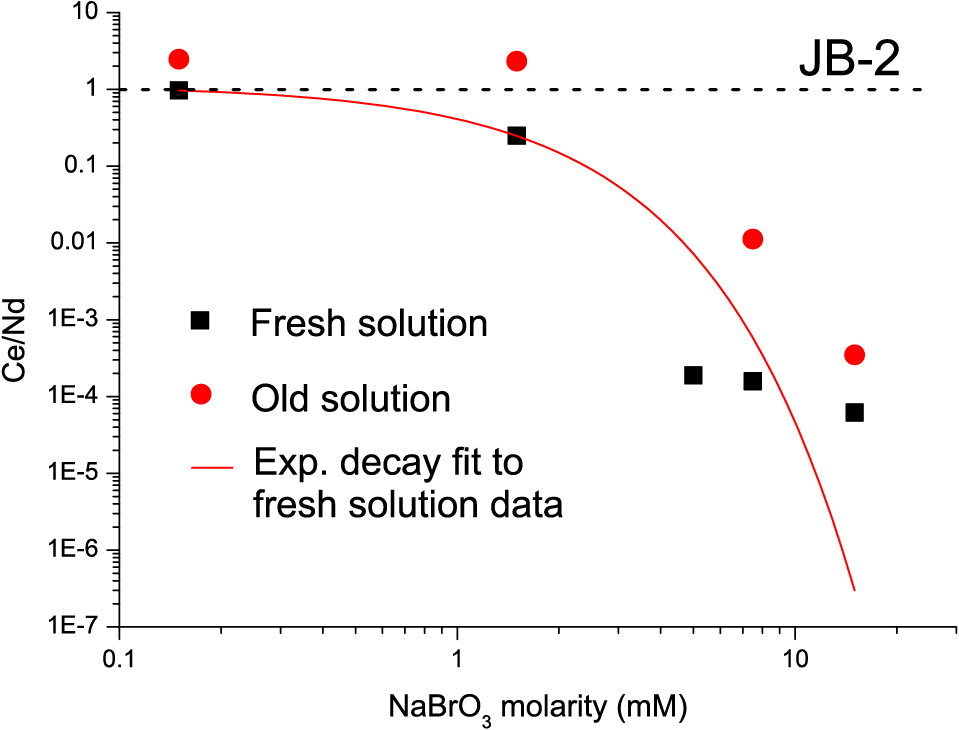
Relationship between Ce/Nd ratio in the Nd fraction (from Ln resin) and NaBrO3 concentration. An exponential decay line has been fitted to through the data obtained using freshly prepared NaBrO3 – HNO3 solution. Circles indicate Ce/Nd ratios of Nd fraction using solution stored for 1 month.
With regard to the effect of long-term storage of the NaBrO3 solution, the results show that the solution degrades over time causing Ce to remain in the Nd fraction with Ce/Nd ratios about one order of magnitude higher than for fresh solution. Furthermore, NaBrO3 causes the HNO3 to degrade over time (for storage ~ 1 month) causing incomplete recovery of Nd (35%–60%). Ce3+ is eluted before Nd from the Ln resin, causing further increase in Ce/Nd ratio, to levels higher in the collected fraction than the original sample (for low concentrations of 0.15 mM and 1.5 mM) (Fig. 5). Therefore, we recommend that the mixed solution should not be stored for more than a few days. However, when only 143Nd/144Nd ratios are to be determined, NaBrO3 solution in milli Q water can be stored for up to a month and can be used to prepare the eluent by mixing with an appropriate amount of HNO3 before column chemistry. In this case, trace amount of Ce may be present in the Nd fraction but is evaporated during heating of filament in TIMS and does not affect the precision or accuracy of data. This is convenient, as it is easier to work with liquid solutions that can be pipetted than with solid reagents that must be weighed. However fresh solution should be prepared when 142Nd/144Nd is analysed.
Acid concentrations used for the Ln resin column were optimised in this study, whereas conventions in previous studies (Yoshikawa et al., 2003; Makishima et al., 2008) were followed for the ion-exchange column and Sr column. Previous studies (e.g., Kagami and Yokoyama, 2016) have used concentrated HNO3 for Ce-free REE elution, whereas we have optimised the concentration to provide the best separation of Nd from all other REEs (i.e., La, Ce, Sm) at this step. 0.2 M HNO3 was selected to recover La and Nd as this provides the best separation while keeping the eluent volume relatively low for Nd (2 mL).
Recovery yields and procedural blanksThe recovery yields for the total procedure were above 90% for Pb, Nd, Ce, Sm, La, and Ba. Sr recovery yield was >80%. The total procedural blanks were measured using isotope dilution TIMS (ID-TIMS). Blanks were prepared and dried alongside other samples to mimic the sample preparation method. Blanks of 98 pg for Sr, 258 pg for Pb and 50 pg for Nd were obtained for the whole procedure including digestion, column and reagent blanks. Most of the blanks are assumed to come from the resins and can be reduced by further cleaning.
Isotopic ratiosThe sample separation procedure was applied for elemental separation and isotopic analysis for nine reference rocks and sediments from GSJ and USGS. Eight aliquots of 40 mg of JB-2 rock powder and single aliquots of 20 mg JG-1a, JG-2, JG-3, JSd-1, JSl-1, and JLk-1 from GSJ and BCR-2 & SDO-1 from USGS were digested for isotopic analysis. Isotopic standards for Sr, Nd and Pb were analysed to ensure instrument stability and external reproducibility. NIST SRM 987 Sr isotopic standard yielded an 87Sr/86Sr ratio of 0.710264 ± 0.000014 (2σ, n = 10). The La Jolla Nd standard yielded a 143Nd/144Nd ratio of 0.511853 ± 0.000011 (2σ, n = 10). 206Pb/204Pb, 207Pb/204Pb, and 208Pb/204Pb ratios from NIST SRM 981 Pb standard was measured as 16.9397 ± 0.0013; 15.4972 ± 0.0014 and 36.7187 ± 0.0024 (2σ, n = 5), respectively.
Each aliquot of the JB-2 was digested, and column chemistry performed individually to determine long term reproducibility and external precision of measurement. JB-2 Sr isotopic analysis yielded an average 87Sr/86Sr ratio of 0.703699 ± 0.000018 (2σ, n = 7) and an average 143Nd/144Nd ratio of 0.513091 ± 0.000008 (2σ, n = 6) (Fig. 6). The standard error of a single measurement for Sr and Nd on our instrument is usually 0.000010 to 0.000015 (2σm). Double spike corrected Pb isotopic analysis yields an average 206Pb/204Pb ratio of 18.3223 ± 0.0097; 207Pb/204Pb ratio of 15.5575 ± 0.0064 and 208Pb/204Pb ratio of 38.2442 ± 0.0162 (2 σ, n = 7). 204Pb has the lowest abundance in natural samples and the uncertainty of measurement is higher than the other isotopes for Pb. Therefore, the precision of Pb isotopic analysis depends on the intensity and stability of the 204Pb ion beam and the error in isotopic ratios is highest along the “204 error line” (Hamelin et al., 1985).

Sr, Nd, Pb isotopic ratios determined from multiple aliquots of GSJ reference rock JB-2. The data shows high reproducibility.
Comparisons of our data with those from previous studies are shown in Figs. 7 and 8 and described in Tables 2 and 3.
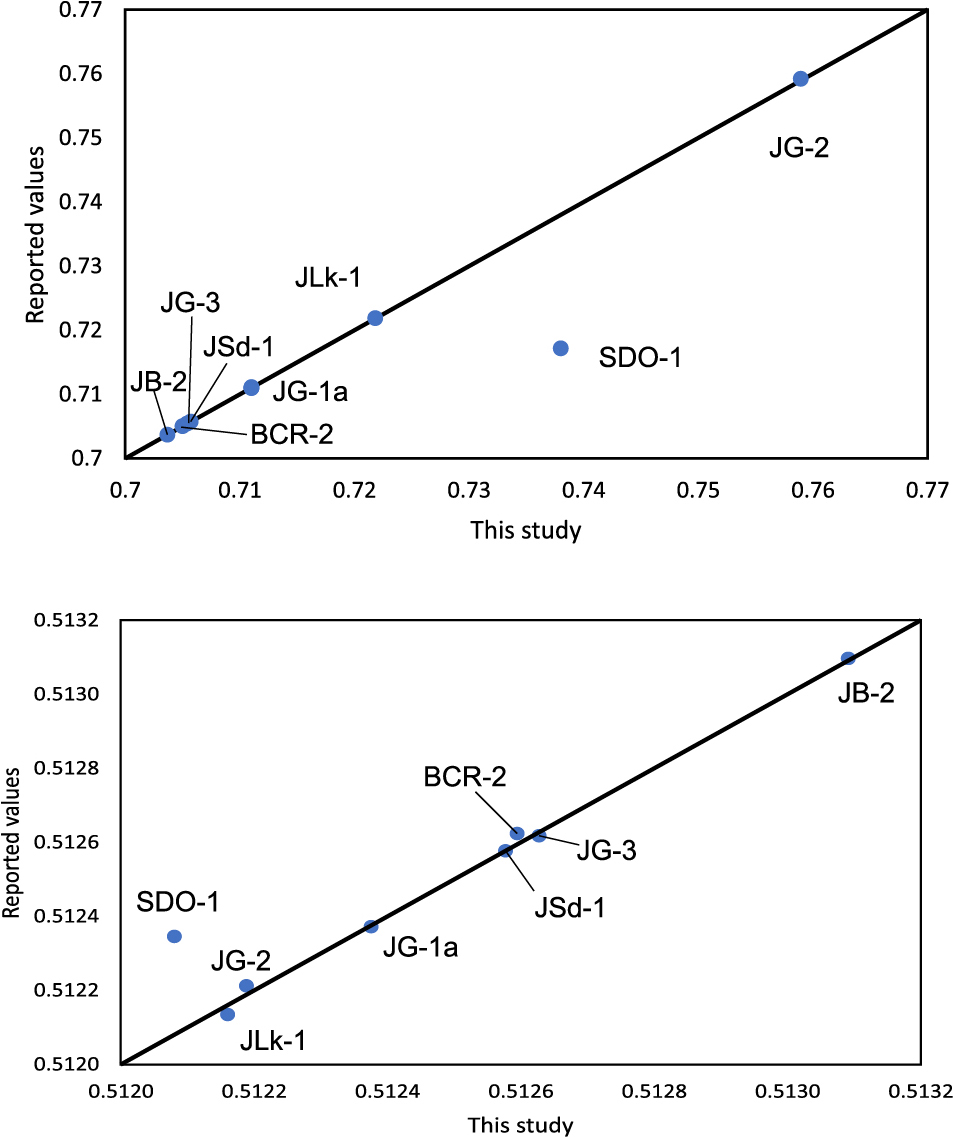
Comparison of Sr and Nd isotopic data obtained from eight silicate reference rocks in this study with reported values. SDO-1 deviates from the only reported value in literature by a significant degree (see text). JSl-1 is not shown in this figure due to lack of reported values.

Comparison of Pb isotopic data obtained from seven silicate reference rocks in this study with reported values. JSl-1 and SDO-1 is not shown in this figure due to lack of reported values.
| Reference rock | 87Sr/86Sr | 2 σm | Reported values (reference) |
143Nd/144Nd | 2 σm | Reported values (reference) |
|
|---|---|---|---|---|---|---|---|
| JB-2 | 1 | 0.703703 | 0.000010 | 0.703703*(1) 0.703691*(2) |
— | — | 0.513097*(1) 0.513096*(2) |
| 2 | 0.703696 | 0.000013 | 0.513087 | 0.000010 | |||
| 3 | 0.703691 | 0.000010 | — | — | |||
| 4 | 0.703699 | 0.000012 | 0.513094 | 0.000012 | |||
| 5 | 0.703686 | 0.000013 | 0.513087 | 0.000012 | |||
| 6 | — | — | 0.513098 | 0.000014 | |||
| 7 | 0.703700 | 0.000010 | 0.513089 | 0.000011 | |||
| 8 | 0.703716 | 0.000012 | 0.513090 | 0.000010 | |||
| (n) | 7 | 7 | 6 | 6 | |||
| Average | 0.703699 | 0.000018 (2σ) | 0.513091 | 0.000008 (2σ) | |||
| JG-1a | 0.711026 | 0.000011 | 0.711093*(1) 0.710975*(3) 0.711023+(4) |
0.512375 | 0.000011 | 0.512372*(1) 0.512384*(3) | |
| JG-2 | 0.758956 | 0.000012 | 0.759197*(1) 0.758560 (5) |
0.512188 | 0.000013 | 0.512225*(1) 0.512212*(6) | |
| JG-3 | 0.705413 | 0.000011 | 0.705426*(1) | 0.512627 | 0.000018 | 0.512618*(1) | |
| JSd-1 | 0.705728 | 0.000010 | 0.705728*(7) | 0.512577 | 0.000009 | 0.512571*(7) | |
| JLk-1 | 0.721836 | 0.000009 | 0.721840*(8) | 0.512160 | 0.000012 | 0.512135*(8) | |
| JSl-1 | 0.711855 | 0.000017 | NA | 0.512549 | 0.000017 | NA | |
| BCR-2 | 0.705011 | 0.000010 | 0.705015*(8) | 0.512594 | 0.000023 | 0.512624*(8) | |
| SDO-1 | 0.738016 | 0.000012 | 0.717146+(9) | 0.512080 | 0.000008 | 0.512346+(9) | |
Note: * = TIMS; + = MC-ICP-MS; — = analysis failed; NA = Data not available
References: (1) Shibata et al. (2003); (2) Li et al. (2015a); (3) Li et al. (2016); (4) Ackerman et al. (2017); (5) Zichao (Per. Comm. to GSJ, 1987); (6) Miyazaki and Shuto (1998); (7) Nath et al. (2009); (8) Jo et al. (2021); (9) Hohl et al. (2022); (10) Miyazaki et al. (2009); (11) Smet et al. (2010); (12) Tanimizu and Ishikawa (2006); (13) Chauvel et al. (2011)
| Reference rock | 206Pb/204Pb | 2 σm | Reported values (reference) |
207Pb/204Pb | 2 σm | Reported values (reference) |
208Pb/204Pb | Reported values (reference) |
||
|---|---|---|---|---|---|---|---|---|---|---|
| JB-2 | 1 | 18.3258 | 0.0019 | 18.3396*(2) 18.3419*(10) 18.3252*(11) |
15.5605 | 0.0016 | 15.5610*(2) 15.5623*(10) 15.5596*(11) |
38.2522 | 0.0038 | 38.2674*(2) 38.2814*(10) 38.2575*(11) |
| 2 | 18.3248 | 0.0016 | 15.5595 | 0.0014 | 38.2498 | 0.0034 | ||||
| 3 | 18.3256 | 0.0013 | 15.5582 | 0.0010 | 38.2485 | 0.0027 | ||||
| 4 | 18.3255 | 0.0021 | 15.5571 | 0.0016 | 38.2447 | 0.0042 | ||||
| 5 | 18.3112 | 0.0015 | 15.5502 | 0.0011 | 38.2259 | 0.0029 | ||||
| 6 | — | — | — | — | — | — | ||||
| 7 | 18.3226 | 0.0028 | 15.5598 | 0.0022 | 38.2463 | 0.0058 | ||||
| 8 | 18.3209 | 0.004 | 15.5573 | 0.0033 | 38.242 | 0.0083 | ||||
| (n) | 7 | 7 | 7 | 7 | 7 | 7 | ||||
| Average | 18.3223 | 0.0097 | 15.5575 | 0.0064 | 38.2442 | 0.0162 | ||||
| JG-1a | 18.6001 | 0.0014 | 18.6223*(3) 18.6057+(12) |
15.6161 | 0.0011 | 15.6140*(3) 15.6102+(12) |
38.7547 | 0.0027 | 38.7793*(3) 38.6874+(12) | |
| JG-2 | 18.6187 | 0.0008 | 18.6050+(12) | 15.6427 | 0.0008 | 15.6345+(12) | 39.0399 | 0.0018 | 38.9829+(12) | |
| JG-3 | 18.3542 | 0.0007 | 18.3531+(12) | 15.5666 | 0.0006 | 15.5641+(12) | 38.4887 | 0.0013 | 38.4480+(12) | |
| JSd-1 | 18.4539 | 0.0010 | 18.4795*(7) 18.4650+(13) |
15.6094 | 0.0008 | 15.6152*(7) 15.6130+(13) |
38.5610 | 0.0021 | 38.5991*(7) 38.5840+(13) | |
| JLk-1 | 18.4396 | 0.0008 | 18.4400+(13) | 15.6416 | 0.0006 | 15.6410+(13) | 38.8009 | 0.0018 | 38.7910+(13) | |
| JSl-1 | 18.6095 | 0.0046 | NA | 15.5722 | 0.0037 | NA | 38.5003 | 0.0094 | NA | |
| BCR-2 | 18.7602 | 0.0087 | 18.7580*(3) | 15.6227 | 0.0073 | 15.6240*(3) | 38.7429 | 0.0181 | 38.7210*(3) | |
| SDO-1 | 24.6216 | 0.0012 | NA | 15.9756 | 0.0008 | NA | 38.7071 | 0.0019 | NA | |
Note: same as Table 2.
Isotopic data obtained from nine reference rocks match with previously reported values (Tables 2 and 3; Figs. 7 and 8). The selected reference rocks include granite, granodiorite, basalt, slate, shale and sediments; and establishes that the proposed method can be successfully applied to various types of silicate rocks to produce accurate and precise isotopic data. Some of the reference rocks (e.g., JG-2) show a large variation in reported data and we have selected reliable reference values to reflect the variation. We report the first isotopic data for two sedimentary reference rocks, i.e., JSl-1 from GSJ and SDO-1 from USGS. Only one report of Nd and Sr isotopic data was found for SDO-1 (Hohl et al., 2022), However the data do not match with our findings. Hohl et al. (2022) uses MC-ICP-MS to determine Sr and Nd isotopic ratios without full elemental separation. We suspect that their method does not eliminate the effect of interfering elements (i.e., Rb on Sr and Sm on Nd) correctly, thus producing erroneous values. Pb isotopic data from JB-2 basaltic rock standard reported in this study differ from some previous studies (Miyazaki et al., 2009; Li et al., 2015a) and is similar to the data reported by Smet et al. (2010). This corroborates the hypothesis that the JB-2 standard is isotopically inhomogeneous (Smet et al., 2010). Concordance of Pb isotopic data for the other samples in this study with published values conclude that the JB-2 (split-9) Pb isotopic data in this study is reliable and reproducible, and not due to contamination or systematic error.
Stability of ion beamOrganic matter from the ion-exchange resins is eluted along with element fractions causing problems during column chemistry and TIMS measurement. Interfering elements such as Rb in Sr fraction and La, Ce, and Sm in Nd fraction were observed during analysis when the eluted fraction was not oxidised with HNO3 and H2O2 before loading on to the next column. We have also observed a reduction in intensity and stability of ion beam for Pb and Nd during TIMS analysis when the final elemental fraction was not oxidised using HNO3 and H2O2 before loading.
Pb, Sr, Nd, Ce, Ba, La, and Sm were separated from single aliquots of acid-digested silicate materials using an efficient and rapid sequential separation method compared to currently available methods (≥3 days). All the element fractions were separated and dried within 2 days when using samples digested for elemental analysis by ICP-MS. This method produces pure element fractions devoid of matrix elements providing long-term and stable ion beams during TIMS analysis, enabling the measurement of minute samples for Sr, Nd, and Pb.
We have quantified the concentration and shelf life of NaBrO3 solution required to produce Ce-free Nd fraction.
Isotopic ratios of Pb, Sr, and Nd of nine reference rocks from GSJ and USGS were determined using TIMS which shows precise and reproducible values. The accurate data from granite, granodiorite, basalt, shale, slate and sediment references establish that this technique can be applied to various silicate rocks and sediments without any problem. The technique is also applicable to sample types other than silicates, such as carbonate, phosphate, soil samples, water samples etc, as is or with minor variations.
We report the first Pb-Sr-Nd isotopic data of reference rock JSl-1 and accurate Pb-Sr-Nd isotopic data of reference rock SDO-1. These data add to the scanty database of sedimentary silicate reference rocks. Our study also confirms the compositional Pb isotopic heterogeneity among different splits of JB-2 rock standard.
BD was supported by the Monbukagakusho scholarship from the Ministry of Education, Culture, Sports, Science and Technology, Japan, for the duration of this research. This work was partially supported by HiPeR, which is selected and supported by Hiroshima University. We thank the Hiroshima University for providing the research facilities. The authors thank the two anonymous reviewers for their valuable comments and suggestions in improving the manuscript. The authors declare no conflicts of interest.