2023 年 57 巻 2 号 p. 85-91
2023 年 57 巻 2 号 p. 85-91
The chemical and isotopic characteristics of waters from typical landslide areas in Niigata and Nagano Prefectures, Japan, are described in this article. The study areas are Mushigame, Nigorisawa, Yomogihira (Niigata Pref.) and Kanayamazawa (Nagano Pref.) where landslides have often occurred. We present the chemical composition and δD-δ18O values of surface and groundwaters from the areas in order to characterize a geochemical nature of the waters involved in the landslides. The waters were of meteoric origin as indicated by the δD-δ18O values. The Mushigame waters were enriched in NaCl, suggesting contribution of fossil seawater trapped in oil-producing mudstone strata in the area. The Nigorisawa and Yomogihira waters are less in Cl– and relatively more enriched in HCO3– and SO42–. The Na-K-Mg relationship of all the waters except the Kanayamazawa waters indicated close equilibration with some minerals such as K-feldspar, K-mica, chlorite and silica in the rocks, suggesting water-rock interaction at deeper depths, although the waters were greatly diluted by surface groundwater on the way to the surface as indicated by their δD-δ18O signatures. Dissolved sulfate is a major anion and was originally produced by oxidation of sulfides in volcanic rocks. Variable δ34S and δ18O values of sulfate suggest reduction of such sulfate to sulfide probably at shallow levels under different reducing conditions followed by re-oxidation, inducing the variability in the isotopic values. Thus, multiple oxidation-reduction processes may be common in the areas.
Landslide, common in mountainous countries like Japan, occurs in a place where steep slopes consisting of mud and unconsolidated rocks develop. Heavy rains and snow-melt water trigger a landslide by increasing pore pressure or reducing shear strength at the sliding planes. It also occurs in the areas where colluvium formed by previous landslides accumulates (Fujita, 1990). Many of the landslides are relocation of debris produced by previous events in a variable scale. Clay-rich layers at the bottom of a stratum contribute to the sliding of the overlying strata. The materials typically contain clay minerals such as montmorillonite which helps downward sliding of the debris. Such areas are wet, and groundwater seepage is often found. The water chemistry is generally correlated to the weathering degree of aquifer rocks, and contains information about hydrological processes (Kim et al., 2014; Marc et al., 2017). Bogaard et al. (2007) reviewed hydro-geochemistry applied to landslide researches, emphasizing the important relationship between pore fluid composition and soil strength. Waters from the early stage of weathering are generally rich in sodium carbonate, whereas sodium sulfate and calcium carbonate waters dominate in later active stages. Thus, the chemical composition of waters from landslide areas is strongly related not only to the local geology but also to other various other factors such as the origin of water, flow paths, time required for deep water to reach the surface and so on.
Masuda (2023) reviewed geochemical characteristics of non-volcanic thermal waters. She stressed that tectonics plays an important role in the formation of the thermal waters, e.g., altered fossil seawater remains as low-temperature mineralized waters in oil and gas fields in Niigata Prefecture and the surrounding areas. Such waters are little contaminated by local meteoric water. Kawaraya et al. (2000) described that δD and δ18O values of groundwaters sampled from wells drilled in bedrocks (shales) at the Yachi landslide area, Akita Prefecture, Japan. Isotopic composition of the waters had smaller variation compared with those of shallow surface waters which mixed during infiltration. The residence time of the waters from drill holes was estimated to be longer than 1 year, while that of the shallow water was as short as only 2 months. Groundwater obtained from the debris had an intermediate residence time of ~6 months, suggesting mixing of the two types of waters. This study gave an approximate time scale for water movement at shallow levels.
The purpose of this study is to supply stable isotopic information of the landslide localities in Niigata and Nagano Prefectures, Japan. Sato (1982) and Sato et al. (1984) published the chemistry of waters obtained from the above areas for the first time. The localities are characterized by poorly consolidated mudstone strata that are cut by small faults. In our work we collected groundwater and surface waters from Mushigame (abbreviated as MSG), Nigorisawa (NGS) and Yomogihira (YMG) landslide areas in Niigata Prefecture and those from Kanayamazawa (KNY) in Nagano Prefecture as a case study of geochemical characterization of landslide waters through isotopic and chemical signatures. The study areas are shown in Fig. 1. The areas (MSG, NGS, YMG) are close to Nagaoka City in Niigata Prefecture and the Otari village in Nagano Prefecture (KNY). We try to understand geochemical processes taking place in the above areas as a case study through additional information on the chemistry and isotopic variations of waters (δD and δ18O) and dissolved sulfate (δ18O and δ34S) of surface and groundwaters collected from the above landslide areas.
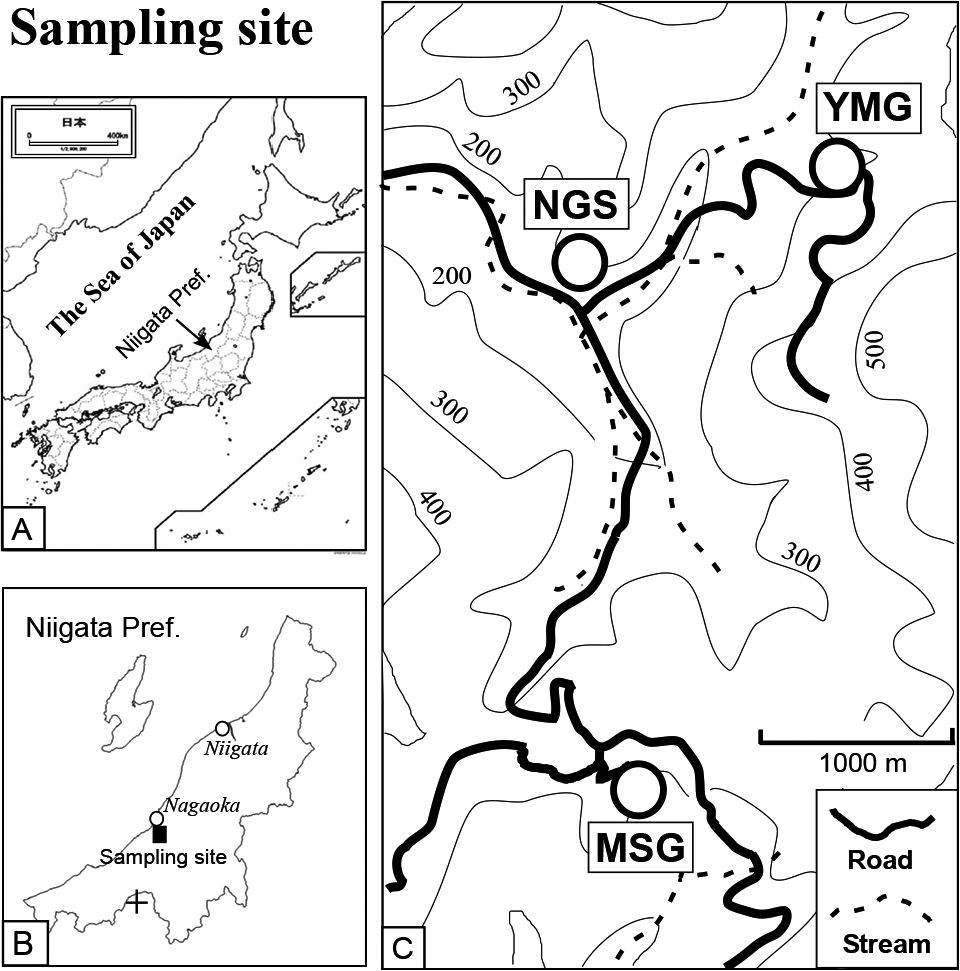
Maps showing Japan (A), Niigata Prefecture (B) and the sampling sites (C). MSG stands for Mushigame, NGS for Nigorisawa and YMG for Yomogihara. These villages are located in Yamakoshi area of Niigata Prefecture. A small cross in (B) indicates the Kanauamazawa site.
The MSG, NGS and YMG landslide areas are found in the Niigata Sedimentary Basin. Geology of the basin is dominated by the Neogene formations which are divided into the Shiiya, Nishiyama, Haizume Formations and the Uonuma Group in the ascending order (Chihara, 1974; Kobayashi et al., 1986). The MSG and YMG sites are in the Shiiya Formation that consists of alternating conglomerate of sandstones and mudstones intercalating felsic volcanic rocks. The sandstones of this formation are a major oil reservoir in this area. A large landslide took place at the MSG site in 1980. The area contains debris flows at upstream and mud flow at downstream, similar characters found in the other landslide areas.
We collected surface waters, groundwaters and well waters (collected at a surface outlet of the wells) in 1984 from the MSG, NGS, and YMG sites which are shown in Fig. 1 and listed in Table 1. Brief descriptions of the water samples and sulfate-pyrite samples are given in Tables 1 and 2, respectively. The landslides at MSG and NGS occurred in 1980 after heavy snowfalls in the previous winter. The annual precipitation at Nagaoka City is well over 2400 mm (data by Japan Meteorological Agency, 2020). Waters from the KNY area (also known as a landslide area) shown as a small cross in Fig. 1b were also sampled (Table 1). The area is located in the upper end of the Urakawa stream, a tributary of the Himekawa river in Nagano Prefecture. Water samples were collected from the points covering the upper to lower ends of each landslide area. The MSG, NGS, and YMG sites located about 10–11 km south of the Nagaoka City are considered to receive much more precipitation due to their higher elevation. Such high precipitation tends to induce a landslide.
| Sample number | Sample Code | Sample description and sampling locality | Temp. deg. C | pH | E.C. mS/cm | HCO3– ppm | SO42– ppm | Cl– ppm | Na+ ppm | K+ ppm | Ca2+ ppm | Mg2+ ppm | δDH2O ‰ | δ18OH2O ‰ | δ34SSO4 ‰ | δ18OSO4 ‰ |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nigorisawa, Mushigame and Yomogihira landslide areas | ||||||||||||||||
| 84406 | NGS-1 | Groundwater from Nigorisawa highest point | 9.4 | 6.7 | 0.70 | 26 | 315 | 6 | 66 | 2.3 | 49 | 19 | –53.1 | –10.1 | –9.9 | –7.6 |
| 84407 | NGS-2 | Water from collective basin No. 2, Nigorisawa highest point | 12.9 | 6.8 | 0.55 | 146 | 123 | 29 | 91 | 1.6 | 23 | 9 | –52.1 | –9.5 | –4.1 | –6.5 |
| 84408 | NGS-3 | Water from collective basin No. 3, Nigorisawa highest point | 12.4 | 7.2 | 1.29 | 291 | 270 | 93 | 287 | 2.0 | 14 | 4 | –52.1 | –9.2 | –10.4 | –7.1 |
| 84409 | NGS-4 | Groundwater from a lateral boring #1, Nigorisawa middle point | 10.6 | 7.8 | 0.35 | 152 | 50 | 11 | 29 | 2.0 | 28 | 11 | –52.7 | –9.1 | 0.7 | –6.8 |
| 84411 | NGS-5 | Groundwater from a lateral boring #2, Nigorisawa lower point | 14.4 | 8.4 | 0.66 | 288 | 27 | 68 | 155 | 1.2 | 6 | 3 | –51.5 | –9.2 | 8.1 | 5.2 |
| 84412 | MSG-1 | NaCl-type groundwater from a well (17 m), Mushigame highest point | 14.4 | — | 6.82 | 533 | 31 | 2258 | 1550 | 7.4 | 45 | 15 | –50.0 | –9.1 | –7.0 | 0.9 |
| 84414 | MSG-3 | Spring water from Mushigame highest point | 17.3 | 8.0 | 1.90 | 339 | 807 | 4 | 297 | 6.6 | 92 | 51 | –54.0 | –9.7 | –15.1 | –7.5 |
| 84415 | MSG-4 | Groundwater from Mushigame middle point | 10.3 | 6.6 | 2.04 | 267 | 648 | 173 | 352 | 7.0 | 76 | 36 | –52.8 | –9.7 | –14.6 | –5.9 |
| 84416 | MSG-5 | Groundwater from collective drainage well #1, Mushigame middle point | 11.1 | 7.5 | 2.21 | 588 | 687 | 35 | 531 | 3.5 | 30 | 12 | –54.5 | –9.7 | –15.3 | –6.5 |
| 84417 | MSG-6 | Groundwater from collective drainage well #2, Mushigame middle point | 10.2 | 7.9 | 2.39 | 589 | 663 | 73 | 602 | 3.1 | 23 | 7 | –53.5 | –9.7 | –14.7 | –6.2 |
| 84418 | MSG-7 | Groundwater near a collective drainage well, Mushigame lower point | 11.6 | 7.3 | 5.33 | 624 | 605 | 1269 | 1235 | 5.9 | 53 | 19 | –49.4 | –9.0 | –13.5 | –5.6 |
| 84419 | YMG-1 | Spring water from Yomogihira lowest point | 14.7 | 8.9 | 0.45 | 212 | 55 | 10 | 109 | 0.8 | 1 | 0 | –49.5 | –8.7 | –12.9 | –5.4 |
| 84420 | YMG-2 | Surface water from Yomogihira lower point near a destroyed house | 26.2 | 7.2 | 1.41 | 102 | 634 | 8 | 126 | 4.7 | 138 | 30 | –50.4 | –8.9 | –24.1 | –11.8 |
| 84421 | YMG-3 | Groundwater from a drill hole #2 (8 m deep),Yomogihira middle point | 14.3 | 7.7 | 0.49 | 314 | 10 | 14 | 109 | 1.6 | 13 | 2 | –47.2 | –8.7 | 3.9 | — |
| 84422 | YMG-4 | Groundwater from a drill hole B-1 (25 m), Yomogihira middle point | 12.6 | — | 0.20 | 79 | 26 | 11 | 40 | 0.8 | 5 | 1 | –51.8 | –9.7 | –5.9 | –2.9 |
| 84423 | YMG-5 | Yomogihira stream | 24.5 | — | 0.18 | 0 | 22 | 19 | 16 | 2.0 | 14 | 3 | –50.5 | –8.9 | — | — |
| Kanayamazawa landslide area | ||||||||||||||||
| 84571 | KNY-1 | Groundwater from Kanayamazawa middle point | 10.3 | 5.0 | 1.32 | — | — | — | — | — | — | — | –78.5 | –12.0 | 1.9 | –8.2 |
| 84572 | KNY-2 | Surface water from Kanayamazawa middle point, left bank. Fe- precipitate | 14.7 | 8.0 | 0.45 | — | — | — | — | — | — | — | –77.1 | –11.7 | 2.7 | –7.3 |
| 84573 | KNY-3 | Groundwater #1 from Kanayamazawa middle point, right bank | 14.0 | 6.0 | 1.07 | — | — | — | — | — | — | — | –65.1 | –10.0 | 2.4 | –9.1 |
| 84574 | KNY-4 | Groundwater #2 from Kanayamazawa middle point, right bank | 10.8 | 7.0 | 1.40 | — | — | — | — | — | — | — | –79.3 | –12.3 | 1.9 | –9.4 |
| 84575 | KNY-5 | Surface water from Kanayamazawa middle point, left bank | 10.9 | 7.8 | 0.93 | 97 | 553 | 1.0 | 10.3 | 2.0 | 165 | 55 | –75.3 | –11.8 | 2.0 | –8.3 |
| 84576 | KNY-6 | Seepage water from talus accumulation, Kanayamazawa middle point, right bank | 10.0 | 5.6 | 1.07 | 2 | 933 | 1.0 | 12.3 | 2.2 | 179 | 110 | –77.6 | –12.1 | 2.4 | –9.4 |
| 84577 | KNY-7 | Seepage water from argillaceous talus accumulation, Kanayamazawa middle point, right bank | 12.7 | 8.0 | 0.66 | 120 | 315 | 1.1 | 12.9 | 1.3 | 126 | 24 | –75.6 | –11.9 | 2.5 | –8.8 |
| 84578 | KNY-8 | Surface water from Kanayamazawa middle point, right bank near TV transmission pole | 7.9 | 7.2 | 0.36 | 60 | 279 | 0.9 | 6.4 | 1.3 | 77 | 32 | –76.9 | –12.2 | 2.6 | –8.3 |
| 84579 | KNY-9 | Surface water from Kanayamazawa middle point, right bank, near a small bridge | 11.7 | 7.4 | 0.74 | 26 | 133 | 1.0 | 5.1 | 0.8 | 37 | 12 | –81.1 | –12.5 | 2.7 | –9.4 |
| 84580 | KNY-10 | Groundwater issuing from serpentinite exposure, Kanayamazawa upper point | 9.4 | 4.8 | 0.34 | 0 | 217 | 0.8 | 5.7 | 1.1 | 61 | 14 | –79.1 | –12.6 | 2.8 | –8.1 |
| 84581 | KNY-11 | Seepage water from talus accumulation, Kanayamazawa upper point, left bank | 9.9 | 5.4 | 0.99 | 0 | 833 | 1.0 | 10.3 | 1.3 | 235 | 56 | –81.1 | –12.3 | 2.6 | –8.7 |
| 84582 | KNY-12 | Surface water from Kanayamazawa uppermost point, left bank | 8.3 | 4.8 | 0.59 | 0 | 379 | 0.9 | 8.7 | 1.0 | 104 | 34 | –81.0 | –12.9 | 2.1 | –9.0 |
| 84583 | KNY-13 | Surface water from Kanayamazawa uppermost point adjacent to KYZ-12, left bank | 8.1 | 4.4 | 0.61 | 0 | 430 | 0.9 | 8.8 | 1.1 | 121 | 35 | –81.3 | –12.9 | 2.6 | –8.7 |
| 84584 | KNY-14 | Stream water, Kanayamazawa uppermost point, left bank | 7.7 | 4.8 | 0.49 | — | — | — | — | — | — | — | –80.8 | –13.0 | 3.0 | –8.3 |
| 84585 | KNY-15 | Stream water, Kanayamazawa uppermost point, left bank next to KYZ-14 | 12.7 | 5.6 | 0.21 | — | — | — | — | — | — | — | –75.7 | –12.0 | 2.3 | –9.9 |
| 84586 | KNY-16 | Seepage water issuing from debris, Kanayamazawa uppermost point, right bank | 11.8 | 8.0 | 1.08 | 71 | 973 | 1.0 | 10.8 | 1.1 | 276 | 74 | –75.8 | –12.3 | 0.4 | –9.0 |
| 84587 | KNY-17 | Stream water #2 from Kanayamazawa uppermost point, left bank | 13.0 | 4.2 | 0.79 | 0 | 527 | 0.8 | 9.1 | 0.8 | 133 | 45 | –75.9 | –12.4 | 2.1 | –9.0 |
| 84588 | KNY-18 | Stream water #1 from Kanayamazawa uppermost point, left bank, next to KYZ-17 | 14.7 | 4.2 | 0.69 | 0 | 418 | 0.7 | 7.3 | 1.0 | 113 | 28 | –72.5 | –11.8 | 2.4 | –8.9 |
| Sample number | Sample Code | Description | δ34SSulfide ‰ | δ34SSO4 ‰ | δ18OSO4 ‰ |
|---|---|---|---|---|---|
| 84424-3 | NGS-7 | Sulfide recovered from sand after SO4 was removed. Nigorisawa | 15.7 | — | |
| 84424-5 | NGS-8 | SO4 in leachate of black mud, Nigorisawa | 7.5 | –8.0 | |
| 84424-1 | NGS-9 | Sulfide in black mud after removal of dissolvable SO4, Nigorisawa | –2.0 | — | |
| 84425 | NGS-6 | SO4 in leachate from Shiiya formation mud stone, Nigorisawa | –31.8 | –4.4 | |
| 84427 | MSG-8 | Pyrite from Mushigame sliding cliff | –4.5 | — | |
| 84429 | MSG-9 | Pyrite aggregate from Mushigame sliding cliff | –5.7 | — | |
| 84589-1 | KNY-1-Py | Pyrite found on the surface of an altered andesite block, Kanayamazawa | 4.6 | — | |
| 84589-2 | KNY-1-Gyp | Gypsum found on the surface of an altered andesite block, Kanayamazawa | 5.8 | –5.5 | |
| 84590-1 | KNY-2-Py | Pyrite found on the surface of an altered andesite block, Kanayamazawa | 2.6 | — | |
| 84590-2 | KNY-2-Gyp | Gypsum found on the surface of an altered andesite block, Kanayamazawa | 4.2 | –6.7 | |
| 84591 | KNY-3-white ppt | White precipitate found on talus accumulation at lower end of Kanayamazawa | 2.4 | –11.7 | |
| 84592-1 | KNY-4-Py | Pyrite aggregate found in fractured andesite, Kanayamazawa left bank | 2.3 | — | |
| 84592-2 | KNY-4-SO4 | SO4 in leachate of altered andesite, found at the Kanayamazawa highest point | 2.5 | –5.8 |
Samples were bottled in a 250 mL plastic bottle after in situ filtration with a 0.45 μm filter for later chemical and isotopic analyses. Chemical analysis of the samples was made by O. Sato in 1985–1986 using the following methods: pH with a glass electrode, HCO3– as alkalinity titration using BCP as an indicator, Ca2+ and Mg2+ with atomic absorption spectrometry, Na+ and K+ with flame photometry, Cl– with colorimetry using mercury thiocyanate, and SO42– with colorimetry using thorium morin for low concentration or turbidimetry for high concentration. The analytical accuracy was within 3~5% of the values given. Ionic balance is calculated to be within 3%. Isotopic analyses of waters and rocks were made by M. Kusakabe in 1985–1986. Hydrogen and oxygen isotopic ratios were determined with mass spectrometry using a zinc reduction method for hydrogen and a H2O-CO2 exchange method for oxygen, respectively. The analytical accuracy was within 1‰ for δD and within 0.1‰ for δ18O. Both δD and δ18O are expressed relative to VSMOW. Sulfur isotopic ratios of dissolved SO4 were determined with mass spectrometry using SO2 prepared by thermal decomposition of BaSO4 under coexisting V2O5 and silica (Yanagisawa and Sakai, 1983). Pyrite was oxidized to SO42– using an aqua regia-Br2 mixture. The 34S/32S ratios of SO2 resulting from thermal decomposition of BaSO4 were measured with a mass spectrometer. The analytical accuracy of δ34S was within 0.2‰. δ18O and δ34S values are expressed relative to VSMOW and VCDT, respectively.
Chemical and isotopic compositions of waters and dissolved sulfates from the studied landslide areas are given in Table 1. Figure 2 is a ternary presentation of the cationic Ca-Mg-(Na + K) relationship (a) and anionic SO4-Cl-HCO3 relationship (b). Cations are plotted along a Ca/Mg = 2 line. Geology of the KNY area is dominated by andesite (Kariya et al., 2012), suggesting that the major source of Ca and Mg in water is dissolution of the felsic rocks which have Ca/Mg = 1.5~3. The KNY samples are low in Na and K compared with the MSG-NGS-YMG samples. Anion composition is highly variable in the MSG-NGS-YMG samples as shown by scattered distribution of the data (Fig. 2b). The points encircled by a dotted circle in Fig. 2b indicate the MSG samples that have high salinity with E.C. greater than 1.9 mS/cm (see Table 1). Two samples close to the Na + K apex show Na+ concentrations exceeding 1200 ppm and those close to Cl apex have Cl– concentrations of 1300~2300 ppm with low Ca2+ and Mg2+ concentrations. These samples indicate that NaCl-type waters exist in deep layers of the MSG area. Such waters may be present in water associated with crude oil at depth as suggested by Masuda (2023). The YMG and most of the NGS waters have a smaller contribution from such deep water. They are enriched in HCO3– and SO42– derived from surface processes, i.e., degradation of organic materials for HCO3– and atmospheric oxidation of pyrite in the rocks for SO42–. The KNY samples have very low Cl concentrations and are plotted along the SO4-HCO3 base line with Cl = ~0 (Fig. 2b). This indicates little or no contribution of deep fluid rich in NaCl in the KNY area. The chemistry of KNY waters is characterized by uniform cationic values (Fig. 2), and probably reflects smaller catchment areas of the site.

Ternary plots for cations (a) and anions (b) of the sample waters. For cations, the MSG samples are enriched in Na and K. The NGS and YMG samples are intermediate and the KNY samples are Na-K poor. Data are plotted around the Ca/Mg = 2 line. For anions, data look dispersed, but the MSG samples plot toward the Cl corner. The MSG plots encircled by dotted line indicates that E.C. values of the samples are greater than 2 mS/cm. The NGS and YMG samples are relatively enriched in HCO3. The KNY samples are low in Cl and enriched in SO4.
Hydrogen (δD) and oxygen (δ18O) isotopic ratios of the sample waters are shown in Fig. 3. The values of the waters are consistent with the spatial distribution of δD-δ18O values for surface and shallow groundwaters from Japan (Mizota and Kusakabe, 1994). They are sandwiched by 2 meteoric water lines, δD = 8δ18O + 10 and δD = 8δ18O + 25, typical lines for Japan. The waters from KNY are lower in δD and δ18O than the MSG, NGS and YMG waters, reflecting that the KNY waters originate from higher altitude than the latter, since meteoric waters from high altitude are known to be more depleted in heavier isotopes than those from lower altitude (Mizota and Kusakabe, 1994). One KNY sample (#84573) has high δD and δ18O values compared to the rest of the KNY samples. This isotopic enrichment may reflect evaporation prior to sampling or during storage.
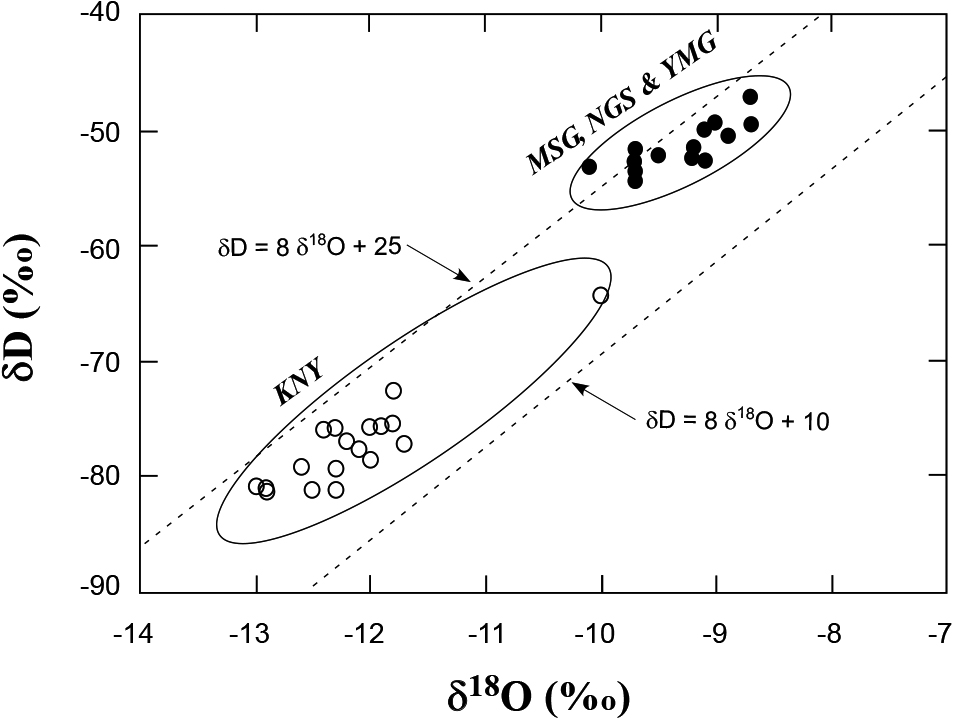
δD and δ18O relationship of the sample waters. The values indicate typical meteoric water, sandwiched by two meteoric water lines in Japan, δD = 8δ18O + 10 and δD = 8δ18O + 25. The KNY waters are plotted slightly lower than MSG-NGS-KNY waters, due to higher altitude of the area.
Figure 4 shows a δ18OSO4 versus δ34SSO4 relationship for dissolved sulfate of the samples. The δ34SSO4 and δ18OSO4 values from MSG, NGS and YMG are variable, i.e., δ34SSO4 ranges from –24 to +8‰ and δ18OSO4 ranges from –12 to +5‰. The variability indicates multiple formation processes of SO42– including intense microbiological activity at each sampling site. Figure 4 indicates that the samples generally show a positive trend between δ18OSO4 and δ34SSO4 values at each site except the NGS samples. The MSG samples that are encircled by dotted line have higher total dissolved solids (TDS) than the others, suggesting that the waters contain a Na-Cl-rich component of deeper origin (Table 1). However, δ18OSO4 and δ34SSO4 values of the MSG samples do not necessarily represent the original isotopic values. They may have changed later in the surficial processes as suggested above. The positive trends may be explained by mixing of bacterially mediated sulfate with sulfate produced by simple oxidation of pyrite in rocks. The point with the highest isotopic values may represent sulfate that remained after biological reduction of sulfate, leaving high δ34S sulfate behind. The point with the lowest isotopic values was found in the YMG group. Since sulfur isotopic fractionation during oxidation of sulfide to sulfate is small, a low δ34SSO4 value of –24‰ might represent sulfate formed through oxidation of sulfide which was strongly fractionated by bacterial reduction in the shallow aquifer. The samples from KNY are plotted close to each other (Fig. 4), i.e., the averaged δ34SSO4 = +2.3 ± 0.6‰ and δ18OSO4 = –8.8 ± 0.6‰ (the numbers after ± indicate standard deviation). These values are consistent with the observed δ34S values of pyrite and δ18O values of gypsum and leached SO4 (Table 2).
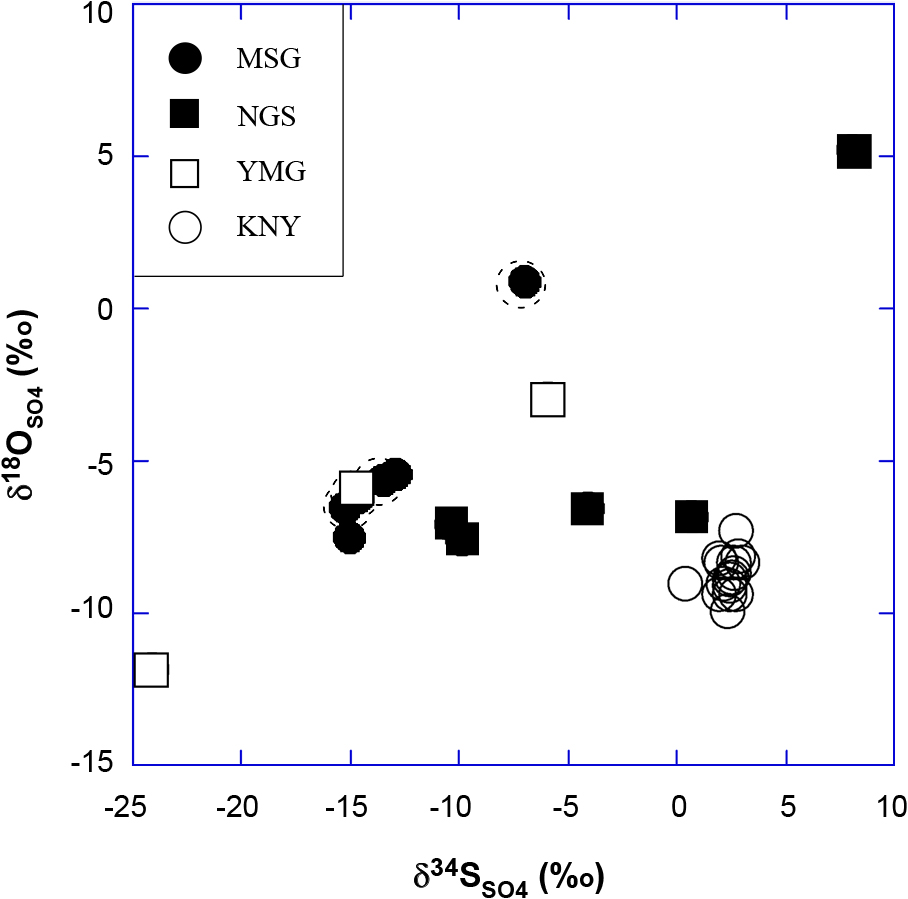
δ18O vs δ34S plot of sulfate dissolved in water. The MSG, YMG, and NGS samples have a positive correlation, whereas the KNY samples plot close to each other at the lower right of the figure.
The Na-K-Mg composition of the samples in this study was converted to Na/1000, K/100 and square root of Mg and plotted on a Na-K-Mg diagram, i.e., so-called the Giggenbach diagram (Fig. 5). The diagram is used to evaluate equilibration of solutes with minerals such as feldspar, mica, chlorite, etc. in surrounding rocks (Giggenbach, 1988). The MSG-1, MSG-7, and MSG-6 are plotted above the equilibrium curve suggesting addition of extra-Na to the rest of MSG waters. It is worth noting that MSG-1 and MSG-7 have high E.C. values enriched in Cl– (2260 and 1270 ppm, respectively) (Table 1). The other samples (MSG-5, NGS-3, NGS-5, MSG-4, MSG-3) are plotted close to the equilibrium curve at temperatures lower than 80°C in the area designated as “partial equilibrium”. Since the MSG-5, NGS-3, NGS-5, MSG-4, MSG-3 samples are low in temperatures (11~14°C) when collected, in situ attainment of chemical equilibrium with rocks is unlikely. The MSG-1, MSG-7, and MSG-6 waters contain oil-associated water and the equilibration with K-feldspar, K-mica, chlorite and silica may have been attained at deep oil layers, although they have been greatly diluted by surface waters during ascent to the surface as shown by δD and δ18O signatures (Fig. 3). The dilution of Cl-rich deep water by SO4-HCO3 surface water is also shown in the anionic distribution (Fig. 2b).
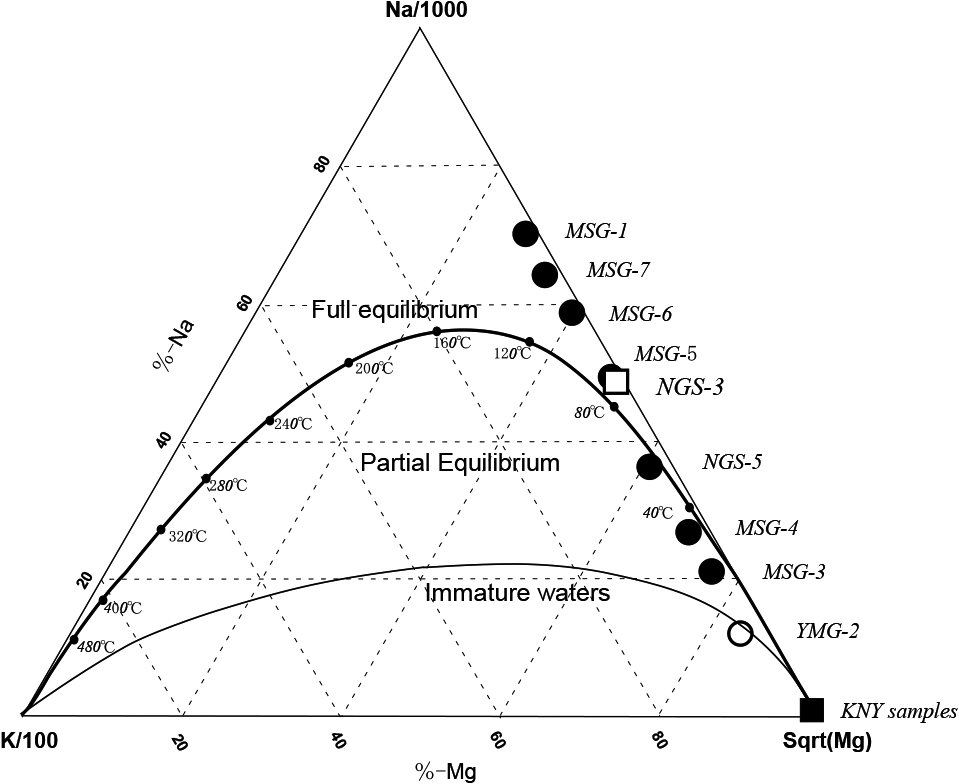
Ternary presentation of Na-K-Mg relationship plotted on a so-called “Giggenbach diagram”. Surprisingly MSG-3, MSG-4, NGS-5, and may be, MSG-5 and NGS-3 are plotted close to an “equilibrium” curve at temperatures lower than 80°C. The other samples are plotted above the equilibrium curve, indicating inclusion of Na-rich water (brine associated with oil?). See text.
Geochemistry of surface- and groundwaters from the MSG, NGS, and YMG landslide areas in Niigata and KNY area in Nagano Prefectures, Japan, was investigated. Their δD and δ18O values are indicative of typical meteoric water in origin. The HCO3– and SO42– were produced by surficial decomposition of organic materials and oxidation of pyrite in the rocks, respectively, and their concentrations in the NGS and KNY waters were generally low. Some of the MSG samples have E.C. values higher than the others. They are enriched in Na+ and Cl– at concentrations as high as 1000~2000 ppm, indicating contribution of deep, saline water. It is conceivable that an aquifer rich in NaCl exists at depth of the MSG area and that such waters are derived from fossil seawater trapped in oil-producing mudstones in the area. Such waters may be pushed out after heavy rains and snow melting in spring. Waters slightly enriched in Na+ and Cl– are also found at NGS (Table 1, and Table 2 in Sato (1982)), suggesting the presence of the MSG-type waters underneath. Large variability of δ34S and δ18O values of dissolved sulfate indicates a biological reduction during infiltration of sulfate that initially formed through oxidation of pyrite in rocks at shallow levels. Such reduction of sulfate to sulfide probably took place at shallow levels under different reducing conditions followed by re-oxidation. These processes induce the variability of the isotopic values. Thus, multiple oxidation-reduction processes may be common in the areas. Regular geochemical monitoring of seepage waters would lead to better understanding of the behavior of groundwater in the landslide areas.
Chemical analysis of the waters was done by O. Sato in 1984–1985 before he deceased in 1990. M. Kusakabe feels sorry to O. Sato for his death and for not publishing this article earlier. Isotopic analyses were done by T. Nogi and M. Kusakabe who worked for the Institute for the Study of the Earth’s Interior, Okayama University at Misasa at that time.