2025 Volume 100 Article ID: 24-00110
2025 Volume 100 Article ID: 24-00110
We aimed to identify new mutants resulting from ONSEN transposition in Arabidopsis thaliana by subjecting nrpd1 mutant seedlings to heat stress. We isolated a mutant with a significantly elongated hypocotyl, named Long hypocotyl in ONSEN-inserted line 1 (hyo1). This phenotype was heritable, with progeny consistently displaying longer hypocotyls than the wild type. Genetic analysis revealed that this trait was due to a single recessive mutation. Further mapping and sequencing identified the insertion of ONSEN into the HY2 gene, a crucial regulator of hypocotyl elongation. The insertion disrupted HY2 transcription, as confirmed by quantitative PCR, leading to the observed phenotype. To assess any influence of the nrpd1 background, we generated lines backcrossed twice to wild-type Col-0, and the results were consistent with those observed in the original mutant lines. Furthermore, we examined the effect of HY2 and HYO1 mutations on flowering time by analyzing the expression levels of FT. The hyo1 mutant exhibited earlier flowering compared to both wild type and the nrpd1 mutant, with increased FT expression levels. This research highlights the impact of ONSEN transposition on gene function and phenotypic variation in A. thaliana, providing new insights into the mutagenic potential of transposons and their role in shaping plant traits.
Transposons make up a large portion of the genomes of plants and animals, and their transposition is one of the driving forces behind the genome evolution of organisms (Kazazian, 2004; Biémont and Vieira, 2006; Bucher et al., 2012; Casacuberta and González, 2013; Fultz et al., 2015). Transposons can be classified into Class I and Class II (Wicker et al., 2007). Class I, also called retrotransposons, is transposed in a “copy & paste” fashion. After synthesizing mRNA, retrotransposons synthesize a cDNA by reverse transcription. The cDNA intermediate is called extrachromosomal DNA; it inserts into a new chromosomal location, thereby increasing the copy number (Bucher et al., 2012). Class II, on the other hand, is called DNA-type transposons and is transposed in a “cut & paste” fashion (Sahebi et al., 2018). Compared to DNA-type transposons, retrotransposon copy number can potentially explode and alter the structure of the host genome.
Transposons are ubiquitous in the genomes of all organisms, yet the majority remain inactive. This inactivity stems partly from accumulated deletions or mutations within their DNA sequences, which render them non-functional. Additionally, host organisms typically repress transposon gene expression through epigenetic modifications, effectively silencing these mobile genetic elements (Wierzbicki et al., 2008; Gao et al., 2010).
Most transposons are repressed in expression, but some are known to be transiently activated by environmental stress (Lisch, 2009; Ito, 2022). ONSEN, a heat-activated retrotransposon, resides within the Arabidopsis genome. It features a heat shock element within its long terminal repeat sequence, which is recognized as a binding site by the heat shock transcription factor HsfA2. This interaction triggers ONSEN activation at 37 °C (Cavrak et al., 2014). In the wild type, transposition of ONSEN is suppressed by an epigenetic mechanism called RNA-directed DNA methylation (RdDM) (Wierzbicki et al., 2008; Gao et al., 2010). In the RdDM pathway, small interfering RNA is synthesized by RNA polymerase IV (Pol IV) and forms an RNA-induced silencing complex that eventually recruits DNA methyltransferases, leading to de novo methylation of target TEs (Matzke and Birchler, 2005). However, when mutants of the RdDM pathway are exposed to heat, transgenerational transpositions of ONSEN are observed (Ito et al., 2011; Matsunaga et al., 2012).
Transposons can alter gene expression and phenotype by integrating into or near target genes. Transgenerational transposition of ONSEN can be triggered in the nrpd1 mutant, which encodes the largest subunit of Pol IV, by subjecting it to heat stress at 37 °C for 24 h (Ito et al., 2011; Matsunaga et al., 2012). Previous studies have found that ONSEN preferentially targets euchromatic regions and affects gene expression (Ito et al., 2016). It has been reported that ABA stress-tolerant mutants were obtained from the ONSEN-inserted population in nrpd1 (Ito et al., 2016). The insertion of ONSEN into ABA-responsive genes such as ABI4 and ABI5 can generate mutants that are insensitive to ABA stress. This demonstrates that ONSEN transposition can confer environmental adaptation to the host plant, highlighting its role in enhancing stress resilience through genetic modification.
In this paper, we present the discovery of a mutant displaying abnormal hypocotyl elongation, attributed to ONSEN transposition triggered by heat stress.
To identify novel mutants resulting from ONSEN transposition, we conducted a phenotypic analysis on a population of ONSEN-inserted lines generated by subjecting nrpd1 mutants to heat stress. This screening led to the isolation of a mutant exhibiting significantly elongated hypocotyls compared to the wild type, which we designated as Long hypocotyl in ONSEN-inserted line 1 (hyo1). To verify the heritability of this trait, seeds were collected from hyo1 and grown. All progeny displayed the same elongated hypocotyl phenotype. At 5 days post-germination on vertical medium, the average hypocotyl length of the mutant was 10.11 mm, markedly longer than the 2.01 mm observed in wild-type plants. In comparison, the hypocotyl length of the original nrpd1 mutant line used to create the ONSEN-inserted population averaged 2.16 mm (Fig. 1A and 1B).

hyo1 has a recessive phenotype
To identify the genetic factor responsible for the elongated hypocotyls, the hyo1 mutant was crossed with a wild-type Ler accession. All F1 progeny exhibited the wild-type phenotype, indicating that the hyo1 trait is recessive. In the F2 generation, approximately one-fourth of the seedlings displayed the elongated hypocotyl phenotype, suggesting that the trait is controlled by a single genetic locus (Fig. 2A).

The elongated hypocotyl phenotype in hyo1 is independent of a mutation in NRPD1
Given that hyo1 originates from an nrpd1 mutant background, we asked whether the elongated hypocotyl phenotype in hyo1 could be attributed to the nrpd1 mutation. While Figure 1 shows hypocotyl length in the nrpd1 mutant alone, we further investigated the nrpd1 mutation within the hyo1 background by genotyping 16 F2 individuals from an nrpd1/Ler cross, focusing specifically on long hypocotyl segregants. This analysis revealed six individuals homozygous for the nrpd1 mutation, seven heterozygous, and three homozygous for the wild type (Fig. 2B). These results indicate that the nrpd1 mutation is not responsible for the extended hypocotyl phenotype observed in hyo1.
Mapping of the gene responsible in hyo1To identify the gene responsible for the elongated hypocotyl phenotype, we conducted rough mapping using Col-0/Ler genetic markers across all five Arabidopsis chromosomes. Initially, we established one marker per chromosome and genotyped DNA samples from 16 F2 individuals. The analysis revealed that the genetic marker NGA162 on chromosome 3 was consistently associated with the Col-0 genotype in all samples, suggesting that the gene responsible is in this region. Further genotyping of 28 F2 DNA samples showed a 27:1 ratio of Col-0 homozygotes to Col-0/Ler heterozygotes for markers NGA172 and NGA162, positioned at 786,296 bp and 4,608,277 bp on chromosome 3, respectively. These findings indicate that the gene responsible for the longer hypocotyl phenotype is situated near these markers on chromosome 3.
ONSEN is inserted in the HY2 geneThe ELONGATED HYPOCOTYL 2 (HY2) gene is situated at 2,803,597–2,805,588 bp, between the genetic markers on chromosome 3. HY2 encodes the principal synthase for PϕB, a crucial cofactor for the photoreceptor phytochrome, and its disruption leads to hypocotyl elongation. In the hy2 mutant, a T-DNA insertion is situated at 472 bp within the HY2 gene (Fig. 3A). PCR analysis, using primers targeting the ends of the HY2 gene, revealed a much larger DNA fragment in hyo1 mutants compared to wild type (approximately 8 kb versus 3 kb, respectively) (Fig. 3B). This suggests the insertion of ONSEN within the HY2 gene region. Further PCR analysis, using primers specific to both the HY2 and ONSEN regions, was performed around the 5' and 3' junctions. On the 3' side, this yielded a 1-kb fragment (Fig. 3C), which was confirmed by sequencing to be a fusion of HY2 and ONSEN sequences (Supplementary Fig. S1). Similarly, analysis of the 5' junction revealed a comparable fusion of HY2 and ONSEN sequences. Segments of nucleotides matched both HY2 and ONSEN, pinpointing the insertion at 1,408 bases within HY2 (Fig. 3A).

To confirm that HY2 disruption was responsible for the hypocotyl elongation phenotype, a complementation test was performed. F1 hybrids from crosses between hyo1 and hy2 mutants displayed significantly elongated hypocotyls compared to the wild type (Fig. 4A and 4B). These results clearly demonstrate that the insertion of ONSEN into the HY2 gene disrupts its function, leading to the observed phenotype in hyo1.
The transcript of HY2 is disrupted by the ONSEN insertionTo assess the impact of ONSEN insertion on HY2 gene transcription in hyo1, we performed quantitative PCR to measure transcript levels downstream of the insertion site. The results revealed a marked reduction in HY2 transcripts in hyo1 compared to both wild type and the control nrpd1 mutant. Similarly, the hy2 mutant, used as a control, also exhibited low levels of HY2 transcripts. These findings indicate that the elongated hypocotyl phenotype in hyo1 is attributable to the disrupted production of HY2 gene transcripts (Fig. 4C).

Comparative analysis of hypocotyl length phenotypes in hyo1 and related mutants
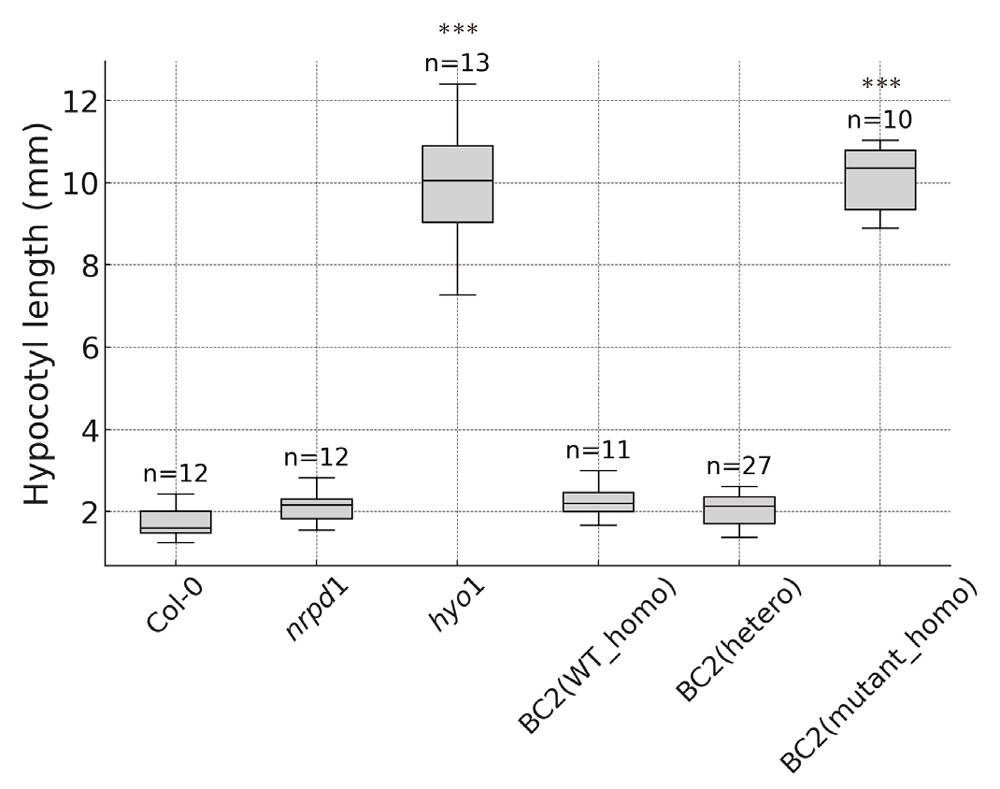
To investigate whether the genetic background, specifically the nrpd1 mutation, influences hypocotyl length in the hyo1 mutant, we backcrossed the hyo1 mutant to the wild-type Col-0 background twice to eliminate any potential effects of the nrpd1 mutation. Hypocotyl length analysis of progeny from these backcrossed plants revealed that, among 48 individuals, 38 displayed the short hypocotyl length typical of the wild type, while 10 exhibited the elongated hypocotyl phenotype characteristic of the hyo1 mutant. Notably, all 10 individuals with elongated hypocotyls were homozygous mutants with an ONSEN insertion in the HY2 gene, while the 38 individuals with short hypocotyls were either wild type or heterozygous at the HY2 locus (Fig. 5). Furthermore, all 48 individuals were confirmed to have reverted to the wild-type genotype at the NRPD1 locus. These results indicate that the elongated hypocotyl phenotype observed in hyo1 mutants is independent of the nrpd1 mutation and is associated with the ONSEN insertion in the HY2 gene.
The hyo1 mutant has an earlier flowering time
To determine if the elongated hypocotyl of the hyo1 mutant influences its flowering time, we compared the flowering times of wild type and hyo1 and nrpd1 mutants. The results revealed that hyo1 mutants flowered significantly earlier, at 16 days after germination, compared to the wild type, which flowered at an average of 24 days. The nrpd1 mutants, used as a control, took 29 days to flower, while the hy2 mutants flowered in 21 days (Fig. 6A). Additionally, we assessed the relationship between flowering time and the number of rosette leaves. The wild type averaged 13 rosette leaves, the nrpd1 mutants averaged 17, and the hyo1 mutants averaged only 4, confirming an early flowering phenotype in hyo1 (Fig. 6B). Notably, the hyo1 mutants flowered even earlier than the hy2 mutants (Fig. 6A and 6C). To examine whether the genetic background, particularly the nrpd1 mutation, influences the flowering time of the hyo1 mutant, the hyo1 mutant was backcrossed twice to a wild-type Col-0 background to eliminate any potential effects of the nrpd1 mutation. Flowering time analysis of progeny from these backcrosses, based on the number of rosette leaves at flowering, revealed that 39 out of 48 plants exhibited flowering times similar to the wild type, while nine plants displayed the early flowering phenotype characteristic of the hyo1 mutant. Notably, all nine individuals with the early flowering phenotype were homozygous mutants carrying an ONSEN insertion in the HY2 gene. In contrast, plants with flowering times comparable to the wild type were either wild type or heterozygous at the HY2 locus (Fig. 6D). Additionally, all 48 individuals reverted to the wild-type genotype at the NRPD1 locus. These findings indicate that the premature flowering phenotype observed in the hyo1 mutant is independent of the nrpd1 mutation and is associated with the ONSEN insertion in the HY2 gene.

To investigate the cause of early flowering in hyo1, we focused on the role of the FT gene, a key regulator of flowering time. Since FT encodes the florigen that promotes flowering, we compared FT transcript levels between hyo1 and wild-type plants using qRT-PCR. The results showed that FT expression was significantly higher in hyo1 than in the wild type (Fig. 6E). This suggests that the early flowering phenotype of hyo1 is attributable to increased FT transcript levels, potentially linking the elongated hypocotyls of hyo1 to enhanced FT expression and accelerated flowering. Additionally, we analyzed samples from a segregating population of hyo1 that had been backcrossed twice to Col-0. Among 48 individuals, FT transcript levels in the 39 plants with short hypocotyls were comparable to those of the wild type. In contrast, the nine plants with elongated hypocotyls exhibited significantly elevated FT transcript levels. Further genotyping revealed that the 39 plants with short hypocotyls were either wild type or heterozygous at the HY2 locus, whereas all nine plants with elevated FT levels and elongated hypocotyls were homozygous mutants at the HY2 locus, with an ONSEN insertion detected in the HY2 gene (Fig. 6E). Additionally, all 48 individuals were verified to carry the wild-type genotype at the NRPD1 locus. These findings suggest that the early flowering phenotype of hyo1 is closely associated with elongated hypocotyls, which may be linked to the presence of the ONSEN insertion in the HY2 locus, resulting in enhanced FT expression and accelerated flowering.
New insertions of transposons generate a diverse array of traits and occasionally act as catalysts for evolutionary processes (Ito et al., 2016; Contreras-Garrido et al., 2024; He et al., 2024). In our previous research, we observed transgenerational transposition of ONSEN in A. thaliana mutants of the RdDM pathway under high-temperature stress (Ito et al., 2011). Building on this, we utilized the nrpd1 mutant, a key RdDM pathway mutant, to screen ONSEN-inserted populations derived from heat-stressed progeny. Remarkably, we identified mutants exhibiting abnormally elongated hypocotyls. The hypocotyl elongation phenotype of hyo1 is striking and distinguishable from the wild type just days after sowing. Subsequent genetic analysis indicated that this elongated hypocotyl phenotype is attributable to a mutation in a single gene. Classical mapping and sequencing identified an ONSEN insertion within the HY2 gene. Consistent with previous findings that ONSEN preferentially inserts within genes, often targeting exons, our experiment revealed ONSEN integration into the sixth exon of the HY2 gene. This demonstrates ONSEN’s potent capability to regulate gene expression.
Hypocotyl elongation in the hyo1 mutant is even more pronounced than in the previously reported hy2 mutant. This may be attributed to the different insertion sites of the T-DNA: while the hy2 mutant has the insertion in the first intron, the hyo1 mutant’s ONSEN is inserted in the sixth exon of the HY2 gene. Despite this difference, transcription analysis shows that both the hy2 and hyo1 mutants exhibit almost complete repression of the HY2 gene. One potential explanation for the pronounced difference in hypocotyl elongation between the hyo1 and hy2 mutants could lie in their genetic backgrounds. The hyo1 mutant was generated through the transposition of ONSEN within an nrpd1 mutant background. Crucially, our data indicate that the elongated hypocotyl phenotype in hyo1 is independent of the nrpd1 mutation, as evidenced by the genotyping of F2 individuals. Although nrpd1 is not directly responsible for the elongation phenotype, it might still exert some influence on hypocotyl length. To address this issue, we conducted multiple backcrosses of hyo1 with the Col-0 wild type to restore the genetic background to that of the wild type. After backcrossing, we analyzed the segregating population to confirm that the elongated hypocotyl phenotype and early flowering trait persisted in the hyo1 background, independent of other genetic influences introduced during the initial mutation process. This confirmed that the observed phenotypes are intrinsic to hyo1 and not perceptibly influenced by residual genetic background effects.
Another possibility is that the insertion of ONSEN in the hyo1 mutant disrupts nearby enhancer or promoter regions, leading to abnormal activation or suppression of HY2 transcription or of the transcription of neighboring genes. Such changes in transcriptional regulation may contribute to the pronounced phenotypes observed in hyo1 compared to hy2. Additionally, epigenetic modifications caused by the ONSEN insertion, such as altered DNA methylation or histone modifications, could influence the expression of HY2 or other related genes. Since the hyo1 mutant was originally generated in the nrpd1 background, some of these epigenetic effects might have been retained even after multiple backcrosses, potentially contributing to the differences in hypocotyl elongation and flowering time between hyo1 and hy2. Further investigation into the transcriptional and epigenetic landscapes surrounding the ONSEN insertion site in hyo1 would help to elucidate these mechanisms.
The hyo1 mutant exhibits markedly accelerated hypocotyl elongation, leading to an earlier flowering time. While the ecological advantages of this phenotype remain uncertain, it is plausible that the ONSEN transposition could drive genome evolution by modulating the flowering time in A. thaliana. This genetic alteration may provide a selective advantage under specific environmental conditions, potentially influencing the evolutionary trajectory of the species. Notably, the early flowering phenotype in hyo1 is accompanied by a significant increase in FT expression, a key regulator of flowering time that encodes the florigen that promotes floral transition. This suggests that the hyo1 mutation enhances the transcriptional activity of FT, potentially linking the mutant’s accelerated flowering to the molecular regulation of this central flowering-time gene. The upregulation of FT could amplify the plant’s ability to adapt to seasonal or environmental cues, such as light or temperature changes, ensuring reproductive success in fluctuating environments. This observation underscores the intricate connection between genetic mutations, hormonal signaling pathways and the timing of key developmental transitions like flowering.
In this experiment, we utilized Col-0 and Ler accessions of wild-type Arabidopsis. The hy2 (SALK_104923C) and nrpd1a-3 (CS66150) mutants, both in the Col-0 background, were sourced from the ABRC Stock Center. Additionally, we generated a line by backcrossing the hyo1 mutant with Col-0 twice. Seeds underwent sterilization by immersion in a chlorine bleach solution with 0.04% Triton X-100 for 5 min, followed by five rinses with sterile water. The sterilized seeds were then sown on 1/2 Murashige and Skoog (MS) medium containing 0.01% agarose, cold-treated in the dark at 4 °C for 2–3 days, and subsequently transferred to an incubator set at 21 °C, where they were grown under continuous light.
Heat stress conditionTo induce transgenerational transposition of ONSEN, nrpd1 mutant seedlings, 7 days post-germination, were subjected to heat stress at 37 °C for 24 h. Seeds collected from these heat-stressed nrpd1 plants were subsequently sown on 1/2 MS plates, cold-treated in the dark at 4 °C for 2 days, and then transferred to 21 °C. Phenotypic analysis was conducted under continuous light conditions to observe the resulting characteristics.
Measurement of hypocotyl lengthSeeds were sown on vertical 1/2 MS plates and subjected to a low-temperature treatment in the dark at 4 °C for 2 days. Following this treatment, the plates were transferred to 21 °C under continuous light. Hypocotyl lengths of seedlings were measured on the 5th day after germination, to assess phenotypic variation.
MappingDNA was extracted from F2 plants derived from crosses between hyo1 and Ler, selecting individuals that exhibited the hypocotyl elongation phenotype. PCR analysis was performed using SSLP (simple sequence length polymorphism) markers to assess genetic associations, allowing us to estimate the chromosomal location of the gene responsible for this phenotype. Mapping primers, including NGA primers, are listed in Supplementary Table S1.
DNA sequencingPCR was performed using primers corresponding to the HY2 and ONSEN regions for FASMAC premix analysis (FASMAC, Kanagawa, Japan).
qRT-PCRTotal RNA was extracted from seedlings grown on 1/2 MS medium under continuous light using TRI Reagent (Sigma-Aldrich, St. Louis, MO, USA). For expression analysis, samples collected 7 days after germination were used to analyze HY2, while samples collected 8 days after germination were used to analyze FT. Post-extraction, RNA was treated with RQ1 RNase-Free DNase (Promega, Madison, WI, USA) and then reverse-transcribed into cDNA using the ReverTra Ace qPCR RT Kit (TOYOBO, Osaka, Japan). qPCR was performed on an Applied Biosystems 7300 Real-Time PCR System using Luna Universal qPCR Master Mix (New England BioLabs, Ipswich, MA, USA). Three biological repetitions were performed, and standard deviation was determined. Expression levels were determined using the Ct method. 18S rRNA was used as an internal control for the analysis of the HY2 gene, while ACT2 was used as the internal control for the analysis of the FT gene. qPCR amplification was carried out using the appropriate primers as listed in Supplementary Table S1.
We thank Ms. Xiaoying Niu for her guidance on the ONSEN transposition induction experiments and overall molecular genetic experiments. This work was supported by the JSPS International Joint Research Program (International Joint Research Acceleration Fund, Type A) Grant Number JP 21KK0263.