2013 年 88 巻 3 号 p. 165-174
2013 年 88 巻 3 号 p. 165-174
The molecular basis of pigmentation variation within and among Drosophila species is largely attributed to genes in melanin biosynthesis pathway, which involves dopamine metabolism. Most of the genetic changes underlying pigmentation variations reported to date are changes at the expression levels of the structural genes in the pathway. Within D. melanogaster, changes in cis-regulatory regions of a gene, ebony, are responsible for the naturally occurring variation of the body pigmentation intensity. This gene is also known to be expressed in glia, and many visual and behavioral abnormalities of its mutants have been reported. This implies that the gene has pleiotropic functions in the nervous systems. In this review, current knowledge on pigmentation variation and melanin biosynthesis pathway are summarized, with some focus on pleiotropic features of ebony and other genes in the pathway. A potential association between pigmentation and behavior through such pleiotropic genes is discussed in light of cis-regulatory structure and pleiotropic mutations.
Our goal has long been to understand the evolution of ecologically relevant traits by integrating many different biological areas such as ecology, genetics, developmental biology, and neurobiology, i.e. from macro to micro scale. Pigmentation is one of the suitable study materials in this respect from both ecological and molecular perspectives. Ecologically, pigmentation could have a direct impact on individual fitness through factors such as camouflage, mate attraction, and temperature adaptation (reviewed in True, 2003; Gray and McKinnon, 2007; Protas and Patel, 2008). From the molecular perspective, pigmentation variation within and among species is widely observed and some causal genetic changes have been identified in many organisms (e.g. Miyagi and Terai, 2013; Suzuki, 2013). Moreover, the pigmentation biosynthesis pathway is one of the well-studied metabolic pathways in animals (reviewed in Sugumaran, 2002), and its key players have been known for a long time in insects (Wright, 1987).
Since there are many comprehensive reviews on molecular basis of pigmentation in insects (True, 2003; Wittkopp et al., 2003; Wittkopp and Beldade, 2009; Protas and Patel, 2008; Kronforst et al., 2012), I refer to them when appropriate. Instead, the main focus of this review is on the studies using naturally inhabiting model organism, D. melanogaster, except where otherwise notified. I briefly summarize our current knowledge on pigmentation variation, melanin biosynthesis pathway, and its role in biogenic amine metabolism. I also intend to look further into the pleiotropic features of one of the genes in the pathway, ebony, especially in relation to behavioral aspects. Finally, I discuss about a potential association between pigmentation and behavior in reference to the structure and evolution of cis-regulatory regions of such pleiotropic gene.
Various types of pigmentation variation within and between closely related species are observed in many insect taxa as well as in Drosophila species (reviewed in True, 2003; Wittkopp et al., 2003; Wittkopp and Beldade, 2009; Kronforst et al., 2012). Among them, an intriguing point one would notice is the existence of numerous reports on closely related species pairs with contrasting appearances in terms of body pigmentation intensity. Those cases include species pairs, D. ananassae - D. pallidosa (Bock and Wheeler, 1972), D. yakuba - D. santomea (Llopart et al., 2002), D. malerkotliana malerkotliana - D. m. pallens (Ng et al., 2008), D. pseudoananassae pseudoananassae - D. p. nigrens (Ng et al., 2008), D. bipectinata - D. parabipectinata (Ng et al., 2008), and D. novamexicana - D. americana (Wittkopp et al., 2003). Actually, D. novamexicana is distinctively pale compared to other D. virilis group species (Spicer, 1991). These species pairs have divergence time short enough that ability to interbreed has allowed genetic analyses and has helped narrowing down or to identify responsive genetic changes underlying the pigmentation differences (Llopart et al., 2002; Wittkopp et al., 2003, 2009; Carbone et al., 2005; Jeong et al., 2008; Ng et al., 2008). Pigmentation divergence in these species within short period of time suggests that pigmentation evolves relatively fast in some species. Short time diversification in abdominal pigmentation pattern is also well described in D. dunni and D. cardini subgroups (Hollocher et al., 2000a, 2000b; Brisson et al., 2006) and in D. montium subgroup (Ohnishi and Watanabe, 1985).
Short time diversification in pigmentation pattern coincides with that among populations from different localities in widely distributed Drosophila species such as D. ananassae (McEvey et al., 1987; Tobari, 1993) and D. elegans (Bock and Wheeler, 1972; Lemeunier et al., 1986; Hirai and Kimura, 1997). The largest source of information on within species variation in pigmentation is in the cosmopolitan species, D. melanogaster. The thoracic and dorsal pigmentation differences in typical dark and light strains from a natural population are indicated in Fig. 1. As shown in Fig. 1A, pigmentation variation can be easily observed in the trident-shaped area on the dorsal side of a thoracic segment (Fig. 1A). The first report of the trident polymorphism from a natural population was by the pioneers of Drosophila geneticists, Morgan and Bridges (1919), followed by Jacobs (1960, 1961), and David et al. (1985). What is particularly noticeable about the studies on pigmentation variation within this species is that a clinal variation in pigmentation intensity is repeatedly observed along altitude or latitude or both (David et al., 1985; Munjal et al., 1997; Pool and Aquadro, 2007; Parkash et al., 2008a, 2008b, 2009; Telonis-Scott et al., 2011). These observations of non-random distribution of the phenotype can be a circumstantial evidence that supports environmental adaptation playing a role in shaping the diverse patterns of body pigmentation.
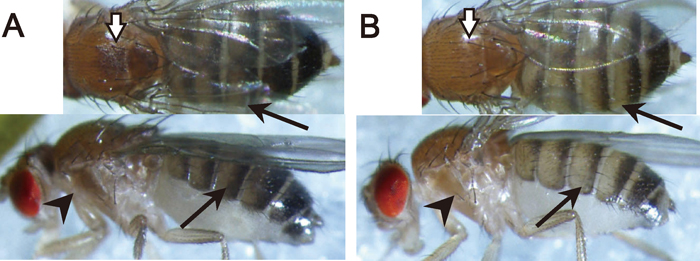
Pigmentation variation within Drosophila melanogaster. A typical dark (A) and light (B) colored individual reared at 20℃. Differences in pigmentation intensity can be observed at thoracic trident (white arrows), abdominal segments (black arrows), and lateral area of thoracic segment (black arrowheads).
Among many environmental factors, temperature is thought to play a substantial role in adaptive changes of the body pigmentation. Thermal budget (melanism) hypothesis in ectoderms argues that a darker pigmentation is more adaptive in colder environments, where it can help absorb heat from solar radiation, while a lighter pigmentation will prevent overheating in a warmer environment (see for review in Clusella Trullas et al., 2007). In D. melanogaster, geographic distributions of pigmentation variation are consistent with this hypothesis (David et al., 1985; Gibert et al., 1996, 2009; Munjal et al., 1997; Pool and Aquadro, 2007; Telonis-Scott et al., 2011; Takahashi and Takano-Shimizu, 2011). Also, phenotypic plasticity of the pigmentation intensity by the developing temperature is in the consistent direction with the hypothesis, which is that the flies becomes darker when grown at lower temperatures (David et al., 1985, 1990; Das et al., 1994; Gibert et al., 1996, 2000, 2009; Munjal et al., 1997; Gibert et al., 2007).
Nevertheless, there are contradictory views on whether body color is effective in regulating body temperature in Drosophila. Hirai and Kimura (1997) showed that between black and brown morphs of D. elegans, the former had slightly but significantly higher body temperature (0.26℃) than the latter under irradiation in the laboratory. However, measurements under natural sunlight had shown that an insect as small as a fruitfly (Diptera 3 mg) cannot keep its internal temperature above ambient, therefore, body color would not make any difference (Willmer and Unwin, 1981). So far, physiological basis of the thermal budget (melanism) hypothesis in Drosophila is still weak.
Regarding other environmental factors, a strong association of pigmentation intensity and desiccation resistance has been reported within and among D. melanogaster populations from India (Parkash et al., 2008a, 2008b, 2009), which suggests that humidity related adaptation may be important in some regions. However, a study on longitudinal cline of pigmentation variation in a different species, D. americana, did not find any association between pigmentation and desiccation resistance (Wittkopp et al., 2011; Clusella-Trullas and Terblanche, 2011). Also, such association was not detected among different strains of D. yakuba (Matute and Harris, 2013). UV resistance is another ecological factor that is thought to affect the adaptive changes in pigmentation intensity (Jacobs, 1985; Majerus, 1998; Matute and Harris, 2013), which awaits further investigations.
Adult pigments in Drosophila as well as in many other insect species are synthesized by the acts of enzymes in the melanin biosynthesis pathway in the developing epidermis (reviewed in Wright, 1987; Wittkopp et al., 2003; True, 2003; Wittkopp and Beldade, 2009; Kopp, 2009; Anderson, 2010; Kronforst et al., 2012). The adult pigment synthesis in Drosophila takes place during the first several hours after eclosion by the activation of ecdysis neuropeptide signaling pathway (Davis et al., 2007). The color of the cuticle is determined by the proportions of different-colored melanin pigments produced during this period, which is likely to be the outcome of the net balance of those structural enzyme activities in the pathway.
The simplified schematic model showing pigment biosynthesis pathway is shown in Fig. 2. There are some inconsistent views regarding the branches leading to the final polymerized products (melanins), shown as broken arrows in Fig. 2A. Some authors have hypothesized that black melanin is derived from Dopa (Wittkopp et al., 2002, 2003; True et al., 2005; Kronforst et al., 2012). Whereas, others have suggested that at least part of the black pigment comes from Dopamine (Wright, 1987; Walter et al., 1996; Gibert et al., 2007). This issue is related to the role of Yellow required for the production of black melanin. Recent studies actually support that this enzyme acts downstream of Dopamine as well as that of Dopa suggesting the latter view (Gibert et al., 2007; Riedel et al., 2011). During the immune response, the black melanin produced through DDC activity seems to be the predominant melanization pathway, although a minor path leading to black melanin from Dopa is also implicated (Nappi and Christensen, 2005; Nappi, 2010).

Simplified schematic models of the proposed biosynthetic pathways in Drosophila. (A) Pathways for producing cuticular pigments, redrawn from figures in Wright (1987), Walter et al. (1996), Wittkopp et al. (2002, 2003), True et al. (2005), Gibert et al. (2007), Kopp (2009), Riedel et al. (2011), and Kronforst et al. (2012). Some inconsistent views exist about whether the majority of the black melanin is produced from dopa or dopamine (pathways indicated by red broken arrows). (B) Pathways for histamine recycling in the visual system; redrawn from Borycz et al. (2002, 2012), True et al. (2005) and Gavin et al. (2007). Core enzymes in the pathways are indicated in blue. Broken arrows indicate paths leading to the final products (colored pigments). NBAD; N-beta-alanyl dopamine, NBAH; N-beta-alanyl histamine, TH; tyrosine hydroxylase, DDC; dopa decarboxylase.
Most of the genetic changes underlying pigmentation variations reported to date are changes at the expression levels of those structural genes (i.e. ebony, yellow, tan, reviewed in Wittkopp et al., 2003; Wittkopp and Beldade, 2009; Protas and Patel, 2008; Kronforst et al., 2012). Changing the cis-regulatory sequences is perhaps the simplest way to adjust the relative activity levels of those enzymes. Thus, this simplicity may be a contributing factor that has enabled fast evolutionary changes of body pigmentation seen in some species, in spite of the fact that many structural genes are highly conserved even among different insect taxa (Kayser, 1985).
It should be added that many recent studies have identified factors outside the core members of the melanin biosynthesis pathway. These factors include transcription factors such as Abdominal-B, bric-à-bric (Kopp et al., 2000; Gibert et al., 2007; Williams et al., 2008), and optomotor-blind (Kopp and Duncan, 1997), a global chromatin regulator network affected by Hsp83 (Gibert et al., 2007), and a cell growth pathway activated by Rheb (Zitserman et al., 2012). Also there is a recent study on microRNA, miR-8, which is required for the proper pigmentation patterning of the adult cuticle (Kennel et al., 2012). Thus, pigmentation is a reflectant of many related developmental systems in flies.
In D. melanogaster, the widely accepted prediction had been that the commonly observed variation in thoracic trident pigmentation intensity is caused by ebony, whose mutants show dark trident pattern on their thoracic segments (Jacobs, 1960; Wittkopp et al., 2002). Some direct evidences linking this gene to the thoracic or abdominal pigmentation variation in natural populations were put forward by an associated linkage pattern in the 5’ upstream region of this gene (Pool and Aquadro, 2007), a genetic mapping result using two wild-derived dark and light strains (Takahashi et al., 2007), and quantitative comparisons of the transcription levels induced by cis-regulatory elements from strains with different pigmentation intensities (Rebeiz et al., 2009). The lower expression of this gene in developing epidermis is associated with darker pigmentation, and the higher expression with lighter pigmentation (Takahashi et al., 2007; Rebeiz et al., 2009). This association is reported in populations from Africa (Rebeiz et al., 2009), Australia (Telonis-Scott et al., 2011), and southern Japan (Takahashi and Takano-Shimizu, 2011).
The summary of cis-regulatory polymorphisms and their association with the pigmentation phenotype are described in Fig. 3. Rebeiz et al. (2009) have performed a precise mutagenesis assay on the cis-regulatory region of ebony, and showed that at least five changes surrounding the core enhancer region have altered transcriptional activity of this gene in African populations. In Iriomote (southern Japan) population, 19 pigmentation-associated sites in the 5 prime region of this gene were identified (Takahashi and Takano-Shimizu, 2011). Interestingly, those sites that had complete association with the phenotype in the Iriomote population were not associated with the phenotype in populations from Uganda and Kenya, and the 5 nucleotide sites that have caused expression level changes in abdomens of African flies were either not segregating or not associated with the pigmentation phenotype in the flies from the Japanese population (Fig. 3; Takahashi and Takano-Shimizu, 2011). These comparisons suggest that there are many potential cis-regulatory mutations that can alter transcriptional activity, and that different mutations could lead to similar phenotypic variation in different populations within this species.

Summary of the cis-regulatory polymorphisms of ebony associated with pigmentation phenotype in populations from Iriomote (southern Japan) and Africa. Positions of Light-type specific SNPs and Indels in Iriomote population are indicated by red arrowheads and red arrows, respectively (data from Takahashi and Takano-Shimizu, 2011). The region resides within an inversion, In(3R)Payne, with which thoracic pigmentation intensity has a strong association in this population. Note that multiple mutations locate within the ~0.9 kb epidermis enhancer element indicated by the blue bar, and are different from the 5 nucleotide sites indicated by black arrowheads that have caused expression level changes of ebony in abdomens of African flies (Rebeiz et al., 2009).
Other than the structural gene, ebony, multiple alleles at a transcription factor bric-à-bric locus, are also likely to contribute to the natural variation in pigmentation pattern in this species (Kopp et al., 2003). This transcription factor plays a role in shaping the sexually dimorphic abdominal pigmentation pattern (Kopp et al., 2000) and mainly affects the width of the dark pigment band on the female posterior abdominal segments, whereas the ebony locus affects the overall abdominal pigmentation intensity outside the band (Rebeiz et al., 2009). It is plausible that close investigations of other structural genes in the melanin biosynthesis pathway or other transcription factor genes acting as pre-pattern formation may be involved in the natural pigmentation variation to some extent.
Another interesting phenomenon is that in Iriomote population from southern Japan, Light-type enhancer haplotype is strongly linked to a cosmopolitan inversion, In(3R)Payne (Fig. 3; Takahashi and Takano-Shimizu, 2011), which is predominant in warmer climatic regions in both hemispheres (Mettler et al., 1977; Knibb et al., 1981; Knibb, 1982; Inoue and Igarashi, 1994; Umina et al., 2005). The latitudinal clines of genetic and phenotypic variation, including In(3R)Payne along the eastern Australian coast, have been extensively studied (reviewed in Hoffmann and Weeks, 2007), and recently, Telonis-Scott et al. (2011) have characterized the clines of pigmentation intensity and ebony expression level that parallels the cline of In(3R)Payne frequency. A common association of In(3R)Payne with the lighter body phenotype in this species awaits further investigations.
Visual defects are among the well-described phenotypes of ebony mutants. It has been known from the early years that the electroretinograms of the mutants are abnormal, lacking the lamina potentials (Hotta and Benzer, 1969; Heisenberg, 1971). Phototactic and optomotor responses were also compared and it was shown that those responses are reduced in ebony flies, whose functional deficit in retinula cells 1–6 was suspected (Heisenberg, 1972). Kyriacou et al. (1978) even concluded that e11 homozygotes were effectively blind.
The molecular function of Ebony in the visual system is now known to be related to the histamine recycling pathway. It is suggested that Ebony functions as an enzyme which conjugates histamine to beta-alanine in the glia close to histamine release sites of photoreceptor cells in the lamina (Borycz et al., 2002; Richardt et al., 2002, 2003; True et al., 2005; Wagner et al., 2007; Gavin et al., 2007). This reaction terminates the action of the histamine released into the synaptic cleft in response to light by removing it by glial cells, where it is converted into carcinine (beta-alanyl histamine) by Ebony (see Fig. 2B for a simplified model).
Thus, Ebony is involved in at least two different biogenic amines, dopamine and histamine, metabolism pathways (Fig. 2). Moreover, tests on in vitro catalytic ability of Drosophila Ebony for other biogenic amines revealed that this enzyme accepts a variety of amines for beta-alanyl conjugation (Richardt et al., 2003). Still unknown functional properties of this enzyme may exist in vivo.
Another apparent phenotype of the ebony mutant is a defect on circadian rhythm. The rhythm defect was first reported in tyrosine aminotransferase activity cycle (Tauber and Hardeland, 1977). Later on, Newby and Jackson (1991) found that 5 of the tested ebony mutants show abnormal locomotor activity rhythms in both constant dark and in light:dark cycles. They also measured eclosion rhythms of the mutants, which was unaffected.
Two microarray studies indicated that ebony transcript has a rhythmic expression in head with a peak in the morning hours (Claridge-Chang et al., 2001; Ueda et al., 2002). Suh and Jackson (2007) elucidated the role of ebony on locomotor rhythmicity in their experiments. They showed that ebony is expressed in a discrete population of glial cells in larval and adult brains and that glial expression of Ebony rescues the altered circadian behavior of the ebony null mutant, e1. It is interesting and yet still rather puzzling that a high constitutive expression of Ebony without rhythmicity can rescue the aberrant locomotor rhythm in e1.
These authors also provided two evidences that support the ebony function in dopaminergic signaling. First, the rhythmic glial expression takes place in cells that are in close proximity to dopaminergic and serotonergic neurons of the larval and adult brains. Second, a genetic interaction exists between ebony and the dopamine transporter gene, DAT. The study emphasizes the importance of glia –neuron communication in coordinating fly behavior.
Rather ambiguous phenotypes of the ebony mutants are those related to the mating behavior. There are reports on the mutants’ disadvantages on mating success, which are the predictable consequences of their visual deficits (Rendel, 1951; Kyriacou et al., 1978; Kyriacou, 1981). Interestingly, most of these studies suggest that mutant males mate more efficiently in the dark or in diminished light conditions. Jacobs (1960, 1961) used wild-derived dark and light flies collected in North Carolina, referring to them as ebony and light flies, respectively, and reported observation in consistent with the laboratory derived ebony mutants. It is noteworthy that Jacobs (1960) observed non-random mating combination of his ebony and light strains such as that the frequency of mated pairs between light females and ebony males was substantially low (13 out of 369 mated pairs). This observation implies that there could be some variation in mate preference associated with the pigmentation variation segregating in a natural population.
Also, there are numbers of descriptions from the earlier studies reporting unusual sexual behavior of ebony mutants that may not be related to visual factors such as less frequent wing extention (Jacobs, 1960), frequent courtship abortion (Crossley and Zuill, 1970), and changes in acoustic parameters of the courtship song (Kyriacou et al., 1978). However, there have not been any rigorous experiments done to comfirm whether these observed mating phenotypes of the mutants are replicable using transgenic flies in a controlled genetic background. Also, since it is well established that dopamine level affects many different aspects of mating behavior (Neckameyer, 1998; Marican et al., 2004; Liu et al, 2008, 2009; Wicker-Thomas and Hamann, 2008), further investigations are awaited to see whether these behavior phenotypes are the indirect consequences of the abnormal neurotransmitter abundances in specific tissues or from other unknown factors.
Dopamine has another pleiotropic function in trachea development, and the expression of its metabolic pathway genes such as ebony, Punch, and Catsup in the developing trachea system of embryo has been reported (Hsouna et al., 2007; Perez et al., 2010). Also, there are series of studies by Neckameyer and colleagues showed that manipulation of dopamine levels affects many other developmental processes such as that in ovary (Neckameyer, 1996) and sensory tissue development (Neckameyer et al., 2001), where the genes in its biosynthesis pathway may also be playing a critical role.
The melanization reaction observed upon injury or on the surface of pathogens and parasites occurs as a part of the innate immune response (see review in Lemaitre and Hoffmann, 2007). And this response also involves phenoloxidase and some enzymes such as Ddc in the dopamine biosynthesis pathway (Fig. 2C and reviews: Nappi and Christensen, 2005; Nappi, 2010). It has also been suggested that N-beta-alanyldopamine has an antimicrobial activity in vitro, and the activation of its synthase, Ebony, was observed in the epidermis of the medfly, Ceratitis capitata, and the yellow mealworm, Tenebrio molitor, upon bacterial infection (Schachter et al., 2007).
I have described many pleiotropic functions of the genes involved in the melanin biosynthesis pathway. Those pleiotropic features can provoke conflicting natural selection pressures from various functional demands. It has been argued that the conflict can be partly resolved by possessing modularly structured cis-regulatory elements that allow evolutionary modifications of specific expression patterns without affecting others. Indeed, such modularly restricted cis-regulatory evolution has been repeatedly demonstrated in genes involved in pigmentation pattern variations among fruitfly species (Gompel et al., 2005; Prud’homme et al., 2006; Jeong et al., 2008). However, those fixed changes among species may represent only a small portion of mutations, which have limited effect on other functions (Stern and Orgogozo, 2008; Gompel and Prud’homme, 2009). But even within species, Rebeiz et al., (2009) have shown that changes in the cis-regulatory region of ebony that affects pigmentation has no effect on its expression level in other tissues, such as wing, haltere, larval mouth hooks, larval anterior spiracles, and larval brain. These results imply modularly restricted non-pleiotropic effects of regulatory mutations. The generality of this conclusion and the possible scenarios of the regulatory evolution (i.e. Monteiro and Podlaha, 2009) await further investigations.
Recent studies have shown that the major causal genetic differences underlying naturally occurring pigmentation variation in D. melanogaster lie in the cis-regulatory region of ebony (Pool and Aquadro, 2007; Takahashi et al., 2007; Rebeiz et al., 2009; Telonis-Scott et al., 2011; Takahashi and Takano-Shimizu, 2011). This leads us to a question why ebony, among other structural genes in the melanin biosynthesis pathway, has been subjected to frequent changes or possibly the target of natural selection in this species. Systematic comparisons among cases in other species (i.e. Kopp, 2009) may yield some answers to the evolvability of the gene network components in the pathway.
There is another evolutionary question about how those pleiotropic features of the pathway has evolved and what was the most ancestral function. Rigorous genomic screenings and phylogenetic analyses of the gene members of the pathway may give some answers to this question.
The modularly structured cis-regulatory elements of a pleiotropic gene seem to be an effective strategy in resolving the conflicting natural selection acting on the gene from different functional demands. However, there must be at least some cases where a simple modular arrangement is not feasible. In these cases, some mutations may affect the efficiencies of multiple functional regulatory elements, which would bring about changes in multiple pleiotropic characters such as pigmentation and behavior. Since within-population variation harbors neutral and slightly deleterious changes, a wider spectrum of mutational effect should be observed than when looking at only the fixed sites between species. Further investigations of the extent of such pleiotropic mutational effect within population may shed light on some new features of the genetic processes leading to species divergence due to associated evolutionary changes of the pleiotropic functions.
A. T. is supported by Grant-in-Aid for Scientific Research on Innovative Areas from the Ministry of Education, Culture, Sports, Science and Technology of Japan.