2016 年 91 巻 2 号 p. 77-84
2016 年 91 巻 2 号 p. 77-84
Stickleback fishes have been established as a leading model system for studying the genetic mechanisms that underlie naturally occurring phenotypic diversification. Because of the tremendous diversification achieved by stickleback species in various environments, different geographical populations have unique phenotypes and genotypes, which provide us with unique opportunities for evolutionary genetic research. Among sticklebacks, Japanese species have several unique characteristics that have not been found in other populations. The sympatric marine threespine stickleback species Gasterosteus aculeatus and G. nipponicus (Japan Sea stickleback) are a good system for speciation research. Gasterosteus nipponicus also has several unique characteristics, such as neo-sex chromosomes and courtship behaviors, that differ from those of G. aculeatus. Several freshwater populations derived from G. aculeatus (Hariyo threespine stickleback) inhabit spring-fed ponds and streams in central Honshu and exhibit year-round reproduction, which has never been observed in other stickleback populations. Four species of ninespine stickleback, including Pungitius tymensis and the freshwater, brackish water and Omono types of the P. pungitius-P. sinensis complex, are also excellent model systems for speciation research. Anthropogenic alteration of environments, however, has exposed several Japanese stickleback populations to the risk of extinction and has actually led to extinction of several populations and species. Pungitius kaibarae, which is endemic to East Asia, used to inhabit Kyoto and Hyogo prefectures, but is now extinct. Causes of extinction include depletion of spring water, landfill of habitats, and construction of river-mouth weirs. Here, we review the importance of Japanese sticklebacks as genetic resources, the status of several endangered stickleback populations and species, and the factors putting these populations at risk.
Stickleback fishes have been established as a leading model system for studying the genetic mechanisms that underlie naturally occurring phenotypic diversification and speciation (Peichel, 2005; Cresko et al., 2007; Kingsley and Peichel, 2007). Fishes belonging to family Gasterosteidae (order Gasterosteiformes) are generally called sticklebacks. Gasterosteidae contains five genera: Spinachia, Apeltes, Gasterosteus, Pungitius and Culaea (Nelson, 2006). Because of the tremendous phenotypic diversification achieved by sticklebacks (particularly Gasterosteus and Pungitius), different geographical populations have unique phenotypes, which makes it difficult to count the exact number of species in this family (Nelson, 2006). In this review, when intrinsic hybrid incompatibility, such as hybrid sterility and inviability, has been found between two groups of fishes, we call them different species; otherwise, we call them populations.
Gasterosteus and Pungitius have achieved tremendous phenotypic diversification and thus provide us unique opportunities for evolutionary research (Wootton, 1976, 1984; Bell and Foster, 1994; McKinnon and Rundle, 2002). During the last two decades, the genetic architectures of phenotypic divergence between populations have been extensively studied (Peichel et al., 2001; Colosimo et al., 2004; Cresko et al., 2004; Albert et al., 2008; Kitano et al., 2009; Shapiro et al., 2009; Greenwood et al., 2011, 2013; Wark et al., 2012; Laine et al., 2013, 2014; Arnegard et al., 2014; Miller et al., 2014). In several cases, genes or even genetic changes that cause morphological divergence have been identified (Colosimo et al., 2005; Miller et al., 2007; Chan et al., 2010; Cleves et al., 2014; O’Brown et al., 2015; Indjeian et al., 2016). cis-Regulatory changes in key developmental genes, such as Eda, Pitx1 and GDF6, contribute to morphological divergence between populations inhabiting contrasting environments (Chan et al., 2010; O’Brown et al., 2015; Indjeian et al., 2016). Furthermore, recent advances in genomic technologies make it possible to find regions under divergent selection between populations in multiple pairs of ecotypes (Mäkinen et al., 2008a, 2008b; Hohenlohe et al., 2010; Jones et al., 2012a, 2012b; Roesti et al., 2014, 2015; Feulner et al., 2015).
Among sticklebacks, Japanese species have several unique characteristics that have not been found in other populations (Mori, 1997; Goto and Mori, 2003), as reviewed in detail below. Anthropogenic alteration of environments, however, has exposed several stickleback populations to the risk of extinction and has actually led to extinction of several populations and species (Mori, 1997, 2003). Therefore, it is urgent to conserve these genetically important resources for evolutionary genetics research. In this paper, we review several unique characteristics of Japanese sticklebacks as well as the current status and conservation needs of endangered stickleback populations.
Japanese marine threespine sticklebacks (Gasterosteus) can be genetically classified into two species (Higuchi and Goto, 1996; Kitano et al., 2007, 2009; Higuchi et al., 2014): Gasterosteus aculeatus and G. nipponicus (Japan Sea stickleback) (Higuchi et al., 2014). Ikeda (1933) was the first to report that marine threespine sticklebacks collected from the east and west coasts of Japan differed morphologically in the heights of their caudal lateral plates (Fig. 1). While marine G. aculeatus are mainly found along the coasts of the Pacific Ocean, G. nipponicus is endemic to the Sea of Japan and the Sea of Okhotsk and is occasionally found in several coastal regions in the northwest of Japan (Fig. 1) (Higuchi and Goto, 1996; Yamada et al., 2007; Cassidy et al., 2013; Higuchi et al., 2014).
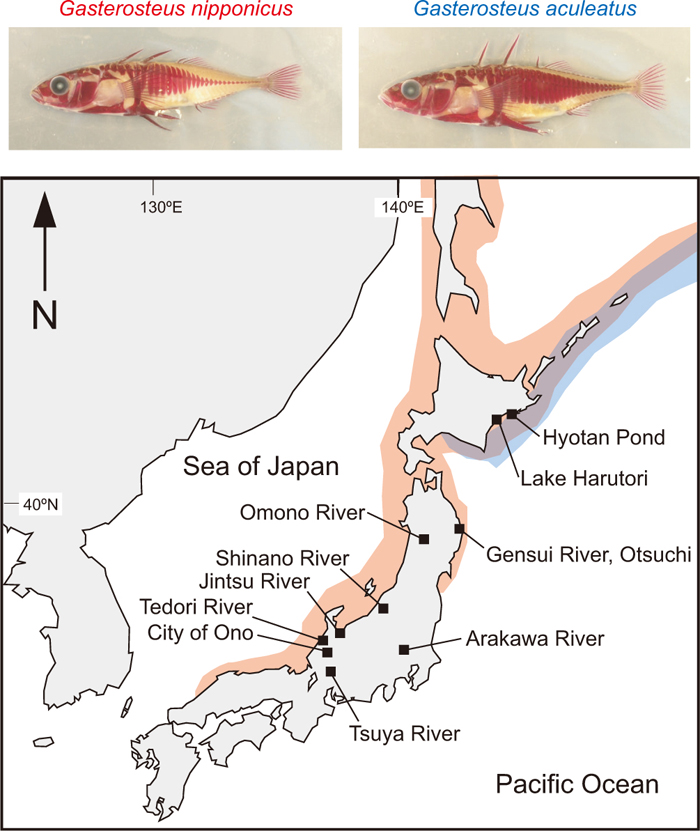
Upper panels indicate that caudal plates are shorter in G. nipponicus (Japan Sea stickleback) than in the Pacific Ocean population of G. aculeatus. Lateral plates were stained with Alizarin red. Pictures are of first-generation, lab-raised fish. Lower panel indicates the distribution of these two species, as well as other locations that are mentioned in this review.
The presence of reproductive isolation in sympatry also supports the notion that the two taxa are distinct species (Higuchi and Goto, 1996; Kitano et al., 2007, 2009). In eastern Hokkaido, for example, G. nipponicus and G. aculeatus are sympatric, but are reproductively isolated by multiple isolating barriers, including eco-geographical isolation (Kume et al., 2005, 2010; Kitano et al., 2009), seasonal isolation (Kume et al., 2005), sexual isolation (Kitano et al., 2009), ecological selection against migrants (Kume et al., 2010), hybrid male sterility (Kitano et al., 2007, 2009) and ecological selection against hybrids (Kitano et al., 2009).
The Japan Sea sticklebacks are invaluable genetic resources for investigating the genetic basis of hybrid male sterility (Kitano et al., 2009). As widely observed in Drosophila (Coyne and Orr, 2004; Presgraves, 2008), X chromosomes play substantial roles in hybrid male sterility between G. aculeatus and G. nipponicus (Kitano et al., 2009). In addition, G. nipponicus has several other characteristics that differentiate it from G. aculeatus. First, G. nipponicus has a neo-sex chromosome system, which was created by Y-autosome fusion (Kitano et al., 2009; Yoshida et al., 2014). Second, the courtship dance performed by G. nipponicus males is different from the zigzag dance that is typical of G. aculeatus males (Ishikawa and Mori, 2000; Kitano et al., 2007, 2008) (Fig. 2). Third, no freshwater populations derived from G. nipponicus have been reported thus far (Higuchi and Goto, 1996; Cassidy et al., 2013; Ravinet et al., 2014), whereas there are many G. aculeatus-derived freshwater populations across the world (Wootton, 1976, 1984; Bell and Foster, 1994). This makes G. nipponicus an excellent model for investigation of the genetic basis for constraints of freshwater colonization (Ravinet et al., 2014; Ishikawa et al., 2016).
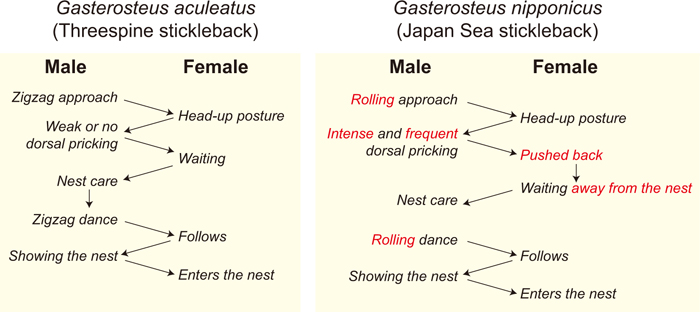
Simplified schema of behavioral chain reactions between males and females during courtship.
In the southern part of Japan, freshwater habitats of G. aculeatus are restricted to spring-fed ponds and streams (Mori, 1997). Although the upper lethal temperatures vary among stickleback populations and can be influenced by the environment’s salinity, sticklebacks are generally cold-water fishes, and optimal temperatures for stickleback growth and reproduction are usually below 20 ℃ (Ikeda, 1933; Wootton, 1984). Because the southern parts of Honshu become hot during summer, the majority of ponds and streams are not suitable for sticklebacks (Mori, 1997); however, some of those fed by cold underground spring water are inhabitable (Mori, 1985, 1997).
For example, in tributaries of Tsuya River in Gifu prefecture (Fig. 3), which are at the southern limit of the natural distribution of freshwater G. aculeatus, the water temperature is constantly near 15 ℃ (Mori, 1985). Freshwater populations in this area are called the Hariyo threespine stickleback (Fig. 3). The constant environment of this area is likely responsible for enabling the threespine sticklebacks to breed almost throughout the year (Mori, 1985); this year-round reproduction is unique to Japanese freshwater populations in spring-fed habitats in Honshu and has never been reported in other threespine stickleback populations (Baker, 1994). As a result, the populations comprise an interesting model system with which to explore the genetic and physiological mechanisms underlying variation in the timing and duration of reproduction.

Tsuya River (upper panel) and a freshwater threespine stickleback, which is called the Hariyo threespine stickleback, in a spring-fed pond in a tributary of Tsuya River (lower panel).
Furthermore, the Hariyo stickleback is a good model system for investigating the genetic basis of convergent evolution. The divergence time between the Hariyo populations and their marine ancestors was estimated to be 0.37–0.43 million years ago based on the analysis of partial sequences of the mitochondrial cytochrome b gene (Watanabe et al., 2003), which is older than that of other freshwater G. aculeatus populations in the world (Hagland et al., 1992; Bell and Foster, 1994; McKinnon and Rundle, 2002). Although similar phenotypic changes are usually observed, such as reduced numbers of armor plates, reduced thyroid hormone levels, shortened spine lengths and increased body depth, the genetic bases for the parallel phenotypic changes often differ between Hariyo and other freshwater stickleback populations (Schluter et al., 2004; Colosimo et al., 2005; Kitano et al., 2010; Jones et al., 2012a; O’Brown et al., 2015). For example, although a reduction of armor plate number is caused by a mutation at the same nucleotide position in a regulatory region of Eda (Schluter et al., 2004; O’Brown et al., 2015), mutations might occur independently in the Hariyo and other freshwater populations, because Hariyo does not have the typical freshwater Eda haplotype that is widely observed in other freshwater populations (Colosimo et al., 2005).
Japanese ninespine sticklebacks are also excellent model systems for speciation research. In Japan, there are currently four species of ninespine sticklebacks (Fig. 4): Pungitius tymensis, and the freshwater, the brackish water and the Omono types of the P. pungitius-P. sinensis complex (Takata, 1987). Another species, P. kaibarae, which is endemic to East Asia, used to inhabit Kyoto and Hyogo prefectures, but is now extinct (Mori, 1997). Pungitius pungitius and P. sinensis were previously distinguished according to lateral plate morphology: fish with continuous rows of lateral plates were classified as P. sinensis and fish with middle gaps in the plate rows were classified as P. pungitius. However, genetic data indicate that the P. pungitius-P. sinensis complex may be more appropriately separated into freshwater, brackish water and Omono types (Takata, 1987; Takahashi and Goto, 2003). Among these, the brackish water type seems to be most genetically similar to North American and European populations of P. pungitius (Takahashi and Goto, 2003).
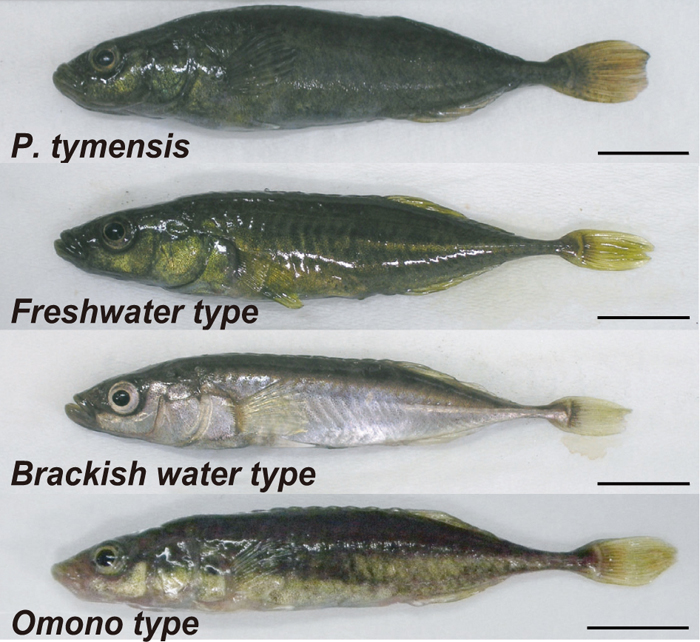
Four ninespine stickleback species (genus Pungitius): P. tymensis and freshwater, brackish water and Omono types of the P. pungitius-P. sinensis complex. Scale bars, 10 mm.
Genetic distinction of these four species is supported by the presence of reproductive isolation between sympatric species. In eastern Hokkaido, for example, the brackish water type, the freshwater type and P. tymensis often inhabit the downstream, midstream and upstream of a single river, respectively, but their distributions often overlap. In those areas, the frequencies of hybrids are low, and reproductive isolation is achieved through a combination of eco-geographical isolation, ecological selection against migrants and hybrid male sterility (Takahashi et al., 2005; Tsuruta et al., 2008; Ishikawa et al., 2013). In several areas along Omono River in Akita prefecture, the Omono type and the freshwater type are sympatric, but strong assortative mating maintains a low frequency of hybridization (Tsuruta et al., 2002).
Importantly, hybrids between the brackish water type and the freshwater type show hybrid male sterility (Takahashi et al., 2005). Hybrids between P. tymensis and the freshwater type or the brackish water type also show hybrid sterility (Kobayashi, 1959) (J. Kitano, personal observations). Thus, these systems offer unique opportunities to test the generality of large-X effects on hybrid abnormality in sticklebacks.
In Honshu, similar to threespine sticklebacks, ninespine sticklebacks inhabit only spring-fed streams and ponds and tend to have longer breeding seasons than those in Hokkaido (Sugiyama and Mori, 2009). One isolated population of the freshwater type can be found in a tributary of Arakawa River in Kanto region and is called the Musashi ninespine stickleback (Kanazawa, 2009). This population lacks lateral plates and has only caudal scutes (Igarashi, 1968). The genetic basis for phenotypic changes observed in the Musashi sticklebacks has not yet been investigated.
Unfortunately, many stickleback populations are now endangered. For example, capture rates of Japan Sea sticklebacks have been decreasing in rivers and estuarine lakes, including Shinano River, Tedori River and Jintsu River (Goto, 2003). This is mainly due to several weirs that were constructed near the mouths of rivers and that block the access of adult sticklebacks to spawning grounds (Goto and Mori, 2003). The weirs may also block descending migration of Japan Sea juveniles, and because Japan Sea sticklebacks have lower abilities to survive in freshwater environments (A. Ishikawa and J. Kitano, unpublished observations), trapping in freshwater environments may be lethal for them. Urbanization and landfill of spawning habitats also occur in many places.
In Honshu, the abundance of freshwater habitats for both threespine and ninespine sticklebacks is also decreasing (Mori, 1997). One notable example is P. kaibarae, which is now extinct in Japan. Two of the main causal factors are the depletion of spring water and the loss of habitats due to landfill. Because urban development often results in disturbances, such as bank protection and overuse of groundwater, the number of inhabitable springs with constantly cold water temperatures is decreasing, and, as a result, the Hariyo threespine stickleback, the Omono ninespine stickleback and the Musashi ninespine stickleback have all become endangered (Mori, 1997; Kanazawa, 2009; Sugiyama and Mori, 2009). It should be noted that spring water may also be important for the recovery of stickleback habitats that are threatened by natural disasters. For example, after a tsunami struck the stickleback habitats of Otsuchi City in Tohoku region on March 11, 2011 (Fig. 5), spring water was observed to supply clean water, which helped to flush out oil and debris that were brought in by the tsunami (Mori, 2013).

A stickleback habitat in Otsuchi City struck by a tsunami in 2011.
Another risk factor for stickleback populations is the straightening of rivers, which generally reduces ecological diversity (Nakamura et al., 2014). As reviewed above, different species exploit different ecological niches, so it is essential to preserve ecologically diverse environments in both coastal lakes and rivers in order for multiple stickleback species to co-exist sympatrically (Kume et al., 2010; Ishikawa et al., 2013).
Recent advances in genome sequencing technologies enable us to investigate the genetic and genomic basis for adaptive evolution and speciation at the molecular level (Nadeau and Jiggins, 2010; Seehausen et al., 2014). Furthermore, genome editing technologies enable us to conduct genetic engineering not only in model organisms but also in natural populations to test phenotypic effects of genetic changes (Jinek et al., 2012). Japanese sticklebacks provide us with excellent models to investigate the genetic mechanisms that underlie phenotypic diversification and speciation.
It should be noted that we could not review all of the Japanese stickleback populations in this paper. For example, some populations of Japanese G. aculeatus, such as the Hyotan Pond and Lake Harutori populations, show partial migration (Mori, 1990; Kitamura et al., 2006): some fish within a population migrate, while others do not. These populations are a valuable resource for investigation of the genetic and physiological basis of variation in migratory behavior (Mori, 1990; Kitamura et al., 2006; Kitano et al., 2012). Although the reasons are unknown, Japanese marine forms of G. aculeatus have also tended to decrease in number.
It is urgent to conserve these invaluable populations and species. For conserving the Japan Sea sticklebacks, it will be necessary to design weirs and dams that enable them to easily migrate between the sea and spawning grounds. Ecological restoration of spawning habitats is also essential. The best way to conserve freshwater populations in spring-fed habitats is to maintain spring waters. Electric pumping of ground water has successfully helped to sustain several Japanese freshwater populations (Mori, 1997). At the same time, it is necessary to explain the importance of sticklebacks to local people: for example, a center for conservation and observation of a freshwater population of threespine stickleback was built in the city of Ono in Fukui prefecture (Fig. 6), where local people, including elementary and high school students, can visit. To conserve these invaluable resources, it will be necessary to conduct further investigation into population risk factors and to coordinate conservation plans with local communities and policymakers.

Hongan Shozu, a spring-fed habitat of threespine stickleback in the city of Ono, and the Stickleback Conservation Center.
This research was supported by Grants-in-Aid for Scientific Research on Innovative Areas from the Ministry of Education, Culture, Sports, Science and Technology to J.K. (23113007 and 23113001), the Environment Research and Technology Development Fund from the Ministry of the Environment (4ZD-1203) to S.M. and J.K., and the NIG Cooperative Research Program (2011- A69 and 2012-A63) to S.M. We thank all members of the Kitano Lab and the Mori Lab for discussion, and two anonymous reviewers for helpful comments on the manuscript.