2018 年 93 巻 5 号 p. 191-198
2018 年 93 巻 5 号 p. 191-198
DNA methylation is an important mediator of gene expression regulation and has been shown to be closely linked to aging. Immune-related genes tend to be influenced by DNA methylation at different ages. To explore DNA methylation changes in the porcine TNFα gene and analyze their potential effects on gene expression, we measured the methylation level of the TNFα promoter and TNFα mRNA expression in the spleen of Meishan piglets at six developmental stages (1, 7, 14, 21, 28 and 35 days old) by bisulfite sequencing PCR and quantitative PCR. The results revealed a trend for TNFα promoter methylation level to increase and mRNA expression to decrease with age. Correlation analysis showed a significant negative association between methylation level and mRNA expression (Pearson’s r = −0.775, P = 4.87E-07). In addition, the transcription factor Sp1 was revealed to bind with the TNFα promoter and regulate TNFα expression. DNA methylation in the TNFα promoter was found to decrease the promoter’s activity, and methylation inhibition could enhance the expression level of TNFα, providing functional evidence that promoter methylation controls TNFα expression. Together, our data provide insights into age-associated changes in promoter methylation of the TNFα gene in the spleen and contribute to our understanding of regulatory mechanisms for TNFα expression in the immune system of pigs.
DNA methylation occurs primarily at cytosine residues of CpG dinucleotides in mammals and is one of the epigenetic mechanisms involved in transcriptional regulation. Emerging evidence indicates that the functions of DNA methylation vary with the distribution of methylation across genomic elements, which makes an intricate relationship between DNA methylation and transcription (Jones, 2012). Gene promoters are susceptible to regulation by cytosine methylation, and promoter methylation has been functionally linked to transcriptional repression of associated genes because it alters the functional state of regulatory elements. Promoter methylation in a number of genes was found to be associated with the status of multiple diseases and with functions of the immune system (Nakagawa et al., 2001; Schoenborn et al., 2007; Steinfelder et al., 2011), which highlights the usefulness of DNA methylation in gene promoters for disease prediction and treatment.
Age-related changes in DNA methylomes have been identified in human peripheral blood mononuclear cells (Heyn et al., 2012), monocytes and T cells (Reynolds et al., 2014), and other tissues (Day et al., 2013), demonstrating the close link between DNA methylation changes and aging. The ITGAL gene promoter has been found to show an age-dependent decline in methylation in T cells (Zhang et al., 2002). The TNFRSF9, SLAMF7 and IL1R2 genes also showed differential methylation status between centenarians and newborns (Heyn et al., 2012). The proinflammatory mediator TNFα, which is involved in a broad range of inflammatory responses and in defense against pathogenic infections (Bradley, 2008), displayed age-associated reduction of CpG methylation in its promoter region, and promoter methylation has been found to be involved in controlling transcription of the TNFα gene (Gowers et al., 2011; Zhang et al., 2013). In addition, genome-wide methylation analysis identified TNFα as a master regulator of a cancer-related network of genes that show altered methylation levels in prostate tumors (Kim et al., 2011). Promoter methylation of TNFα has potential as an inflammation marker for hypocaloric diet-induced weight loss (Campión et al., 2009). These findings indicate the involvement of promoter methylation in TNFα expression regulation, as well as the significant role of TNFα methylation in the immune system and its potential as an epigenetic marker for diseases.
The spleen is involved in both innate and adaptive immune responses through its role in blood filtering, phagocytosis, and lymphocyte circulation and activation, making it a vital organ for immune hemeostasis (Mebius and Kraal, 2005). Furthermore, spleen macrophages are the major source of spleen tumor necrosis factor during endotoxemia (Rosas-Ballina et al., 2008). The aim of this study was to investigate longitudinal changes in promoter methylation of the porcine TNFα gene and to analyze the effects of such changes on gene expression. We revealed longitudinal changes in TNFα promoter methylation and gene expression in the spleen of Meishan pigs at six developmental stages (1, 7, 14, 21, 28 and 35 days old). Association analysis indicated a negative association between promoter methylation and gene expression. Functional assays were then performed to provide further evidence for the repressive effects of promoter methylation on gene expression. Overall, our study provides insights into age-associated changes in promoter methylation of the TNFα gene in porcine spleen tissue and contributes to our understanding of regulatory mechanisms for TNFα expression in the immune system.
In this study, five litters of Meishan pigs were collected from the Meishan Pigs Conservation Breeding Company (Jiangsu, China) and used as the experimental animals. All the pigs were raised in the same conditions, allowed free access to feed and water, and weaned at the age of 28 days old. At six different developmental stages (1, 7, 14, 21, 28 and 35 days old), one animal was selected from each litter. A total of thirty animals (five animals per group) were used for subsequent experiments, and animals at the same developmental stage had similar characteristics (e.g., size and weight). Animals were humanely sacrificed via intravenous injection of sodium pentobarbital. Spleen samples were collected and stored in liquid nitrogen for subsequent nucleic acid isolation. The animal study proposal was approved by the Institutional Animal Care and Use Committee (IACUC) of the Yangzhou University Animal Experiments Ethics Committee (permit number: SYXK (Su) IACUC 2012-0029). All experimental methods were conducted in accordance with the relevant guidelines and regulations.
Nucleic acid isolationGenomic DNA and total RNA were extracted using the TIANamp Genomic DNA kit (Tiangen Biotech, Beijing, China) and TRIzol reagent (Thermo Fisher Scientific, Waltham, MA, USA), respectively, following the vendors’ protocols. The quantity and quality of DNA and RNA were measured with a NanoDrop 2000c Spectrophotometer (Thermo Fisher Scientific). The integrity was further checked by electrophoresis in 1% agarose gel.
DNA methylation measurementGenomic DNA was bisulfite-modified using the EZ DNA Methylation-Gold Kit (Zymo Research, Orange, CA, USA) and the converted DNA was PCR-amplified using ZymoTaq PreMix (Zymo Research) in accordance with the provided guidelines. Primers used for PCR amplifications were: forward 5’-GGAAATAATGGGAAATGGGAG-3’, reverse 5’-TTTCAAAAAAACTCAAATCCAACTA-3’. The PCR products were electrophoresed in 1% agarose gels and purified using a TIANquick Midi purification kit (Tiangen Biotech) following the manufacturer’s instructions. The purified products were then ligated into Trans1-T1 Phage Resistant Chemically Competent Cell (TransGen Biotech, Beijing, China). The successful construction of cloning plasmids was identified by colony PCR and more than ten positive clones for each subject were randomly selected for sequencing. The methylation level of each CpG site was quantified using the BiQ Analyzer program (Bock et al., 2005).
Quantitative PCRTotal RNA of the samples was purified and reverse transcribed into cDNA using a PrimeScript RT reagent Kit with gDNA Eraser (Takara Biotechnology (Dalian), China) according to the manufacturer’s guidelines. mRNA expression of TNFα was quantified by quantitative PCR (qPCR) on an ABI 7500 system (Applied Biosystems, Foster City, CA, USA). The assays were performed in a 20-μl mixture containing 10 μl of 2× SYBR Premix Ex Taq II, 0.4 μl of 50× ROX Reference Dye II, 1 μl of cDNA template, 1 μl of each forward and reverse primer, and 6.6 μl of deionized water. The thermal conditions were as follows: 95 ℃ for 15 s, followed by 40 cycles of 95 ℃ for 5 s, 60 ℃ for 30 s. The primers for TNFα were: forward 5’-CGACTCAGTGCCGAGATCAA-3’, reverse 5’-CCTGCCCAGATTCAGCAAAG-3’; those for Sp1 were: forward 5’- GCCATACCCATCAACCCTG-3’, reverse 5’-CCCCAATTCTTCCCACCAC-3’. The GAPDH and β-actin genes were used as internal references. The primers for GAPDH were: forward 5’-ACATCATCCCTGCTTCTACTGG-3’, reverse 5’-CTCGGACGCCTGCTTCAC-3’; those for β-actin were: forward 5’-TGGCGCCCAGCACGATGAAG-3’, reverse 5’-GATGGAGGGGCCGGACTCG-3’ (Dong et al., 2016). Each sample was analyzed in triplicate, and relative gene expression was calculated using the 2-ΔΔCt method (Livak and Schmittgen, 2001).
Transcription factor binding site predictionMEME software (Bailey, 2002) was used to identify motifs in the promoter sequence analyzed in this study. The motifs were then compared against the JASPAR database (Mathelier et al., 2016) using Tomtom software (Tanaka et al., 2011) to screen potential transcription factor binding sites (TFBSs) present in the sequences.
Chromatin immunoprecipitation (ChIP) assayChIP assay was conducted using the Pierce Agarose ChIP kit (Thermo Fisher Scientific) following the manufacturer’s guidelines. An amount of 80 mg sheared spleen sample or about 2 × 106 cells was placed in cold phosphate-buffered saline and then fixed with formaldehyde. Immunoprecipitation was performed with anti-Sp1 antibody or anti-ELK4 antibody (Abcam, Shanghai, China) or rabbit IgG antibody. The primers used for ChIP-PCR to measure the enriched DNA were: forward 5’-GGGCAGGGAGGGTAGGAA-3’, reverse 5’-GTGAGAAGAGGGCGGGGA-3’.
Knockdown of transcription factor Sp1Sp1 was knocked down by RNA interference using siRNA. The siRNA sequence was designed using RNAi Designer online software (http://rnaidesigner.thermofisher.com/rnaiexpress/). A scrambled siRNA that targets no known genes was used as a negative control. The siRNA sequences were: Sp1 siRNA: UCUUCUGCCUACUGCACUUTT; control siRNA: UUCUCCGAACGUGUCACGUTT. PK15 cells were transfected for 48 h with the siRNAs using Lipofectamine 2000 (Thermo Fisher Scientific) according to the manufacturer’s protocols. The cells were then collected for quantification of Sp1 and TNFα expression by qPCR assays.
Promoter activity assayThe promoter sequence analyzed in this study was synthesized and inserted into the pGL3-Basic luciferase reporter vector to generate porcine TNFα luciferase constructs. For mutant plasmid construction, the putative Sp1 binding site 5’-TCCCCGCCCTC-3’ was changed to 5’-TCCAGGTCCTC-3’. PK15 cells were cotransfected with pRL-TK vector and either the wild type construct, the mutant construct or the null vector (control), using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA), in 24-well plates following the manufacturer’s protocols. At 24 h of transfection, promoter activity was measured using the dual-luciferase reporter assay system (Promega, Madison, WI, USA) according to the manufacturer’s guidelines.
Methylated promoter plasmid construction and 5-azacytidine treatmentA 417-bp fragment in the promoter region of the TNFα gene was PCR-amplified with primers (forward 5’-GGACTAGTAGGGAGGGTAGGAAGTATC-3’, reverse 5’-CATGCCATGGTGTCTTTTTCAGAGGGGCT-3’) that included SpeI and NcoI restriction sites. The PCR products were purified and inserted into a CpG-free firefly luciferase reporter vector (pCpGL-basic), kindly provided by Dr. M. Klug and Dr. M. Rehli (Klug and Rehli, 2006). The constructed plasmid was then used to transfect BW23473 competent cells for plasmid amplification. Correct insertion of the construct was verified by Sanger sequencing. SssI methyltransferase (NEB, Beijing, China) was used to methylate all cytosine residues at CpG sites following the manufacturer’s protocol. The methylated constructs were purified by TIANquick Midi purification kit (Tiangen Biotech) and quantified using the NanoDrop ND-2000c Spectrophotometer (Thermo Fisher Scientific). PK15 cells were cotransfected with 100 ng pCpGL-basic vector, either with or without the insert, together with 2 ng of pRL-TK vector as an internal control reporter using Lipofectamine 2000 (Invitrogen) in 96-well plates according to the manufacturer’s protocol. Luminescence was quantified at 24 h of transfection using the dual-luciferase reporter assay system (Promega), following the manufacturer’s guidelines. For inhibition of DNA methylation, the PK15 cells were treated with or without 1 μM 5-azacytidine (5-Aza) for 48 h. The expression level of TNFα was measured using the qPCR assay described above.
Statistical analysisData are presented as mean ± standard deviation. Differences between two groups were compared using Student’s t-test. Pearson correlation analysis was conducted to test the associations between promoter methylation and gene expression. Differences were defined as significant at the level of P < 0.05. All statistical analyses were performed using the R (version 3.3.0) program.
The methylation levels of the TNFα promoter region (chr7: 23699294-23699808, Sscrofa11.1) in porcine spleen tissue of five animals at each of six developmental stages were analyzed using bisulfite sequencing PCR. The methylation status of individual CpG sites is shown in Supplementary Fig. S1. The findings indicated a trend for TNFα promoter methylation level to increase with age (Fig. 1). Analyses of differences among the six developmental stages revealed that the methylation level in 35-day-old animals was significantly higher than that in 1- (95% confidence interval (CI): 0.17 – 0.26, P = 4.43E-06), 7- (95% CI: 0.14 – 0.25, P = 4.49E-05), 14- (95% CI: 0.12 – 0.23, P = 1.52E-04) and 21-day-old (95% CI: 0.02 – 0.12, P = 6.86E-03) animals. The methylation level in 28-day-old animals was also significantly higher than that in 1- (95% CI: 0.16 – 0.24, P = 5.48E-06), 7- (95% CI: 0.14 – 0.24, P = 9.77E-05), 14- (95% CI: 0.11 – 0.22, P = 3.10E-04) and 21-day-old (95% CI: 0.02 – 0.11, P = 6.86E-03) animals. Furthermore, the methylation level in 21-day-old animals was significantly higher than that in the 1- (95% CI: 0.09 – 0.18, P = 1.52E-04), 7- (95% CI: 0.06 – 0.17, P = 1.09E-03), and 14-day-old (95% CI: 0.04 – 0.16, P = 4.29E-03) animals.

Methylation level of the TNFα gene promoter in the spleen of pigs at different age (days). For each stage, five individuals were used for methylation analysis. Boxes represent the interquartile range between the first and third quartiles, and the bold line indicates the median. Whiskers indicate the minimum and maximum within 1.5 times the interquartile range from the first and third quartiles. **P < 0.01.
qPCR assays were performed to quantify the expression level of TNFα in the spleen tissue. As shown in Fig. 2, the expression level of TNFα tended to decrease with the age of animals. In 1-day-old animals, TNFα expression was significantly higher than that in 14- (95% CI: 0.27 – 0.43, P = 9.36E-06), 21- (95% CI: 0.28 – 0.47, P = 1.69E-05), 28- (95% CI: 0.34 – 0.49, P = 8.33E-06) and 35-day-old (95% CI: 0.32 – 0.52, P = 1.16E-05) individuals. In addition, the expression level of TNFα in 7-day-old animals was significantly higher than that in 14- (95% CI: 0.17 – 0.35, P = 2.80E-04), 21- (95% CI: 0.19 – 0.39, P = 1.76E-04), 28- (95% CI: 0.25 – 0.42, P = 1.49E-04) and 35-day-old (95% CI: 0.23 – 0.44, P = 8.52E-05) animals. TNFα expression in 14-day-old animals was significantly higher than that in 28-day-old animals (95% CI: 0.02 – 0.13, P = 0.02). These results revealed an inverse trend between TNFα expression level and promoter methylation status.

Expression level of the TNFα gene in the spleen of pigs at different age (days). For each stage, five individuals were used for gene expression analysis. Boxes represent the interquartile range between the first and third quartiles, and the bold line indicates the median. Whiskers indicate the minimum and maximum within 1.5 times the interquartile range from the first and third quartiles. *P < 0.05, **P < 0.01.
Pearson correlation analysis was next performed to test the association between TNFα promoter methylation and gene expression. As shown in Fig. 3, the methylation level and mRNA expression displayed a significant negative association (Pearson’s r = −0.775, P = 4.87E-07). We then analyzed the correlation between the methylation level of each of the 15 CpG sites in the promoter (see Fig. 5) and TNFα expression. The results indicated that the methylation level of all the CpG sites showed an inverse association with TNFα expression, with four CpG sites (CpG-6, CpG-8, CpG-11 and CpG-15) displaying a statistically significant negative association with the expression level of TNFα (Table 1).
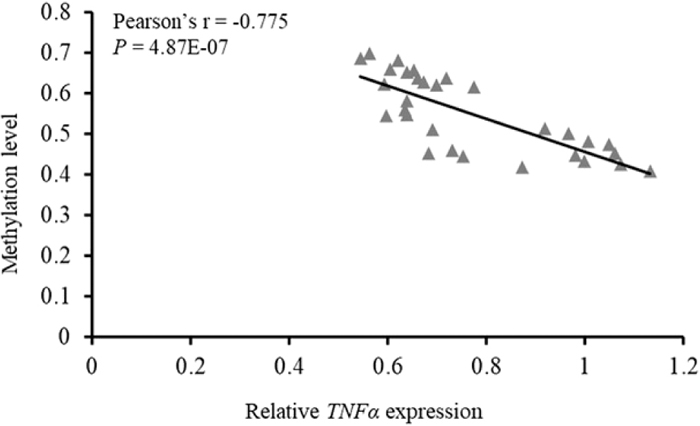
Correlation between porcine TNFα gene promoter methylation status and mRNA expression level.

Prediction of putative TFBSs in the porcine TNFα gene promoter sequence. The underlined sequences are putative TFBSs and below are the potential transcription factors. Numbers 1 – 15 mark the positions of CpG dinucleotides (bold type) CpG-1 – CpG-15.
| CpG site | Pearson’s r | P value |
|---|---|---|
| CpG-1 | −0.253 | 0.176 |
| CpG-2 | −0.308 | 0.104 |
| CpG-3 | −0.237 | 0.206 |
| CpG-4 | −0.190 | 0.314 |
| CpG-5 | −0.236 | 0.209 |
| CpG-6 | −0.398 | 0.029 |
| CpG-7 | −0.206 | 0.275 |
| CpG-8 | −0.506 | 0.005 |
| CpG-9 | −0.246 | 0.189 |
| CpG-10 | −0.253 | 0.176 |
| CpG-11 | −0.382 | 0.037 |
| CpG-12 | −0.325 | 0.079 |
| CpG-13 | −0.317 | 0.088 |
| CpG-14 | −0.267 | 0.154 |
| CpG-15 | −0.406 | 0.025 |
We functionally tested the effect of TNFα promoter methylation on gene expression using luciferase assays. The TNFα promoter sequence was cloned into a CpG-free luciferase reporter plasmid, which was constructed for investigating the impact of CpG methylation on promoter transcriptional activity in transfection experiments. PK15 cells were transfected with methylated or control constructs and luciferase activity was quantified. The results demonstrated that methylation of the TNFα promoter sequence significantly decreased luciferase reporter gene expression by 41.9% (95% CI: 0.33 – 0.48, P = 0.001), indicating a repressive effect of TNFα promoter methylation on gene expression (Fig. 4A). 5-Aza was used to analyze the effect of methylation inhibition on gene expression. As shown in Fig. 4B, DNA methylation inhibition enhanced the TNFα expression level in PK15 cells (95% CI: 0.23 – 0.42, P = 0.027). These findings provide functional evidence for the link between promoter methylation and TNFα expression.

Effects of DNA methylation (A) and methylation inhibition (B) on TNFα promoter activity and gene expression. PK15 cells were transfected with methylated or control TNFα promoter constructs which contained the promoter sequence analyzed in this study. At 24 h of incubation, luciferase activity was measured. For inhibition of methylation, PK15 cells were treated with or without 1 μM 5-Aza for 48 h. TNFα expression was quantified using qPCR assays. Bars denote the means ± standard deviation (N = 6). *P < 0.05; **P < 0.01.
A total of six transcription factors (THAP1, EGR2, ELK4, NFATC1, Sp1 and TFAP2B) whose binding sites contain CpG dinucleotides were predicted to bind in the promoter sequence of the TNFα gene (Fig. 5). Correlation analysis revealed that three CpG sites located in the TFBSs showed significantly inverse associations (CpG-6, Pearson’s r = −0.399, P = 0.029; CpG-8, Pearson’s r = −0.506, P = 0.005; CpG-15, Pearson’s r = −0.406, P = 0.026) between their methylation level and TNFα expression, indicating the potential roles of DNA methylation at these CpG sites in regulating TNFα expression by affecting the binding of these transcription factors to the promoter sequence. Further experiments were performed for Sp1 and ELK4, which are predicted to bind at CpG-6 and CpG-8 and are expressed in the spleen tissue (Li et al., 2017). Binding of only Sp1 with the promoter sequence of TNFα was found by the ChIP-PCR assay (Supplementary Fig. S2). Moreover, an approximately 1.6-fold enrichment of Sp1 was observed in TNFα promoter sequence in 5-Aza-treated PK15 cells, indicating the repression of DNA methylation of the TNFα promoter on its interaction with transcription factor Sp1 (Supplementary Fig. S3). A luciferase assay further showed that promoter sequence containing the putative Sp1 binding site could enhance the promoter activity, which was higher than that of the control plasmid and the mutant construct (Fig. 6A). To analyze the potential effects of Sp1 on TNFα expression, knockdown of Sp1 was conducted by siRNA, which achieved a knockdown efficiency of approximately 73% (Supplementary Fig. S4). Furthermore, our results showed that knockdown of Sp1 significantly reduced the expression level of TNFα (Fig. 6B). These findings indicate that Sp1 binds with the TNFα promoter and that their interaction plays a regulatory role in TNFα expression.

Effects of Sp1 on TNFα promoter activity (A) and gene expression (B). Relative luciferase activity was calculated as the ratio of firefly luciferase activity to Renilla luciferase activity. WT: wild type construct with one putative Sp1 binding site; MT: construct with mutations in the putative Sp1 binding site. Bars represent the means ± standard deviation (N = 6); *P < 0.05.
DNA methylation is an important mediator of gene expression regulation. Growing evidence shows significant alterations in DNA methylation related to aging, which is closely pertinent to changes in the innate immune system including impairment of defensive tissues and deterioration of immune cell function (Gomez et al., 2005). TNFα has crucial roles in immunomodulation and inflammatory responses (Beutler and Cerami, 1989), which underlines the importance of unveiling the mechanisms involved in its expression regulation. It has been shown that the production of tumor necrosis factor is primarily mediated at the transcriptional and post-transcriptional levels (Sariban et al., 1988). In addition, mucin 1 modulates the interaction of several factors involved in chromatin remodeling with the TNFα promoter, and thus controls its expression (Cascio et al., 2014). We herein identified an increase in methylation status of the TNFα promoter with age and a negative association between the TNFα promoter methylation and TNFα mRNA expression in porcine spleen, which is largely consistent with previous reports (Gowers et al., 2011; Zhang et al., 2013). However, Gowers et al. revealed an age-related loss of TNFα promoter methylation, indicating different methylation patterns of TNFα promoter that may be due to species-specific and tissue-specific differences. DNA methylation changes with aging may be caused by factors such as altered expression or function of DNA methyltransferases and demethylases, diet, and immune capacity at different developmental stages. Further, DNA methylation status is mediated by the methyltransferase enzymes, of which DNMT3A and DNMT3B are essential for establishing DNA methylation patterns, and DNMT1 can maintain an established pattern of DNA methylation. Changes in DNA methylation can affect expression and function of targeted genes and are intimately linked with histone acetylation, which is important in the aging process (Richardson, 2003). Our findings provided insights into the epigenetic regulation of TNFα expression at the transcriptional level and a potential link of longitudinal methylation changes to porcine spleen function.
The promoter region is a crucial genomic element in regulating gene expression. The inverse relationship between DNA methylation in a promoter and gene expression has been widely recognized. Blocking the binding of transcription factors to their promoter sequences is one of the mechanisms involved in repressing gene expression (Klose and Bird, 2006). We predicted the CpG sites located within the putative binding sites of six transcription factors (THAP1, EGR2, ELK4, NFATC1, Sp1 and TFAP2B) and demonstrated significant inverse correlations between methylation of three CpG sites (CpG-6, CpG-8 and CpG-14) and TNFα expression. The binding of Sp1 has been discovered to be methylation-sensitive, and promoter methylation of the human TNFα gene can result in reduced binding of Sp1 and thus in suppression of the promoter’s activity (Clark et al., 1997; Pieper et al., 2008). NFATC1 is involved in the transcriptional regulation of CD40L gene expression by the mechanism of epigenetic chromatin remodeling (Pham et al., 2010). These findings indicate that the methylation status of the TNFα promoter influences its interaction with these transcription factors, thus controlling TNFα expression. However, the mechanisms associated with interaction between the TNFα promoter sequence and transcription factors warrant further investigation.
In this study, we focused on six developmental stages of piglets before 35 days old, during which the animals are prone to be infected with various pathogens such as enterotoxigenic Escherichia coli K88 and F18, porcine epidemic diarrhea virus and reproductive and respiratory syndrome virus. Recent studies have identified a collection of immune-related genes that show age-associated methylation alterations, suggesting that these genes tend to be influenced by DNA methylation at different ages (Zhang et al., 2002; Heyn et al., 2012; Dong et al., 2015). As the spleen plays crucial roles in immune responses, it has been widely used to identify immune-related genes involved in pathogenic infections in pigs (Chen et al., 2009; Li et al., 2010). TNFα is one of the important proinflammatory cytokines that are triggered in host immune responses. We here revealed an inverse relationship between TNFα promoter methylation and TNFα mRNA expression in the spleen and provided functional evidence in vitro, which expanded our understanding of the regulatory mechanisms involved in TNFα expression in immune responses and indicated the potential of TNFα promoter methylation to be used as an epigenetic marker of responses to pathogenic infections. Given that DNA methylation status is likely to be affected by environmental exposure, the animals were raised in the same conditions to provide evidence for the link of methylation changes with aging.
In summary, we here identified age-associated changes in the methylation status of the TNFα promoter in porcine spleen and a negative relationship between promoter methylation and gene expression. In addition, the transcription factor Sp1 was revealed to bind with the TNFα promoter and regulate TNFα expression. We also provided functional evidence for the repressive effect of promoter DNA methylation on TNFα expression. Our findings provide insights into the trend for promoter methylation alterations of the porcine TNFα gene in pigs of different ages and contribute to our understanding of the regulatory mechanism of TNFα expression in porcine spleen.
This work was supported by the Undergraduate Science and Technology Innovation Fund of Yangzhou University (X201707011800), the National Natural Science Foundation of China (31702082, 31772560), the China Postdoctoral Science Foundation (2017M621842, 2018T110564), the Qing Lan Project of Yangzhou University and the Priority Academic Program Development (PAPD) of Jiangsu Higher Education Institutions.