2020 年 95 巻 6 号 p. 303-314
2020 年 95 巻 6 号 p. 303-314
yata mutants of Drosophila melanogaster exhibit phenotypes including progressive brain shrinkage, developmental abnormalities and shortened lifespan, whereas in mammals, null mutations of the yata ortholog Scyl1 result in motor neuron degeneration. yata mutation also causes defects in the anterograde intracellular trafficking of a subset of proteins including APPL, which is the Drosophila ortholog of mammalian APP, a causative molecule in Alzheimer’s disease. SCYL1 binds and regulates the function of coat protein complex I (COPI) in secretory vesicles. Here, we reveal a role for the Drosophila YATA protein in the proper localization of COPI. Immunohistochemical analyses performed using confocal microscopy and structured illumination microscopy showed that YATA colocalizes with COPI and GM130, a cis-Golgi marker. Analyses using transgenically expressed YATA with a modified N-terminal sequence revealed that the N-terminal portion of YATA is required for the proper subcellular localization of YATA. Analysis using transgenically expressed YATA proteins in which the C-terminal sequence was modified revealed a function for the C-terminal portion of YATA in the subcellular localization of COPI. Notably, when YATA was mislocalized, it also caused the mislocalization of COPI, indicating that YATA plays a role in directing COPI to the proper subcellular site. Moreover, when both YATA and COPI were mislocalized, the staining pattern of GM130 revealed Golgi with abnormal elongated shapes. Thus, our in vivo data indicate that YATA plays a role in the proper subcellular localization of COPI.
In eukaryotic cells, transmembrane proteins and secreted proteins are synthesized in the rough endoplasmic reticulum (ER) and then transported to their own destinations by intracellular vesicular trafficking (Bonifacino and Glick, 2004). Transport vesicles are surrounded by coat proteins, whose type varies among distinct cellular locations (Bonifacino and Lippincott-Schwartz, 2003). These coat proteins enable the efficient formation of transport carriers and incorporation of specific cargos into the vesicles. In the early steps of vesicular trafficking between the ER and the Golgi, vesicles are coated by coat protein complex I (COPI) or II (COPII) (Miller and Schekman, 2013; Arakel and Schwappach, 2018; Béthune and Wieland, 2018). COPI-coated vesicles transport proteins retrogradely from the Golgi to the ER, whereas COPII-coated vesicles transport proteins anterogradely from the ER to the Golgi. Proper regulation of vesicular trafficking between the ER and the Golgi is considered to be important in the retrieval of ER- and Golgi-resident proteins as well as appropriate quality control of synthesized proteins (Jin et al., 2017; Dell’Angelica and Bonifacino, 2019; Kokubun et al., 2019). Impairment of the early steps of vesicular trafficking has been suggested to cause some human genetic disorders.
We previously identified a Drosophila yata mutant that showed phenotypes in the compound eye, wing and brain (Sone et al., 2009). Homozygotes of the null allele of the yata gene, yataKE2.1, show various phenotypes such as morphological abnormalities in the compound eye and wing, progressive reduction of brain volume and shortened lifespan. Electron microscopic examination of the internal morphology of the compound eye revealed that the yata mutation enhanced the formation of an abnormal cellular structure that formed as a bleb-like cellular protrusion and contained membranous organelles that had the appearance of lysosomes, autophagosomes and late endosomes (Arimoto et al., 2020). The yata gene has been suggested to be ubiquitously expressed. It encodes a protein that has no transmembrane domains and has a catalytic domain of a protein kinase. The kinase domain of YATA is predicted to be catalytically inactive because some of the amino acid residues that are essential for catalytic activity are not conserved. The yata mutant was originally identified as a locus that genetically interacted with the null allele of the Appl gene, Appld. The Appl gene encodes the orthologous protein of mammalian APP, which is a causative molecule of Alzheimer’s disease (Luo et al., 1990; Poeck et al., 2012; Cassar and Kretzschmar, 2016). Double null mutants of yata and Appl showed exacerbated phenotypes in brain volume reduction and shortened lifespan. APPL proteins are synthesized in neuronal cell bodies and then transported to synaptic terminals by means of vesicular trafficking (Torroja et al., 1996, 1999). Our immunohistochemical analysis using an anti-APPL antibody revealed the aberrant accumulation of APPL in neuronal cell bodies in the pupal brain in yata mutants. This accumulation of APPL overlapped with a marker of the ER. In a Drosophila Alzheimer’s disease model in which human mutant APP was ectopically expressed in larval motor neurons, RNAi-mediated knockdown of yata resulted in partial suppression of the anterograde transport of APP to synapses and of phenotypes caused by APP such as abnormal morphology of neuromuscular synapses and abnormal electrophysiological properties of neuromuscular synapses (Furotani et al., 2018). In addition to APPL, synaptic transport of another synaptic protein, Fasciclin II, which is a synaptic homophilic cell adhesion molecule orthologous to mammalian NCAM (Lin et al., 1994; Schuster et al., 1996), is reduced in yata mutants. However, synaptic transport of Synaptotagmin I, which is a membrane-bound protein localized on synaptic vesicles (Littleton et al., 1993a, 1993b), was not affected in the yata mutants, suggesting that yata mutation affects only a subset of proteins that are transported by vesicular trafficking.
yata orthologs occur in a variety of eukaryotes including yeast, plants, nematodes and mammals. Null mutation of the murine yata ortholog, Scyl1, causes motor neuron degeneration (Schmidt et al., 2007). Because neural-specific, but not skeletal muscle-specific, deletion of Scyl1 causes motor dysfunction, Scyl1 is thought to function autonomously in the nervous system (Pelletier et al., 2012). Because deletion of Scyl1 also caused the mislocalization and accumulation of the TAR DNA-binding protein of 43 kDa (TDP-43), mice with Scyl1 deletion are thought to share features with human neurodegenerative diseases, including amyotrophic lateral sclerosis. Mutation of human Scyl1 has been identified as a cause of a genetic disease that results in liver failure, peripheral neuropathy, cerebellar atrophy and ataxia (Schmidt et al., 2015; Lenz et al., 2018; Shohet et al., 2019). A previous mass spectrometry-based screen identified βCOP, a subunit of COPI, as the binding partner of SCYL1 (Burman et al., 2008). SCYL1 directly binds with COPI and colocalizes with COPI in the ER–Golgi intermediate compartment (ERGIC) and the cis-Golgi, which are the sites where buds of COPI-coated vesicles are formed. In cultured cells, RNAi-mediated knockdown of Scyl1 resulted in the inhibition of COPI-mediated retrograde trafficking of the KDEL receptor protein from the Golgi to the ER. Further study also identified class II Arfs, which are GTPases that are involved in the formation of COPI-coated vesicles (D’Souza-Schorey and Chavrier, 2006; Popoff et al., 2011), as another binding partner of SCYL1 (Hamlin et al., 2014). SCYL1 has been suggested to function as a scaffold protein for components of the COPI coat. In cultured cells, overexpression or knockdown of Scyl1 resulted in abnormal morphology of the Golgi and ERGIC, possibly due to loss of the scaffolding function of SCYL1 for COPI (Burman et al., 2010; Hamlin et al., 2014). In patients with a genetic disease caused by a mutation in Scyl1, enlargement of the Golgi has been observed in fibroblasts (Schmidt et al., 2015).
In this study, we used immunostaining with anti-YATA and other marker antibodies to examine the subcellular localization of YATA in the Drosophila pupal brain. Our observations obtained using confocal microscopy and structured illumination microscopy (SIM) revealed colocalization among YATA, COPI and a cis-Golgi marker. Our further analysis using transgenically expressed YATA indicate that it has an in vivo role in the proper subcellular localization of COPI.
Flies were raised on yeast–cornmeal medium (7.5% cornmeal, 3.8% yeast, 9.4% glucose, 3.0% wheat germ, 0.24% n-butyl p-hydroxybenzoate, 0.09% calcium chloride, 1.1% potassium tartrate hemihydrate and 0.9% agar). All fly stocks were maintained at 25 ℃ under a 12:12 hour light–dark cycle. We used Canton-S as the wild type. The UAS-YFP-KDEL fly strain (Zhu et al., 2003) was a gift from Dr. Matthew P. Scott. The UAS-Arf4-GFP (stock number 65866) and OK107-Gal4 (stock number 854) (Lee et al., 1999) strains were obtained from the Bloomington Drosophila Stock Center. The UAS-YATAwt (F001645), UAS-YATAwt-HA (F001011) and UAS-Sar1-HA (F001192) strains were obtained from the FlyORF, Zurich ORFeome project (Bischof et al., 2013). For the UAS-HA-YATAwt and UAS-HA-YATAAAAL strains, plasmids were constructed using PCR-based methods. We used a previously described nucleotide sequence of the 3×HA epitope tag (amino acids: YPYDVPDYA) (Kumar et al., 2000). We performed PCR amplification using forward primers containing an XhoI site, the 5-bp Kozak sequence of the yata gene, an ATG codon, the sequence encoding the 3×HA tag and the first 19 bp of the open reading frame of the yata gene and a reverse primer corresponding to part of the yata gene in which the original XhoI site was disrupted. The sequences of the forward primers were 5′-TATGACGTCCCGGACTATGCAGGATCCTATCCATATGACGTTCCAGATTACGCTATGTGGTCGTTCTTCTCGC-3′ (HA-Yata-Fw1) and 5′-CGCTCGAGACATAATGTACCCATACGATGTTCCTGACTATGCGGGCTATCCCTATGACGTCCCGGACTATGC-3′ (XhoI-HA-Fw) and the sequence of the reverse primer was 5′-CGCACGGATCCTCCTCCAGACTGCCCCACTCC-3′ (yata-Rv-delXhoI). We performed another PCR amplification using a forward primer corresponding to the part of the yata gene with the disrupted original XhoI site and either of two reverse primers. The reverse primer used to construct HA-YATAwt contained a sequence corresponding to the last 15 bp of the open reading frame of the yata gene, a TAA stop codon and an XbaI site. The reverse primer used to construct HA-YATAAAAL contained a sequence corresponding to the last 26 bp of the open reading frame of the yata gene, in which the encoded amino acid sequence was mutated from KK to AA, a TAA stop codon and an XbaI site. The sequence of the forward primer was 5′-GGAGTGGGGCAGTCTGGAGGAGGATCCGTGCG-3′ (yata-Fw-delXhoI) and the sequences of the reverse primers were 5′-GTCTAGATTAAAGCTTTTTGGCGCC-3′ (yata-stop-XbaI-Rv) and 5′-GTCTAGATTAAAGCGCTGCGGCGCCCAGCTTCATGG-3′ (yata-KK>AA-XbaI-Rv). The amplified DNA fragments were combined and subcloned into the vector pJFRC7 (Pfeiffer et al., 2010). After the sequence of the entire DNA fragment was checked, transgenic flies were created by inserting the sequence into the attP40 site at position 25C on the second chromosome (Markstein et al., 2008).
Anti-YATA antibodyAnti-YATA antiserum was raised against a fusion protein that consisted of a portion of the YATA protein (amino acids 504 to 803) and glutathione S-transferase derived from the pGEX4T-1 vector (GE Healthcare). After the fusion protein was expressed in the Escherichia coli strain BL21 and purified, it was injected into rats for immunization.
MicroscopyFor confocal microscopy, pupal brain dissection and immunostaining were performed essentially as previously described (Nakayama et al., 2014). Female pupae at 72 h after puparium formation were collected and rinsed in 70% ethanol for 30 s. After they were washed in phosphate-buffered saline (PBS), the brains were dissected in PBS. The brains were fixed in 4% paraformaldehyde in PBS for 1 h on ice and washed in PBS with 0.4% Triton X-100. The samples were incubated with the antibodies in PBS with 0.4% Triton X-100 overnight at 4 ℃. The antibodies used were rat anti-YATA (diluted 1:100), rabbit anti-GFP (Invitrogen, A-6455, diluted 1:500), rabbit anti-GM130 (diluted 1:200) (Yano et al., 2005), guinea pig anti-αCOP (a subunit of COPI; referred to hereafter as anti-COPI) (diluted 1:400) (Kitazawa et al., 2012), rabbit anti-Syntaxin 16 (Abcam, ab32340, diluted 1:100) (Simonsen et al., 1998; Charng et al., 2014) and mouse anti-HA (BioLegend, 16B12, MMS-101R, diluted 1:1,000). The anti-COPI antibody was a gift from Dr. Yoshihiro Inoue. The samples were then washed in PBS with 0.4% Triton X-100 and incubated with secondary antibodies in PBS with 0.4% Triton X-100 overnight at 4 ℃. The secondary antibodies used were FITC-conjugated anti-rabbit IgG antibody (Jackson ImmunoResearch, 711-095-152, diluted 1:200), Cy3-conjugated anti-guinea pig IgG antibody (Jackson, 706-165-148, diluted 1:200), Cy5-conjugated anti-rat IgG antibody (Jackson, 712-175-150, diluted 1:200) and Cy5-conjugated anti-mouse IgG antibody (Jackson, 715-175-150, diluted 1:200) The samples were then washed in PBS with 0.4% Triton X-100 and mounted in Vectashield with DAPI (Vector Laboratories, H-1200). A coverslip was mounted on other coverslips used as pillows. Images were collected with a confocal microscope (Olympus, FV-1000) equipped with a UPlanSApo 60×/1.35 oil-immersion objective. All signals in triple-stained samples were scanned separately. Images were processed with Photoshop (Adobe). For SIM, wild-type pupae were collected at 72 h after puparium formation and the brains were dissected. Whole-mount brains were fixed and stained with antibodies. The antibodies used were rabbit anti-GM130 (diluted 1:200), guinea pig anti-COPI (diluted 1:400) and rat anti-YATA (diluted 1:100). The secondary antibodies used were Alexa 488-conjugated anti-rat IgG antibody (Invitrogen, A-21208, diluted 1:200), Cy3-conjugated anti-guinea pig IgG antibody (Jackson, 706-165-148, diluted 1:200) and Cy5-conjugated anti-rabbit IgG antibody (Jackson, 711-175-152, diluted 1:200). Samples were observed with a SIM microscope (Zeiss, ELYRA S.1) equipped with a Plan-Apochromat 63×/1.4 oil-immersion objective. Photos were processed using Photoshop (Adobe).
To assess the molecular function of the YATA protein, we examined its subcellular localization using an anti-YATA antibody. We stained whole-mount wild-type pupal brains with this antibody and examined the localization of YATA by confocal microscopy. We examined pupal brains 72 h after puparium formation because this is the stage at which impaired anterograde trafficking of the APPL protein and aberrant accumulation of COPII were previously observed in yata mutants (Sone et al., 2009). Localization of YATA was detected as punctate signals throughout the cortical regions of the brain (Fig. 1A). These signals were absent in the yataKE2.1 homozygous null mutant, confirming that the antibody specifically labeled the YATA protein. Close examination revealed that punctate signals of YATA were localized in the cytoplasmic regions that surrounded DAPI-stained nuclei (Fig. 1B). These data suggested that YATA is localized in restricted subcellular sites in the cytoplasm of neuronal cell bodies.
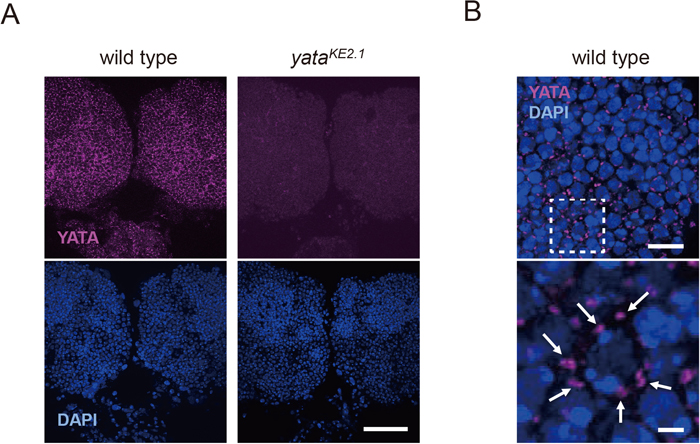
Subcellular localization of YATA revealed by immunostaining with an anti-YATA antibody. (A) A pupal brain at 72 h after puparium formation stained with an anti-YATA antibody (magenta). Nuclei were stained with DAPI (blue). The YATA signal was absent in homozygotes of the yata null allele, yataKE2.1. Scale bar: 50 μm. (B) Magnified images of a pupal brain stained with an anti-YATA antibody (magenta) and DAPI (blue). A higher-magnification image of the boxed area in the upper panel is shown in the lower panel. YATA signals were observed in the cytoplasm surrounding the nuclei (arrows). Scale bars: 10 μm (upper panel) and 2 μm (lower panel).
Next, we compared the localization of YATA with that of marker antibodies for organelles that are involved in vesicular trafficking. We induced the expression of YFP-KDEL (Zhu et al., 2003), a marker of the ER, using OK107-Gal4, a specific driver for mushroom bodies (Lee et al., 1999), with the Gal4-UAS system (Brand and Perrimon, 1993) and examined its localization by staining with anti-GFP antibody to observe mushroom bodies. YFP-KDEL labeled the regions that surrounded the round-shaped nuclei (Fig. 2, arrows) and some regions near the nuclei (Fig. 2, arrowheads), both of which appeared to be the ER. Localization of YATA was shown by costaining to partially overlap with the YFP-KDEL signal, but the patterns and shapes of the signals were different. We costained YATA with GM130, a marker of the cis-Golgi (Kondylis et al., 2001; Yano et al., 2005), and observed colocalization of YATA and GM130 (Fig. 2, arrows). We also costained YATA with a subunit of COPI, the protein complex that coats secretory vesicles traveling retrogradely from the Golgi to the ER. Colocalization of YATA and COPI was observed (Fig. 2, arrows). We further costained YATA with Syntaxin 16 (Syx16), a marker of the trans-Golgi (Simonsen et al., 1998; Charng et al., 2014). The patterns and shapes of the signals of YATA and Syx16 were similar, but not completely overlapping; instead, they seemed to be adjacently localized (Fig. 2, arrowheads). We induced the expression of GFP-Arf4 (Arf102F), a GTPase involved in the assembly of COPI-coated vesicles, and evaluated colocalization by staining tissues with anti-GFP and anti-YATA. Arf4-GFP showed mainly punctate signals that overlapped with YATA signals. Finally, we induced the expression of Sar1 conjugated with an HA epitope tag. Sar1 is a GTPase involved in the assembly of COPII-coated vesicles that travel anterogradely from the ER to the Golgi. We examined the localization of Sar1-HA by immunostaining with an anti-HA antibody. Sar1-HA was localized as relatively strong punctate signals that overlapped with YATA signals. In addition, Sar1-HA also localized relatively weakly in regions surrounding the nucleus that seemed to be the ER. Collectively, these data suggest that YATA is highly colocalized with COPI and with a marker of the cis-Golgi.

Costaining for YATA and organelle markers. Pupal brains at 72 h after puparium formation were stained with antibodies for YATA (magenta) and six organelle markers (green). Merged (top panels), magenta and green images are shown. The organelle markers used show the expression of YFP-KDEL stained by anti-GFP antibody for the ER, anti-GM130 antibody for the cis-Golgi, anti-COPI antibody for retrogradely transported vesicles from the Golgi to the ER, anti-Syntaxin 16 (Syx16) antibody for the trans-Golgi, the expression of Arf4-GFP as stained by anti-GFP antibody for retrogradely transported vesicles from the Golgi to the ER, and the expression of Sar1-HA as stained with anti-HA antibody for anterogradely transported vesicles from the ER to the Golgi. YFP-KDEL labeled the regions that surrounded the nuclei (arrows) and some regions near the nuclei (arrowheads), both of which appeared to be the ER. Colocalization of punctate signals was observed for GM130 and YATA (arrows), and also for COPI and YATA (arrows). Adjacently located signals were observed for Syx16 and YATA (arrowheads). Colocalization of punctate signals was observed for Arf4-GFP and YATA (arrows). The strong punctate signal for Sar1-HA also colocalized with YATA (arrows). Scale bar: 2 μm.
To further assess the subcellular localization of YATA and its relevance to the localization of COPI and a cis-Golgi marker, we utilized SIM. We triple-stained pupal brains with antibodies against the cis-Golgi marker GM130 as well as COPI and YATA. In SIM images, the GM130, COPI and YATA signals showed distinct patterns (Fig. 3A, 3B). COPI and YATA signals were observed to overlap with GM130 signals and to partially occupy regions containing GM130 signals (Fig. 3B, arrowheads). The COPI and YATA signals showed distinct but partially overlapping patterns. These data suggest that COPI and YATA are localized at specific sites within the cis-Golgi, and also that COPI and YATA partially colocalize.

Colocalization of GM130, COPI and YATA revealed by SIM observation. (A) Pupal brains at 72 h after puparium formation were triple-stained with anti-GM130 antibody (blue), anti-COPI antibody (green) and anti-YATA antibody (magenta) and observed by SIM. Higher-magnification images of the boxed region are shown in (B). Scale bar: 2 μm. (B) Higher-magnification images captured by SIM. The GM130, COPI and YATA signals showed distinct patterns. The COPI and YATA signals occupied part of the region containing the GM130 signal. The COPI, GM130 and YATA signals partially overlapped (arrowheads). Scale bar: 0.5 μm.
Next, we examined whether overexpression of YATA affects the localization of COPI. To reliably evaluate the effect of ectopic expression of YATA, we induced the expression of YATA under the control of the OK107 Gal4 driver, which is specific for the mushroom body (Lee et al., 1999). We induced the expression of YATAwt, which comprises a full-length open reading frame encoding YATA with no epitope tag (Fig. 4). We triple-stained pupal brains with anti-COPI, anti-GM130 and anti-YATA and observed the marginal region of the mushroom body and adjacent brain areas. We compared the signals between the inside of the mushroom body, where YATA was ectopically expressed, and the outside of the mushroom body, where YATA was not ectopically expressed, within a single image. In Fig. 5A, the region that lies to the left of the dotted line and contains square 1 is the region outside the mushroom body where YATAwt was not ectopically expressed. In this region, endogenous YATA was expressed but no anti-YATA signal was detected because the image was acquired with a high gain value to preferentially visualize the strong anti-YATA signals of the regions where YATAwt was ectopically overexpressed. The region that lies to the right of the dotted line and contains square 2 is the region inside the mushroom body where YATAwt was ectopically overexpressed. In this region, strong anti-YATA signals were observed. Their patterns contained punctuate signals, similar to the signals observed for endogenous YATA, as well as relatively weak signals in other cytoplasmic perinuclear regions. Next, we compared the localization patterns of COPI and GM130 between the regions inside and outside the mushroom body. The anti-GM130 antibody signal was punctate and indistinguishable between the inside and outside of the mushroom body. In the region outside the mushroom body, where YATAwt was not overexpressed, the anti-COPI antibody signals were observed as a punctate pattern that seemed to overlap with the anti-GM130 signals. In the region inside the mushroom body, where YATAwt was overexpressed, anti-COPI signals were also observed as a punctate pattern, but their intensity was clearly increased. The differences in the intensities of the COPI signals were clearly observed by comparing the areas outside and inside the mushroom body within single images. These changes in the COPI staining intensity were reproducibly observed (n = 4). These data suggest that overexpression of YATA increases the localization of COPI to the cis-Golgi.

Summary of the analysis of transgenic flies ectopically expressing YATA and its modified derivatives. YATA is an 873-amino acid (a.a.) protein that contains a kinase-like domain, three HEAT repeats and a coiled-coil domain. YATAwt-HA has a 3×HA tag conjugated to the C-terminus. HA-YATAwt and HA-YATAAAAL have a 3×HA tag conjugated to the N-terminus. In HA-YATAAAAL, the C-terminal sequence was mutated from AKKL to AAAL. The results obtained regarding colocalization with GM130 and effects on COPI localization are summarized.

Effect of ectopic expression of YATA and its modified derivatives on the localization of COPI and GM130. (A) Pupal brain at 72 h after puparium formation stained with COPI (red), GM130 (green) and YATA (white). YATAwt was expressed in the mushroom bodies by OK107 Gal4. The marginal region between the mushroom body and the adjacent brain region was observed. The region to the right of the dashed line is inside the mushroom body, where YATAwt was expressed, while the region to the left is outside the mushroom body, where YATAwt was not expressed. Higher-magnification images of the boxed regions 1 and 2 are shown in the lower panels. Expression of YATAwt increased the localization of COPI. The signal for endogenous YATA expressed in the region outside the mushroom body cannot be seen because the image was acquired to visualize signals indicating overexpressed YATA. (B) YATAwt-HA was expressed in the mushroom bodies. The brain was stained with COPI (red), GM130 (green) and HA (white) antibodies. The COPI and GM130 signals were indistinguishable between inside and outside the mushroom bodies. (C) HA-YATAwt was expressed in the mushroom bodies. HA-YATAwt was diffusely mislocalized. In the regions where HA-YATAwt was expressed, COPI mislocalization and an abnormally elongated pattern of GM130 expression (arrows) were also observed. (D) HA-YATAAAAL was expressed in the mushroom bodies. HA-YATAAAAL was mislocalized. COPI and GM130 signals were indistinguishable between the areas inside and outside the mushroom bodies. Scale bar: 10 μm.
Our data suggest that YATA colocalizes with and influences the localization of COPI. These findings are consistent with previous results that showed that SCYL1, the mammalian ortholog of YATA, binds COPI (Burman et al., 2008). SCYL1 binds COPI at its C-terminal amino acid sequence (RKLD-COO-). This sequence resembles the KKXX-COO- motif, which is found in the C-terminus of type 1 integral membrane ER-resident proteins and functions as a COPI-binding motif to target ER-resident proteins to COPI-coated retrogradely transported vesicles (Nilsson et al., 1989; Cosson and Letourneur, 1994; Dominguez et al., 1998). A previous study suggested that the interaction between SCYL1 and COPI was substantially reduced when the C-terminal 10 amino acids were deleted or the C-terminal sequence was mutated to AALD-COO- (Burman et al., 2008). Because the Drosophila YATA protein contains an AKKL-COO- sequence in its C-terminus, we examined whether mutating the C-terminal sequence of the YATA protein would disrupt the effect of YATA on COPI localization.
We conjugated a 3×HA tag sequence just after the C-terminus of the wild-type YATA protein. We induced this YATAwt-HA protein under the control of the mushroom body-specific OK107 Gal4 driver. We triple-stained pupal brains with anti-COPI, anti-GM130 and anti-HA and observed the marginal region of the mushroom body and adjacent brain areas. We compared the signals between the inside of the mushroom body, where YATAwt-HA is expressed, and the outside, where it is not expressed, within a single image. Expression of YATAwt-HA, as revealed by staining with an anti-HA antibody, was observed only within the mushroom body, located to the right of the dotted line (Fig. 5B). Localization of YATAwt-HA showed a punctate pattern that appeared similar to the localization pattern of endogenous YATA. The punctate pattern of the anti-GM130 antibody signal was indistinguishable between inside and outside the mushroom body. Similarly, the punctate pattern of the anti-COPI antibody signal was also indistinguishable between the inside and outside of the mushroom body, and we found that the COPI and GM130 signals colocalized. These data suggest that this modified YATA protein, in which the C-terminus was masked by an HA tag, did not cause an increase in the localization of COPI even when overexpressed and that the C-terminus of YATA is necessary to exert its effect on COPI.
Next, we conjugated a 3×HA tag before the N-terminus of the YATA protein, because we sought to assess the effect of introducing amino acid substitution mutations to its C-terminal sequences. We induced the HA-YATAwt protein under the control of the mushroom body-specific OK107 Gal4 driver, and triple-stained pupal brains with anti-COPI, anti-GM130 and anti-HA and observed the marginal region of the mushroom body and adjacent brain parts. HA-YATAwt was expressed only in the mushroom body, located to the right of the dotted line (Fig. 5C). Unexpectedly, we found that the HA-YATAwt protein was diffusely distributed throughout the cytoplasm surrounding nuclei. These data suggest that HA-YATAwt was mislocalized because of the addition of the HA tag at the N-terminus. We also examined the effect of the expression of mislocalized HA-YATAwt on the localization of COPI and GM130. In the regions outside the mushroom body, where HA-YATAwt was not expressed, COPI and GM130 signals showed normal punctate patterns. However, in the regions where HA-YATAwt was expressed, COPI did not show a clear punctate pattern; instead, only weak, diffuse signals were observed. In addition, in the regions where HA-YATAwt was expressed, the staining pattern of the cis-Golgi marker anti-GM130 often showed abnormal elongated shapes (Fig. 5C, arrows). These differences in the COPI signals and in the shape of the Golgi as revealed by the GM130 signal could be clearly observed by comparing the areas outside and inside the mushroom body within single images. These changes in COPI staining and GM130 signal pattern were reproducibly observed (n = 4). These data suggest that YATA was mislocalized and could not colocalize with the cis-Golgi marker when its N-terminus was masked by the HA tag. They also suggest that the expression of mislocalized YATA can cause the mislocalization of COPI and an abnormal morphology of the localization of a cis-Golgi marker.
Finally, we conjugated a 3×HA tag before the N-terminus of the YATA protein and mutated the C-terminal amino acid sequence from AKKL-COO- to AAAL-COO-. We induced the HA-YATAAAAL protein under the control of mushroom body-specific OK107 Gal4 driver. Staining with anti-HA antibody revealed that the HA-YATAAAAL protein was diffusely distributed, similar to the HA-YATAwt protein (Fig. 5D). We also examined the effect of expressing mislocalized HA-YATAAAAL on the localization of COPI and GM130. In the regions outside the mushroom body, where HA-YATAAAAL was not expressed, the COPI and GM130 signals showed a normal punctate pattern. In the regions inside the mushroom body, where HA-YATAAAAL was expressed, neither COPI nor GM130 signals seemed to be changed compared with the regions where HA-YATAAAAL was not expressed. These data suggest that the expression of mislocalized YATA cannot direct the mislocalization of COPI when the C-terminal sequence of YATA is mutated.
In this study, we examined the subcellular localization of the YATA protein by immunostaining with an anti-YATA antibody and observation using confocal microscopy. Anti-YATA signals were observed to have a punctate pattern in the cytoplasm surrounding nuclei. These punctate signals colocalized with the signals for GM130, a marker of the cis-Golgi, and for COPI. Further analysis using SIM revealed that COPI and YATA were localized in a subset of regions within the GM130 punctae. YATA and COPI partially colocalized. These data suggest that the cis-Golgi, which is labeled by anti-GM130 antibody, contains several distinct subregions where YATA and COPI are localized. Subregions where GM130 colocalized with both YATA and COPI may be sites for the assembly and budding of COPI-coated secretory vesicles traveling from the Golgi to the ER. Consistent with this interpretation, when the expression of Arf4-GFP was induced, its localization showed mainly punctate signals that overlapped with the localization of YATA. A previous study has shown that Arf4-GFP is localized in punctae that seem to be the sites of formation of COPI-coated vesicles in Drosophila embryos (Lee et al., 2015), although it has not been proven that localization of Arf4-GFP completely overlaps with that of the endogenous Arf4 protein. On the other hand, when YFP-KDEL expression was induced, it was found to be localized in the regions that surrounded the nuclei and in some regions near the nuclei. The localization of YATA partially overlapped the YFP-KDEL signal, but the patterns and shapes presented by the signals were different. The C-terminal KDEL sequence is the ER retention motif, which is recognized by the KDEL receptor and functions in the retrieval of ER-resident proteins from the Golgi (Munro and Pelham, 1987; Capitani and Sallese, 2009). In Drosophila neurons, staining with an anti-KDEL antibody and the localization of RFP-KDEL and Lysozyme-GFP-KDEL all reveal localization in the regions that surround the nuclei and in some regions near the nuclei (Sone et al., 2009; Charroux and Royet, 2014; Wong et al., 2014; Oswald et al., 2015). In addition, a previous immunoelectron microscopic study showed that a secreted protein, Hikaru genki, is localized in the region surrounding the nucleus in Drosophila neurons (Hoshino et al., 1996). These observations suggest that the regions we found to be labeled by YFP-KDEL represent the ER. When the expression of Sar1-HA was induced, Sar1-HA showed a relatively strong punctate pattern of localization and a relatively weak pattern of perinuclear localization that seemed to correspond to the ER. A previous study has shown that Sar1-GFP is localized in the perinuclear region and that it also appears as relatively strongly stained punctae in Drosophila S2 cells (Ivan et al., 2008). Costaining with dSec16 suggested that the relatively strongly stained punctae represent the ER exit sites at which COPII-coated vesicles are formed. Because the localization of Sar1-HA resembles that of Sar1-GFP, the punctate signals that arise from Sar1-HA seem to represent the ER exit sites, although it has not been proven that localization of Sar1-HA completely overlaps with that of endogenous Sar1 protein. YATA colocalized with the punctate Sar1-HA signal. Notably, the punctate signals arising from anti-YATA antibody labeling may not represent the entire areas of YATA localization. When YATAwt was overexpressed with no epitope tag, relatively weak staining was also observed in the perinuclear regions of the cytoplasm in addition to the relatively strong punctate signals that were found to colocalize with COPI and the cis-Golgi marker. Therefore, its COPI-related function may not be the only molecular function of YATA. In addition, the site of assembly of COPI-coated vesicles on the cis-Golgi and the site of assembly of COPII-coated vesicles on the ER may be spatially close to each other.
We also assessed whether overexpression of YATA affects the localization of COPI. Our results suggest that ectopic overexpression of YATAwt with no epitope tag increased the localization of COPI. This is consistent with the hypothesis that YATA directs COPI to the subregion of the cis-Golgi where COPI-coated vesicles are assembled, because an increased amount of YATA may be capable of directing an increased amount of COPI to this specific subregion of the cis-Golgi. However, in cultured cells, overexpression of SCYL1 conjugated with GFP caused an expanded morphology of the cis-Golgi, as revealed by labeling with anti-GM130 antibody (Hamlin et al., 2014). These differences may be due to species-specific differences, differences between cultured cells and living animals, an effect of the conjugated GFP, or differences in gene dosage.
Previous studies have shown that SCYL1 binds COPI at its C-terminal amino acid sequence (RKLD-COO-), which resembles the KKXX-COO- motif that functions as a COPI-binding motif in ER-resident proteins (Nilsson et al., 1989; Cosson and Letourneur, 1994; Dominguez et al., 1998; Burman et al., 2008). Because the Drosophila YATA protein has an AKKL-COO- sequence at its C-terminus, we examined whether mutating the C-terminal sequence of the YATA protein would influence the effect of YATA on COPI localization. While overexpression of YATAwt increased the localization of COPI, the induction of YATAwt-HA, which has a 3×HA tag sequence at its C-terminus, did not affect COPI. These data suggest that the C-terminal sequence of YATA is required for its effect on COPI. We also tested the effects of HA-YATAwt and HA-YATAAAAL expression, both of which have a 3×HA tag at their N-terminus. We unexpectedly found that these N-terminally tagged YATA proteins did not exactly colocalize with the cis-Golgi marker but instead showed diffuse localization in the cytoplasm. These data suggest that the N-terminal sequence of YATA is required for its proper subcellular localization. Although the reason for the importance of the N-terminal sequence is unknown, mislocalization of N-terminally tagged YATA enabled us to examine the effect of mistakenly localized YATA on the localization of COPI. Our data show that the expression of mislocalized HA-YATAwt caused the mislocalization of COPI, while the expression of mislocalized HA-YATAAAAL did not. These data suggest that YATA functions to direct COPI to the proper subcellular site and that the C-terminal sequence of YATA is required for this function. Our data regarding the localization of a cis-Golgi marker show that the Golgi exhibited an abnormal elongated shape when HA-YATAwt was expressed but that this effect was not observed when HA-YATAAAAL was expressed. These data suggest that the expression of mislocalized YATA protein that retains its original C-terminal sequence caused the cis-Golgi to acquire abnormal morphology. These observations are consistent with previous results showing that the overexpression or knockdown of Scyl1 resulted in an expanded morphology of the Golgi in cultured cells (Burman et al., 2010; Hamlin et al., 2014) and that the Golgi was enlarged in fibroblasts of patients with a genetic disease caused by mutation of Scyl1 (Schmidt et al., 2015).
Our data reveal a function of YATA, namely its involvement in the regulation of subcellular localization of COPI, and provide a basis for further in vivo genetic explorations of the mechanisms and physiological importance of COPI-mediated vesicular trafficking in Drosophila. However, the relevance of the COPI-regulating function of YATA to the phenotypes observed in yata null mutants is still unclear. yata mutants show impaired anterograde trafficking of a subset of proteins including APPL and Fasciclin II, although Synaptotagmin I trafficking is not affected (Sone et al., 2009; Furotani et al., 2018). In addition, the aberrant accumulation of Sec23, a component of COPII, was observed in yata mutants. One possibility is that the impairment of COPI-mediated retrograde trafficking from the Golgi to the ER disrupts the quality control system and metabolism of proteins synthesized in the ER, as previously suggested (Hamada et al., 2004; Mimura et al., 2007, 2008; Jin et al., 2017; Kokubun et al., 2019), because the retrograde trafficking system is necessary to recover some of the proteins that play roles in the ER and are transported from the ER to the Golgi. Alternatively, YATA could have another function in addition to the regulation of the subcellular localization of COPI in which it could affect the anterograde trafficking of some proteins. The important question of why the trafficking of only a subset of proteins is affected in yata mutants also remains unresolved. The fact that null mutants of yata are not lethal suggests that there are other molecules whose functions overlap with that of yata. Paralogs of yata that exist in the Drosophila genome and belong to the family of genes that encode SCYL family pseudokinases (Pelletier, 2016) are candidates for such molecules. Further detailed analysis of the phenotypes observed in yata mutants and their relevance to the formation of COPI-coated vesicles and the regulation of intracellular vesicular trafficking will be necessary in the future.
We thank Dr. Yoshihiro Inoue for providing the anti-COPI antibody and Dr. Matthew P. Scott for the UAS-YFP-KDEL fly strain. We thank Ms. Ayumi Komatsu for technical assistance. We also thank FlyORF and the Bloomington Drosophila Stock Center for providing Drosophila strains. This work was supported by Japan Society for the Promotion of Science KAKENHI (grant numbers 26350982, 23500401 and 20K06881 to M. S.) and the Joint Usage/Research Program of the Medical Research Institute, Tokyo Medical and Dental University.