2017 年 86 巻 4 号 p. 543-551
2017 年 86 巻 4 号 p. 543-551
‘Sagan-Ruby’ is the first grapefruit (Citrus paradisi) cultivar to be developed in Japan and is used for food, cosmetics, and other purposes owing to its favorable characteristics, such as the absence of harmful pesticides and its long shelf life. The desired qualities of grapefruit depend on the specific use, and these qualities are influenced by the metabolite composition of the fruits. However, little is known about the influence of the growing environment or harvest period on the metabolite composition of the ‘Sagan-Ruby’ grapefruit. Therefore, we harvested fruits that were grown either in a plastic house without artificial heating or outdoors with rain cover from December, 2014 to April, 2015, on a monthly basis, and we investigated the composition of the primary metabolites such as sugars, organic acids, and amino acids, in the juice and peel of the fruit using gas chromatography mass spectrometry (GC/MS). We detected a total of 53 and 68 compounds in the juice and peel, respectively, and the first and second components of the principal component analyses of the detected metabolites of both juice and peel were associated with the growing environment and harvest period, respectively. Since we observed that glucose, fructose, sucrose, and citric acid were more concentrated in the juice of outdoor-grown fruits than in that of the house-grown fruits, especially in March and April, it is likely that the sweetness and acidity of the fruits are dependent on the growing environment. Similarly, the primary metabolite contents, including succinic acid and other organic acids, were higher in peels from outdoor-grown fruits. In addition, we also observed that the contents of proline, phenylalanine, and other amino acids in the juice increased continuously from December to April, and many sugars, including glucose and fructose, gradually decreased in peels from December to February and were lower from February to April. These results indicated that quality of the ‘Sagan-Ruby’ grapefruit varies with the harvest period.
Grapefruit (Citrus paradisi) is one of the most popular citrus fruits in Japan, but since it is typically cultivated in subtropical regions and is difficult to grow in Japan, most of the grapefruit consumed in Japan is imported. In fact, 100960 t of grapefruit were imported into Japan during 2015 (Overview of Foreign Trade of Agricultural, Forestry, and Fishery Products of Japan (2015), http://www.maff.go.jp/j/tokei/kouhyou/kokusai/houkoku_gaikyou.html), and the fruit was third among the most imported fruits, after bananas and pineapples, and first among imported citrus fruits. In addition, 95% of the grapefruit in Japan was imported from North America and South Africa. To suppress the deterioration in fruit quality during long-term transport, agrochemicals are sprayed on the surface of citrus fruits, and some of these agrochemicals have been suggested to have detrimental health effects (Hiraga and Fujii, 1984) and have been shown to remain in citrus fruits even after storage and processing (Tsumura-Hasegawa et al., 1992). Since the safety of imported foods has recently become a growing concern in Japan, the demand for domestically-grown grapefruit that are not treated with post-harvest agrochemicals is rising.
The new grapefruit cultivar ‘Sashika-ichigou’ (‘Sagan-Ruby’), which was developed by Saga University (Saga, Japan), is the first cultivar to be bred and variety-registered in Japan (registration no. 22466). The cultivar has excellent cold-resistance and is capable of withstanding the climate of Japan. It is expected that this grapefruit cultivar will be used safely for various purposes without worrying about residual agrochemicals. In addition, this cultivar has a long shelf life and can be shipped domestically from April to June, when the supply of citrus fruits in Japan is usually low. We are now developing products using ‘Sagan-Ruby’ grapefruit as a raw material by taking advantage of its increased food safety and long shelf life. The weight of fruits is over 300 g, which is large enough to eat fresh, and juices, liqueurs, and sweets that contain ‘Sagan-Ruby’ juice are already being consumed. Several cosmetics and fragrances that use the essential oil extracted from the peel have also been developed.
The required quality and specific characteristics of grapefruit vary widely depending on the intended end-use. For example, sweetness, acidity, and bitterness are important when consuming the fruit either fresh or in drinks, whereas aroma is more important when using the fruit for cosmetics and fragrances. In addition, since grapefruit juice is reported to contain bioactive compounds that have high antioxidant potential and have a positive influence on plasma lipid metabolism (Gorinstein et al., 2004), the functional aspects of grapefruit are also attracting attention. A wide variety of metabolites, such as sugars, organic acids, and flavonoids, influence the quality of grapefruit. Therefore, an analysis of the metabolite composition of ‘Sagan-Ruby’ would be valuable for product development. During the cultivation, fruits begin ripening on the trees in early winter, but can be kept on the trees until spring in order to extend the shelf life and improve the fruit quality (Suzuki et al., 1997). The changes with time in the chemical constituents of citrus fruit during the course of maturation and ripening have been studied (Bermejo and Cano, 2012; Iwagaki et al., 1981; Takebayashi et al., 1993), but there is no information about changes with time in the metabolite composition of grapefruit grown in Japan. Furthermore, in Japan, citrus fruits are grown both outdoors and in plastic houses, with the aim of stabilizing and improving fruit quality, and although the influence of growing environment on fruit quality and metabolite composition has been studied in other citrus species (Izumi, 1999; Kamota, 1987; Morinaga and Sykes, 2001; Sakamoto and Okuchi, 1968; Sawamura et al., 1983; Takagi et al., 1994; Takebayashi et al., 1992), no studies have compared the metabolite composition of grapefruit grown outdoors to that of grapefruit grown in plastic houses in Japan until the present study.
Recently, metabolite profiling has been widely used to analyze the characteristics and qualities of agricultural products, and gas chromatography-mass spectrometry (GC/MS) is suitable for the unbiased profiling of primary metabolites in plants (Roessner et al., 2000). In fact, GC/MS-based metabolite profiling has already been applied to tomatoes (Solanum lycoperstcum), strawberries (Fragaria × ananassa), and peaches (Prunus persica), in order to analyze the dynamics of metabolite composition during fruit development (Fait et al., 2008; Lombardo et al., 2011; Mintz-Oron et al., 2008; Zhang et al., 2011). The present study focused on the low molecular weight metabolites of grapefruit, including sugars, organic acids, amino acids, and other primary metabolites, which together are directly responsible for sweetness, acidity, and other characteristics and also indirectly influence bitterness, aroma, as well as the functionality of secondary metabolites. The objective of the study was to evaluate the suitability of ‘Sagan-Ruby’ grown in different environments and harvested at different stages of ripening as foods and processing ingredients.
The ‘Sashika-ichigou’ (‘Sagan-Ruby’) grapefruit trees were grafted to Citrus unshiu in 2002 (14 years ago) and grown either in a plastic house without artificial heating in northern Saga, Japan, or in an outdoor field with rain cover in southern Saga, Japan. Fruits were picked at approximately month-long intervals from December, 2014 to April, 2015 (December 8, January 6, February 5, March 5, and April 9). Each month, we collected fruits from three individual trees as replicates and collected three fruits per replicate. After collection, the fruits were weighed and then separated into flesh and peel. The flesh was squeezed and filtered to obtain juice, and the juice of the three fruits from each tree were pooled, while the peels were separated into albedo and flavedo, and similar sized peel fragments of the three fruits from each tree were pooled into combined albedo and flavedo samples, which were then freeze-dried and powdered.
Extraction and derivatizationThe metabolite analyses were conducted as described by Roessner et al. (2000). For the juice, 200 μL filtered juice was mixed with 600 μL methanol, 200 μL chloroform, and 10 μL ribitol solution (10 mg·mL−1; used as the internal standard) by vortexing for 5 min. Subsequently, 400 μL distilled water was added, the samples were centrifuged at 10000 × g for 5 min to separate the polar and nonpolar phases, and 200 μL of the upper polar phase was transferred from each sample to a new plastic tube. For the peels, 20 mg of either albedo or flavedo powder was suspended in a mixture of 480 μL methanol, 160 μL chloroform, 160 μL distilled water, and 8 μL ribitol solution (10 mg·mL−1), and the slurry was mixed for 10 min. Subsequently, ~320 μL distilled water was added, the samples were centrifuged, and 200 μL of the upper polar phase was transferred from each sample to a new tube. All of the extracts were dried completely using a centrifugal evaporator, and for oxime-derivatization, the dried samples were resolved with 20 μL methoxyamine hydrochloride (pyridine solution, 40 mg·mL−1) and incubated at 30°C for 120 min. Finally, 10 μL of each sample was transferred to an individual glass vial containing an insert (0.2 mL, within a 1.5-mL glass vial), mixed with 40 μL N-methyl-trifluoroacetamide (MSTFA, reagent for producing a silylated derivative), and incubated at 37°C for 30 min.
GC/MS experiments and data analysesThe derivatized sample (1 μL) was injected into an Agilent GC/MSD 5977A, using the 1:5 split mode. The type of column, oven temperature, and other GC/MSD parameters were set for metabolite identification using the Fiehn GC/MS metabolomics RTL library (Agilent Technologies, Inc., California, USA). The identification and quantitative estimation of each of the metabolites were conducted using MassHunter software (Agilent Technologies, Inc.). Before statistical analysis, the data were normalized using the peak area of the internal standard, ribitol. The peak areas of all compounds detected in the juices and peels were subjected to the principal component analysis (PCA) and other multivariate analysis. Significantly differences (P-value calculated by ANOVA was lower than 0.05) were detected among growing environments (outdoor field or plastic house, each group consisted of 15 samples) or harvest months (December, January, February, March, or April, each group consisted of 6 samples). These analyses were conducted using R ver 3.1 (https://www.r-project.org/) and MetaboAnalyst 3.0 (http://www.metaboanalyst.ca/faces/home.xhtml). The contents of glucose, fructose, sucrose, citric acid, and malic acid in juice were calculated by comparing their peak areas with those of compounds with known concentrations.
The ‘Sagan-Ruby’ trees grown in the outdoor field yielded heavier fruits than those grown in the plastic house, except for December (Table 1), and during the harvesting period (December to April) no marked increases in fruit weight were observed in either of the growing environments, except for that of the outdoor-grown fruits from December to January. Meanwhile, the ratio of peel weight to whole fruit weight remained constant from December to March in the outdoor-grown fruits and from December to February in the house-grown fruits and subsequently increased until April in the fruits from both growing environments. Therefore, the fruit weight and peel ratio were mainly constant, with only a few exceptions, indicating that the growth and development of the fruit were already complete by early winter and that later harvesting only increases the duration of the fruit’s ripening period. In contrast, the peel color of the fruit depended on the harvested period, with a lemon yellow in December that gradually changed to reddish yellow in February, returning to pale yellow in April (Fig. 1).

Effect of the growing environment on the temporal changes in ‘Sagan-Ruby’ grapefruit whole fruit weight and separated peel weight.

Changes in the color of ‘Sagan-Ruby’ grapefruit peels during the ripening period. Photographs of peels from fruits grown in an outdoor field (upper) or in a plastic house (lower) and harvested in December (left), February (center), and April (right).
A total of 53 compounds (15 amino acids, 14 organic acids, 15 sugars, and 9 unidentified compounds) were detected from the grapefruit juices, and a total of 68 compounds (15 amino acids, 17 organic acids, 18 sugars, 4 others, and 14 unidentified compounds) were detected from the grapefruit peels (albedo and flavedo). ANOVA between outdoor-grown fruits and house-grown fruits in all harvest months showed that the content of 24, 23, and 25 compounds were significantly influenced by growing environments in the juice, albedo, and flavedo, respectively (Table 2). ANOVA among fruits harvested in different month, regardless of growing environment, showed that the contents of 25, 25, and 27 compounds were significantly influenced by the harvest period in the juice, albedo, and flavedo, respectively.
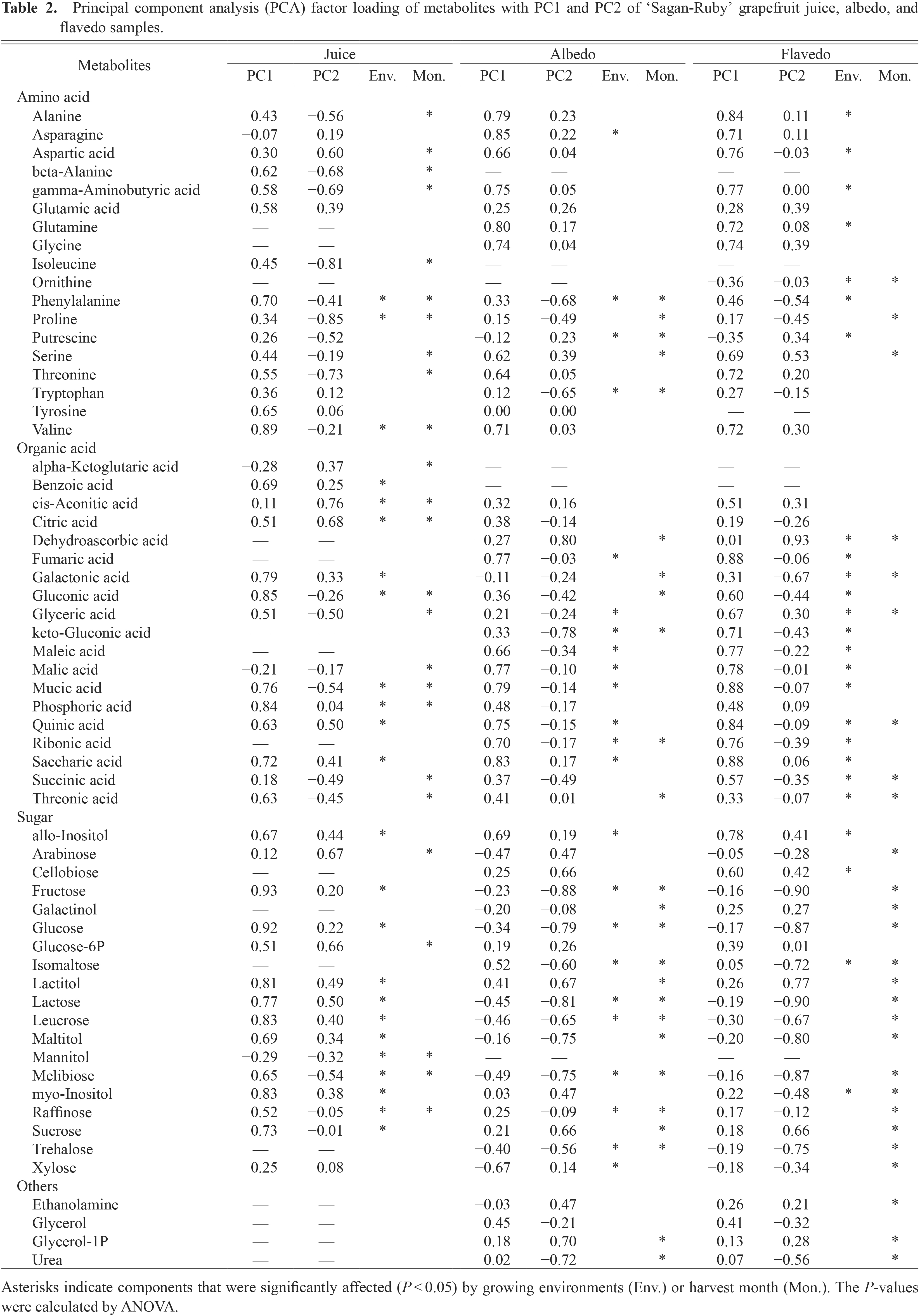
Principal component analysis (PCA) factor loading of metabolites with PC1 and PC2 of ‘Sagan-Ruby’ grapefruit juice, albedo, and flavedo samples.
In PCA of the juice metabolites, the first principal component (PC1) accounted for 38% of the total variance and mainly discriminated between the samples grown in the outdoor field and those grown in the plastic house (Fig. 2A). Meanwhile, the second principal component (PC2) accounted for 20% of the total variance, and the December and April samples had higher and lower PC2 scores, respectively (Fig. 2A), whereas the January, February, and March samples had intermediate PC2 scores, suggesting that the changes in metabolite contents were proportional to the duration of the ripening period of fruits (from December to April) and were reflected in PC2.

Sample scores of the first (PC1) and second (PC2) principal components from a principal component analysis (PCA) of metabolites detected in ‘Sagan-Ruby’ grapefruit juice (A), albedo (B), and flavedo (C).
In contrast, in the albedo and flavedo, PC1 only accounted for 22% and 25% of the total variance, respectively, although it still mainly discriminated between samples from different growing environments (Fig. 2B, C), and PC2 accounted for 18% and 16% of the total variance in the metabolite contents of the albedo and flavedo samples, respectively. Furthermore, we also found that the albedo samples from December, January and April, and February and March had relatively high, intermediate, and low PC2 scores, respectively, and that flavedo samples from December, April, and the three months in between had high, moderately high, and moderately low PC2 scores, respectively (Fig. 2B, C). These results indicated that PC2 reflected the change in metabolites and that the individual metabolites either increased from December to February and decreased from February to April (ridge-shaped pattern) or decreased from December to February and increased from February to April (valley-shaped pattern), with a greater change from December to February than from February to April. Meanwhile, the third principal component (PC3) accounted for 13% and 12% of the total variance of the metabolite contents of albedo and flavedo and mainly discriminated the April samples from the other samples.
Since PC1 and PC2 sufficiently reflected the differences in the metabolites of ‘Sagan-Ruby’ grapefruit from different growing environments and harvest periods, we used factor loading scores that were calculated along with each of the PCs to determine which metabolites were influenced by environmental and seasonal changes (Table 2). According to the PC1 loading scores of the juices, many of the identified metabolites were more prevalent in juices from outdoor-grown fruits, and the PC2 loading scores of the juices indicated that the content of many amino acids and some organic acids increased with the duration of the ripening period, whereas the content of other organic acids decreased. At each of the harvest periods, glucose, sucrose, saccharic acid, quinic acid, and other metabolites, which had positive PC1 scores and were significantly affected by the growing environment, were more concentrated in juices from outdoor-grown fruits (Fig. 3). From January to February, the contents of these metabolites were elevated in the juice of outdoor-grown fruits and from February to March were much lower in the juice from house-grown fruits. The contents of phenylalanine, proline, threonine, mucic acid, and other metabolites, which had negative PC2 scores and were significantly affected by the harvest period, increased with the duration of fruit ripening, and from February to April, those that had positive PC1 scores increased more in the juice from the outdoor-grown fruits than that from the house-grown fruits.

Effect of the growing environment and harvesting period on the metabolite contents of ‘Sagan-Ruby’ grapefruit juice, albedo, and flavedo. Only the metabolites for which we observed significant effects of growing environment or harvest period (refer to Table 2) are shown. Colors indicate the relative metabolite contents of each sample point in relation to the mean content of all samples; dark blue indicates the lowest ratios, and dark red indicates the highest ratios. The metabolites with similar patterns are arranged close together.
Meanwhile, the PC1 and PC2 loading scores of the peels indicated that the differences in metabolite contents caused by the growing environment and harvest period were similar in the albedo and flavedo tissues (Table 2). Some amino acids and many organic acids were more concentrated in the peels of outdoor-grown fruits, whereas the contents of many sugars in peels were only affected by the harvest period. The contents of quinic acid, malic acid, and other metabolites, which had positive PC1 scores, were higher in the peels of outdoor-grown fruits, and the contents of glucose, fructose, and other sugars, which had negative PC2 scores, gradually increased from December to February and then gradually decreased from February to April (Fig. 3). Exceptionally, sucrose had positive PC2 scores and exhibited a valley-shaped change with time. The concentrations of sugars, which had negative PC1 scores, were higher in the albedo of house-grown fruits, and the content of xylose, arabinose, and other sugars, which had negative PC2 and PC3 scores, continuously increased in the flavedo from December to April.
In the juices, we found that glucose, fructose, and sucrose yielded relatively high GC/MS peaks compared to the other sugars, which suggested that the sweetness of ‘Sagan-Ruby’ grapefruit juice is greatly affected by these sugars, while the content of sucrose was higher than that of glucose or fructose (Fig. 4). The environmental and seasonal changes in glucose were the same as those of fructose and were similar to those of sucrose, and the growing environment only had a small effect on the content of these sugars from December to February. However, from February to March, we found that levels of these three sugars decreased in the house-grown fruits, which resulted in the differences in the sugar contents in fruits from the two growing environments in March and April. Meanwhile, the level of citric acid in the juices was extremely high when compared to the levels of malic acid and other organic acids, and the content of citric acid was more than several ten-fold that of malic acid (Fig. 4), which suggested that citric acid was the main contributor to the acidity of ‘Sagan-Ruby’ grapefruit juice. The influence of the growing environment and harvest period on citric acid was similar to their influence on glucose. On the other hand, the content of malic acid in juices exhibited a valley-shaped change with time.

Effect of growing environment and harvest period on the glucose, fructose, sucrose, citric acid, and malic acid contents of ‘Sagan-Ruby’ grapefruit juice.
To investigate whether growing environment and harvest period contributed to the variation in primary metabolites of ‘Sagan-Ruby’ grapefruit, we conducted metabolite profiling of the fruit and peel of ‘Sagan-Ruby’ grapefruit using GC/MS. A PCA of the detected metabolite contents revealed that the most influential factor for the metabolite composition of both juice and peel was the growing environment (Fig. 2).
The differences between the PC1 scores of juices from outdoor-grown fruits collected in December and January and those of house-grown fruits were smaller than differences between the PC1 scores of juices from outdoor-grown fruits collected in February, March, and April and those from house-grown fruits (Fig. 2A). This suggests that the influence of growing environment on the metabolite composition of the juice is more pronounced during late winter and early spring. It is possible that the difference in weight of fruit from the two growing environments, which was small in December and large thereafter (Table 1), affects these different metabolite contents of the juices.
In contrast, the metabolite composition of peels from fruit collected in early winter was sensitive to the growing environment (Fig. 2B, C), and we found that ridge- and valley-shaped changes with time were more prevalent in the albedo and flavedo samples than in the juice samples (Fig. 3). In addition, the difference between the metabolite contents of the peel samples from April and those from other months was represented by PC3, which indicated that the metabolic mechanism in peels was greatly different between fruits harvested during early and mid-winter and fruits harvested during late winter and early spring. From February to April, both the metabolite change in a direction opposite to the change from December to February, and a metabolite change unique to this period, were observed in peels.
Effect of growing environment and harvest period on the metabolite composition of juiceIn Japan, the influence of growing environment on the quality of citrus fruits has been investigated using satsuma mandarin (Citrus unshiu), and it has been reported that satsuma mandarin grown in plastic houses have better yield, sugar content, and fruit quality than those grown outdoors (Suzuki et al., 1997). However, Sawamura et al. (1983) reported that the amount of sugars, organic acids, and other metabolites was substantially lower in juices from house-grown satsuma mandarin than in juices from satsuma mandarin grown outdoors, and with the exception of satsuma mandarin, the sugar content of citrus grown in plastic houses is not different from that of citrus grown outdoors (Kamota, 1987). In the present study, we demonstrated that the content of sugars, organic acids, amino acids, and other metabolites were lower in the juices of house-grown ‘Sagan-Ruby’ grapefruits (Table 2). The concentrations of many metabolites, including glucose, fructose, and citric acid, were higher in juices from outdoor-grown fruits, owing to the reduction in the metabolites in the juice of house-grown fruits from February to March. Average air temperature near the cultivated region was constant from December of 2014 to February of 2015 and increased in March and April of 2015 (Japan Meteorological Agency, http://www.jma.go.jp/). It is predicted that the higher temperature of plastic houses enhances the respiration activity of fruit flesh and the metabolism of sugars and organic acids as substrates, and in addition, several reports have demonstrated that high temperatures during fruit development reduce the acidity and total sugar content of juice from satsuma mandarin (Sakamoto and Okuchi, 1968; Takagi et al., 1994).
The present study revealed that the content of phenylalanine, proline, gamma-aminobutyric acid (GABA), and various amino acids in juices increased with the duration of the ripening period (Table 2; Fig. 3). Arginine, alanine, glutamine, proline, and GABA are reported to increase with development and maturation of satsuma mandarin fruit (Iwagaki et al., 1981). These results suggest that the change with time of amino acids in ‘Sagan-Ruby’ is similar to that of other citrus fruits. From late winter to early spring, the ripening and aging of the fruit progressed, and the decreased production of new proteins and increased degradation of old proteins may have promoted the accumulation of amino acids. The present study demonstrated that the content of many organic acids and sugars remain relatively constant during fruit ripening, especially in outdoor-grown fruit (Fig. 3), and similar patterns have been reported in grapefruit cultivated in the Mediterranean region (Bermejo and Cano, 2012). Takebayashi et al. (1993) explained that citrus species and cultivars are divided on the basis of patterns in their accumulation of sugar during fruit development and maturation. In this scheme, grapefruit is categorized into a group that contains citrus fruits in which the increase with time and final accumulation of sugars are small. Our results correspond to this grouping.
Effect of growing environment and harvest period on the metabolite composition of peelIn the albedo and flavedo, we found that the seasonal changes in metabolite contents were often ridge- or valley-shaped (Fig. 3). In the subtropics, citrus fruits degreen during the cool winter season and, when left on the tree, some varieties regreen during the ensuing spring (Hortensteiner, 2006). The peel color of ‘Sagan-Ruby’ changed from yellow to reddish yellow and then returned to yellow as the ripening period progressed (Fig. 1). Thus, it is considered that the change with time of metabolites in peels is associated with degreening and regreening processes, which, in turn, are strongly influenced by the sugar (mainly reducing sugars) concentration in peels (Hortensteiner, 2006). In the present study, the content of several kinds of sugars in the albedo and flavedo, including glucose and fructose, increased from December to February and decreased from February to April. The low and high concentrations of sugars promote the regreening and degreening of peels, respectively (Ahmed, 2009; Iglesias et al., 2001; Takagi et al., 1994). It is possible that the peel color change of ‘Sagan-Ruby’ was induced by the ridge-shaped change with time of sugar contents. Although sucrose exhibited a valley-shaped change with time in both albedo and flavedo, sucrose has a relatively small effect on citrus degreening compared to reducing sugars (Takagi et al., 1994), and the content in ‘Sagan-Ruby’ peels is lower than either glucose or fructose (data not shown). These results suggest that sucrose has an insignificant effect on the change of peel color when compared to glucose and fructose.
One of the amino acids, ornithine, was not detected in the peels from fruits collected during December and January, and the ornithine content of the flavedo was significantly elevated from February to April (Fig. 3). In higher plants, ornithine is the precursor of arginine, which serves as a main nitrogen storage compound, and increases in ornithine content induce tolerance to salt and drought stresses (Kalamaki et al., 2009). In addition, ornithine is utilized in the biosynthesis of polyamine, alkaloids, and other plant secondary metabolites (Shargool et al., 1988). In the present study, it is unclear whether the elevation of ornithine content was the result of preparation for the synthesis of secondary metabolites or if the elevation of ornithine content results from the suspension of biosynthesis pathways that utilize ornithine as a substrate. However, the reduced content of proline, an ornithine metabolite, in the peels of fruits collected from February to April supports the second hypothesis, that is, ornithine content is elevated as a result of suspended biosynthesis.
Effect of the growing environment and harvest period on quality of ‘Sagan-Ruby’ grapefruitSince the flesh of ‘Sagan-Ruby’ grapefruit is mainly used for eating raw or as an ingredient in the production of juice and liquor, the characteristics of sweetness, acidity, and bitterness are probably the most important. In grapefruit juice, sweetness and acidity depend on the content of sugars such as glucose, fructose, and sucrose, and organic acids, especially citric acid. The contents of glucose, fructose, and citric acid in juices squeezed from outdoor-grown fruit were about 1.2 to 1.6 times those in juices squeezed from house-grown fruit during March and April, and other sugars and organic acids tended to be found at high content in juices of outdoor-grown fruit (Figs. 3 and 4). Thus, it is likely that outdoor-grown fruits have a ‘stronger taste’ than house-grown fruits. In satsuma mandarin, house-grown fruits were preferred over outdoor-grown fruits, even though the content of sugars and organic acids was lower (Sawamura et al., 1983). This evaluation is thought to be attributed to the increased sugar-acid ratio that results from the reduced acidity of the house-grown fruits. Therefore, the house-grown fruit may also be considered ‘sweeter’ than the outdoor-grown fruit. In fruit cultivation, drought stress is known to reduce fruit weight and to increase the sugar content of fruit (Suzuki et al., 1997). Plastic houses are suitable for managing drought stress and producing high-quality fruits. Although the sugar content of juice from house-grown fruits was lower in the present study, proper irrigation would likely improve the quality of house-grown ‘Sagan-Ruby’ grapefruits.
Compared to sugars and organic acids, the number of reports that have investigated the relationship between amino acids and citrus taste is small. Takebayashi et al. (1993) reported that good-tasting citrus fruits have higher concentrations of proline. In the present study, we found that the content of proline in juices increased about two times from December to April, and the content of other amino acids was increased with the duration of fruit ripening. Thus, it is predicted that the taste of ‘Sagan-Ruby’ is influenced by the harvest period. In addition, the content of GABA in juices more than doubled from December to April. GABA is reported to possess anti-stress activity, and the GABA content of grapefruit is the second highest among citrus fruits, following oranges (Citrus sinesis; Shimizu and Sawai, 2008). When ‘Sagan-Ruby’ grapefruit with high GABA content is required, fruits harvested in the early spring would be ideal.
The bitterness of citrus fruits is caused by a variety of secondary metabolites, such as flavonoid and limonoid (Hasegawa et al., 1995). Unfortunately, we were unable to analyze the composition of secondary metabolites. However, we found that the contents of phenylalanine, benzoic acid, and quinic acid, which are associated with the metabolism of phosphoenolpyruvate to flavonoid, in juices squeezed from outdoor-grown fruit were about 1.4, 1.2, and 1.8 times those in juices squeezed from house-grown fruit, respectively. Moreover, the content of phenylalanine in juices more than doubled from December to April. These results suggest that both growing environment and harvest period affect the composition of secondary metabolites. Therefore, the profiling of secondary metabolites will be useful in the future to further evaluate the quality of ‘Sagan-Ruby’ grapefruit.
In conclusion, the present study demonstrated that both the growing environment and harvest period influence the metabolite composition of ‘Sagan-Ruby’ grapefruit. The sweetness and acidity were higher in the outdoor-grown fruit, and many kinds of metabolites that affect fruit qualities exhibited seasonal changes as the ripening period progressed. The data presented here will be useful for determining the optimal growing environment and harvest period for various ‘Sagan-Ruby’-derived products.