2018 年 87 巻 4 号 p. 481-489
2018 年 87 巻 4 号 p. 481-489
Actinidia kolomikta (Maxim. & Rupr.) Maxim., a wild kiwifruit relative native to East Asia, has several desirable characteristics, such as strong cold tolerance, precocity, and high levels of vitamin C, and has therefore attracted horticultural interest. In this study, the interspecific cross compatibility of the diploid Actinidia kolomikta with one cultivated species [A. deliciosa (A. Chev.) C. F. Liang & A. R. Ferguson], three native Actinidia species [A. arguta (Siebold & Zucc.) Planch. ex Miq., A. polygama (Siebold & Zucc.) Maxim., and A. rufa (Siebold & Zucc.) Planch. ex Miq.], and one endemic variety [A. arguta (Siebold & Zucc.) Planch. ex Miq. var. hypoleuca (Nakai) Kitam.], was evaluated to see if interspecific hybridization using A. kolomikta could improve edible Actinidia crop breeding programs. There was no fruit setting two weeks after anthesis in the tested cross combinations when A. kolomikta was the male parent. In contrast, all five crosses using A. kolomikta as the female parent produced 100% fruit setting two months after pollination. The crosses with different ploidy levels such as combinations between diploid A. kolomikta and hexaploid A. deliciosa, or diploid A. kolomikta and tetraploid A. arguta, produced a few viable seeds. In contrast, the crosses with diploid species such as A. arguta var. hypoleuca, A. polygama, and A. rufa, produced many viable seeds and seedlings. From these results, the cross compatibilities of A. kolomikta with the other tested species were categorized as follows: no fruit setting (A. arguta × A. kolomikta and A. polygama × A. kolomikta); fruit setting with few viable seeds (A. kolomikta × A. arguta and A. kolomikta × A. deliciosa); and normal fruit setting with viable seeds (A. kolomikta × A. arguta var. hypoleuca, A. kolomikta × A. polygama, and A. kolomikta × A. rufa). These results could be a basis for future edible Actinidia crop breeding programs using A. kolomikta and interspecific hybridization.
Actinidia kolomikta (known as Miyama matatabi in Japanese) is native to East Asia, including China, Japan, North and South Korea, and the Russian far East. It is a highly cold-tolerant species that is related to the cultivated kiwifruit A. deliciosa and A. chinensis Planch. In Japan, A. kolomikta is distributed across relatively cold temperate regions in northern Honshu (the main island) and Hokkaido, Japan. This species has several desirable characteristics such as a compact growth habit and a short growing season to achieve fruit maturity (Ferguson and Seal, 2008). Actinidia kolomikta fruits are rich in taste and flavor and have been found to contain high levels of vitamin C (ascorbic acid) (Chesoniene et al., 2004; Latocha et al., 2010). A recent study reported that A. kolomikta fruit had a higher antioxidant activity than those produced by A. arguta and A. chinensis (Zuo et al., 2012). Therefore, A. kolomikta has potential as a new type of berry crop or as a breeding material to improve kiwifruit so that they are suitable for cultivation in cold temperate climates.
Breeding based on the evaluation of wild genetic resources is important if A. kolomikta is to be commercially cultivated. Previously, we evaluated wild A. kolomikta accessions in Japan and described wide variations in fruit traits including fruit size, shape, soluble solids content, and ascorbic acid content, which showed that A. kolomikta was an important and diverse genetic resource (Asakura and Hoshino, 2016). We also reported that endosperm culture could be used to produce a triploid plant regeneration system (Asakura and Hoshino, 2017a), and that immature seeds from A. kolomikta could be used to create an efficient plant regeneration system (Asakura and Hoshino, 2017b) that may be applicable in future breeding programs for this species.
Crop improvement of A. kolomikta using cross breeding techniques requires further information on the interspecific cross compatibility of this species with other related species. Hirsch et al. (2001) crossed A. kolomikta with kiwifruit, A. chinensis, and A. deliciosa, and obtained hybrid plants by embryo rescue. Guthrie et al. (2007) crossed A. kolomikta with seven other species, including cultivated kiwifruit, and produced several hybrids.
One cultivated species (A. deliciosa), three native Actinidia species (A. arguta, A. polygama, and A. rufa), and one endemic variety (A. arguta var. hypoleuca), related to A. kolomikta, were used in this study. In Actinidia, all known species are dioecious (Fraser et al., 2009). Hence, cross pollination is generally required for fruit setting, although some selections of A. arguta are reported to be self-fruitful, or parthenocarpic (Mizugami et al., 2007).
Kiwifruit, A. deliciosa, native to Central and South China, have been cultivated mainly in warm temperate regions on Honshu, and in Shikoku and Kyushu in Japan. Actinidia arguta and A. polygama, called Sarunashi and Matatabi in Japanese, respectively, are mostly found in mountainous regions throughout Japan where they are widely distributed except for the Ryukyu Islands (the southernmost islands). In contrast, A. rufa, called Shima sarunashi in Japanese, is native to lowland evergreen forests in subtropical to warm-temperate regions from the Ryukyu Islands to Shikoku, Kyushu, and western Honshu. Actinidia arguta var. hypoleuca, called Urajiro matatabi in Japanese, is sometimes treated as a distinct species (Actinidia hypoleuca Nakai) and is endemic to the warm Pacific hill areas of Shikoku, Kyushu, and the south western part of Honshu. There is wide ploidy variation in Japanese A. arguta, including diploid (2n = 2x = 58; called A. arguta var. hypoleuca), tetraploid (2n = 4x = 116), hexaploid (2n = 6x = 174), heptaploid (2n = 7x = 203), and octoploid (2n = 8x = 232) variations (Kataoka et al., 2010). However, A. kolomikta, A. polygama, and A. rufa seem to be mainly diploid (2n = 2x = 58).
As for A. arguta and A. rufa, interspecific cross compatibility with other native and cultivated species in Japan has been evaluated, and interspecific hybrids have been produced between these species and cultivated kiwifruit (Kataoka et al., 2003, 2014; Kokudo et al., 2003; Matsumoto et al., 2011). However, relatively little information is available on the interspecific cross compatibility of A. kolomikta with other Actinidia species grown in Japan. In this study, we evaluated the interspecific cross compatibility of A. kolomikta with one cultivated species (A. deliciosa), three native Actinidia species (A. arguta, A. polygama, and A. rufa), and one endemic variety (A. arguta var. hypoleuca) as a basis for future edible Actinidia crop breeding programs using A. kolomikta and interspecific hybridization.
One cultivated species (A. deliciosa), four native Actinidia species (A. arguta, A. kolomikta, A. polygama, and A. rufa), and one endemic variety (A. arguta var. hypoleuca), were used in this study. Female A. kolomikta [accession name: AKN1, diploid (2n = 2x = 58)] plant, one A. arguta [accession name: AATD1, tetraploid (2n = 4x = 116)] plant, one A. polygama [accession name: APSP1, diploid (2n = 2x = 58)] plant, and male A. kolomikta [accession name: AKN2, diploid (2n = 2x = 58)] plant originally came from Hokkaido and were cultivated at the Experiment Farm, Field Science Center for Northern Biosphere, Hokkaido University, Sapporo, Hokkaido, Japan. As pollen sources, A. arguta [tetraploid (2n = 4x = 116), one accession from Hokkaido, Japan], A. arguta var. hypoleuca [diploid (2n = 2x = 58), one accession from Tokyo, Japan], A. deliciosa [estimated hexaploid (2n = 6x = 174); the pollen grains (anonymous accession) purchased from Kokkaen Co., Izumi, Japan], A. polygama [diploid (2n = 2x = 58), one accession from Tokyo, Japan], and A. rufa [diploid (2n = 2x = 58), one accession from Okinawa, Japan] were used. The A. arguta, A. arguta var. hypoleuca, A. polygama, and A. rufa pollen grains were collected from their natural habitats mentioned above during the flowering seasons in 2015 and 2016. The pollen was stored at 4°C in a refrigerator until needed. Pollen germination was assayed using a liquid medium containing 0.01% (w/v) yeast extract, 0.01% (w/v) H3BO3, 0.01% (w/v) CaCl2, 0.0007% (w/v) KH2PO4, and 10% (w/v) sucrose as reported by Hirano and Hoshino (2009), before it was first used. Using this method, pollen grains showing more than a 35% pollen germination rate were used for the following pollination experiments.
Using these plant materials, the following experiments were conducted at the Experiment Farm, Hokkaido University, from 2015 to 2017.
Crossing experiment using A. kolomikta as the male parentOne female A. arguta (accession name: AATD1, tetraploid) plant and one A. polygama (APSP1, diploid) plant were pollinated with pollen collected from one male A. kolomikta plant (accession name: AKN2, diploid). Flowers on the female parents were covered with paraffin bags from one day before flowering until seven days after pollination to prevent contamination by other pollen. To confirm whether the female parents used were parthenocarpic or not, non-pollinated flowers on the female parents were also covered with paraffin bags from one day before until seven days after anthesis. These were then used as the controls. The fruit setting rate and the number of seeds obtained were recorded two months after pollination.
Crossing experiment using A. kolomikta as the female parentOne female A. kolomikta plant (accession name: AKN1, diploid) cultivated at the Experiment Farm, Hokkaido University, was pollinated with pollen from the following species: Control (A. kolomikta with open pollination), A. arguta (tetraploid, one accession from Hokkaido, Japan), A. arguta var. hypoleuca (diploid, one accession from Tokyo, Japan), A. deliciosa [hexaploid, unknown accession(s)], A. polygama (diploid, one accession from Tokyo, Japan), and A. rufa (diploid, one accession from Okinawa, Japan). Other experimental procedures were the same as in the experiments described above.
Seed germination in vitroThe seeds obtained from mature fruits were sterilized with sodium hypochlorite solution (1% active chlorine) with two drops of polyoxyethylene sorbitan monolaurate (Tween 20) for 10 min, rinsed 3 times in sterilized distilled water, and then sown in vitro on half-strength MS medium (Murashige and Skoog, 1962) after incubation at approximately 4°C in a refrigerator for three months. The cultures were incubated at 22°C under continuous light (35 μmol·m−2·s−1), and the germination rate was recorded after they had been cultured for two months.
Embryo cultureOur preliminary study indicated that most of the seeds obtained from the A. kolomikta × A. deliciosa and A. kolomikta × A. arguta crosses were empty. A similar result for the A. kolomikta × A. deliciosa cross was reported by Hirsch et al. (2001), who obtained hybrid plants of A. kolomikta × A. deliciosa via an embryo rescue technique. Therefore, embryos were only selected aseptically and cultured in vitro on half-strength MS medium after confirming their presence by dissecting the seeds from these two crosses under a dissection microscope. The cultures were incubated at 22°C under continuous light (35 μmol·m−2·s−1), and the number of embryos that grew into plantlets was recorded after they had been cultured for two months.
Observation of the young plants obtainedAn acclimatization process was initiated approximately one to two months after germination. The young plants, after removing the culture medium from the roots using tap water, were transplanted into peat pellets (Jiffy-7®; Sakata Seed Co., Yokohama, Japan) and grown in an incubator at 22°C under continuous light (35 μmol·m−2·s−1) for approximately one month before they were transferred to a greenhouse. After acclimatization, plant, leaf and stem morphological characteristics were observed.
Estimation of ploidy levels by flow cytometric analysis in young plants obtained from the interspecific crossesFlow cytometric analysis was used to estimate the ploidy level in the plants obtained from the interspecific crosses. Leaves were collected from the shoot tips and prepared for flow cytometric analysis using the protocol described by Asakura and Hoshino (2016). Nuclear samples were extracted from the leaves and stained with 4',6-diamidino-2-phenylindole (DAPI). The nuclear fluorescence was then measured using a ploidy analyzer (Partec PA; Partec, Münster, Germany). Leaves from the diploid A. kolomikta and hexaploid A. deliciosa plants were used as internal controls when the plants obtained from the cross between diploid A. kolomikta and hexaploid A. deliciosa were analyzed. However, leaves from the tetraploid A. arguta were used as internal controls when the plants obtained from other cross combinations were analyzed. More than 5000 cells were analyzed.
In total, 15 flowers from the A. arguta female plant and 25 flowers from the A. polygama female plant were pollinated with A. kolomikta pollen. In these crosses, all the ovaries had fallen off within two weeks after full anthesis, and no seeds were obtained when A. kolomikta was used as the male parent (Table 1). In the control experiment, A. arguta and A. polygama plants that had not been pollinated did not set fruit.

Crossing experiment using A. kolomikta as a male parent.
In the control treatment (no pollination), all the ovaries had fallen off within two weeks after full anthesis, and no seeds were obtained (Table 2). In contrast, the five crosses tested using A. kolomikta as the female parent, as well as open pollination treatment, showed 100% of fruit set two months after anthesis (Table 2). In the open pollination treatment, the number of mature seeds per fruit was 101.8. Most of the seeds from the crosses with different ploidy levels, such as hexaploid A. deliciosa or tetraploid A. arguta, did not contain embryos or endosperm. Therefore, these empty seeds were discarded and excluded from the seed count. However, at the end of the experiment a few mature seeds containing embryos and endosperm were obtained (13 and 21, respectively). Consequently, the numbers of mature seeds per fruit were low (0.9 and 1.4, respectively) compared to those observed in the open pollination treatment (Table 2). In contrast, the crosses with diploid species, such as A. arguta var. hypoleuca, A. polygama, and A. rufa, produced many mature seeds (1519, 551, and 492, respectively), and the number of mature seeds per fruit was comparable to that observed in the open pollination treatment (101.3, 91.8, and 98.4, respectively).
Crossing experiment using A. kolomikta as a female parent.
Seed germination was observed in the crosses with diploid A. arguta var. hypoleuca and A. polygama, but the germination frequency was relatively low (8.5 and 9.8%, respectively, Table 3) compared to that observed in the open pollination treatment (48.9%). The cross with diploid A. rufa led to a seed germination frequency of 45.5%, comparable to that observed in the open pollination treatment.
Germination of seeds obtained from interspecific crosses.
Only one embryo grew into a plantlet from the 13 cultured embryos obtained from the cross with hexaploid A. deliciosa (Table 4). No plantlets were obtained from the 21 cultured embryos obtained from the cross with tetraploid A. arguta.
Culturing of the embryos obtained from interspecific crosses.
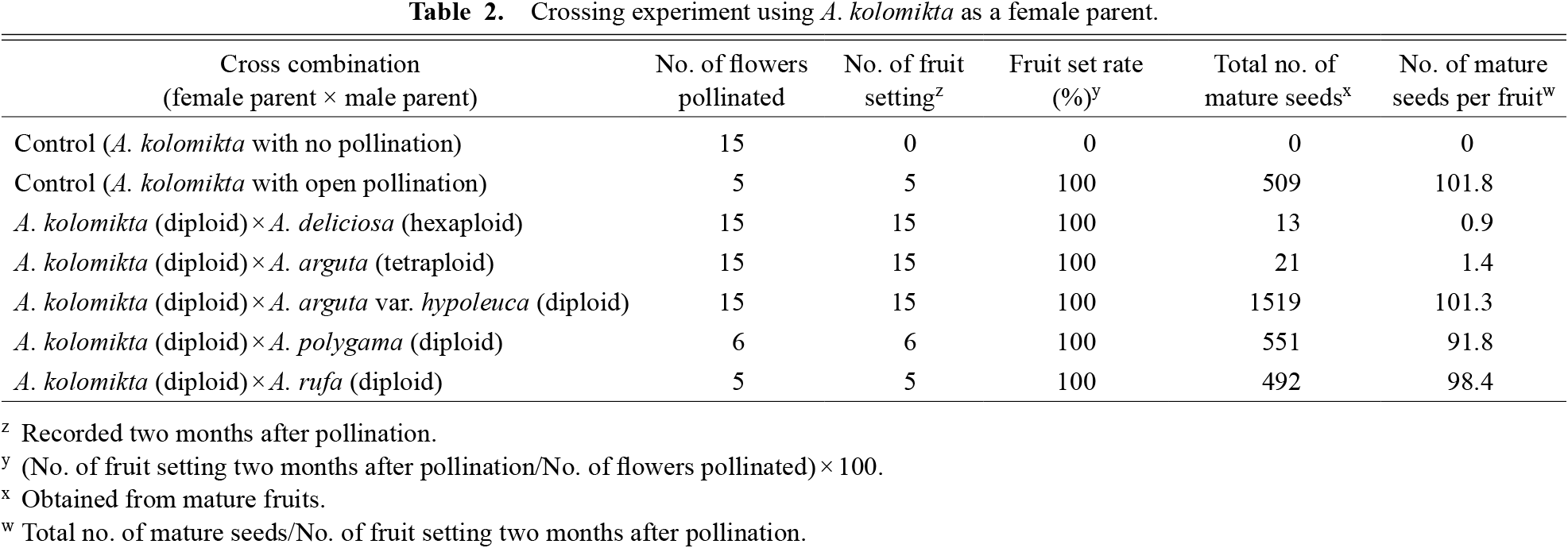
A tiny, weak seedling was obtained by culturing the embryo obtained from the cross between diploid A. kolomikta and hexaploid A. deliciosa (Fig. 1). The seedling died one month after acclimatization. Other plants obtained from the crosses with A. arguta var. hypoleuca (Figs. 2A–2C), A. polygama (Figs. 2D–2F), and A. rufa (Figs. 2G–2I) showed leaf and stem morphological characteristics that were mixed or intermediate between the parents. These plants showed relatively vigorous growth, especially those obtained from the cross with A. rufa.

An anomalous plantlet obtained by culturing the embryo obtained from an interspecific cross between A. kolomikta and A. deliciosa on half-strength MS medium. The plantlet was weak and died one month after acclimatization. Bar = 5 mm.
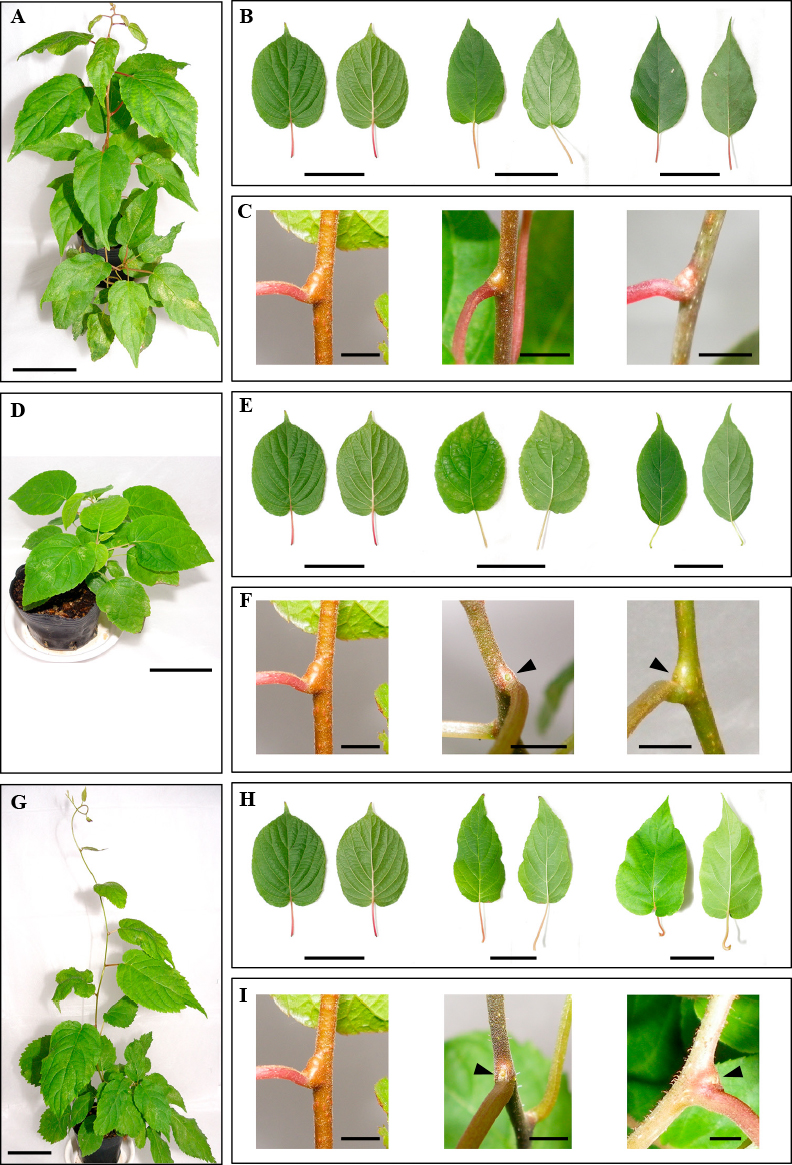
Observations of young plants obtained from interspecific crosses between A. kolomikta and other native Actinidia species grown in Japan. (A) A young plant obtained from an interspecific cross between A. kolomikta and A. arguta var. hypoleuca; (B) young plant leaf comparisons with the parent species. Left: A. kolomikta. Middle: a young plant leaf obtained from the interspecific cross. Right: A. arguta var. hypoleuca; (C) young plant stem comparisons with the parent species. Left: A. kolomikta. Middle: a young plant obtained from the interspecific cross. Right: A. arguta var. hypoleuca; (D) a young plant obtained from the interspecific cross between A. kolomikta and A. polygama; (E) young plant leaf comparisons with the parent species. Left: A. kolomikta. Middle: a plant leaf from the interspecific cross. Right: A. polygama; (F) young plant stem comparisons with the parent species. Left: A. kolomikta. Middle: a young plant obtained from the interspecific cross. The dormant buds are semi-concealed in the pulvini (arrowhead) as in A. polygama. Right: A. polygama. The dormant buds are semi-concealed in the pulvini (arrowhead); (G) a young plant obtained from interspecific cross between A. kolomikta and A. rufa; (H) young plant leaf comparisons with the parent species. Left: A. kolomikta. Middle: a young plant obtained from the interspecific cross. Right: A. rufa; (I) young plant stem comparisons with the parent species. Left: A. kolomikta. The young shoot is generally glabrous, and dormant buds are concealed in the pulvini. Middle: a young plant obtained from the interspecific cross. The young shoot is sparsely hairy, and dormant buds are semi-concealed (arrowhead) as in A. rufa. Right: A. rufa. The young shoot is generally hairy and dormant buds are semi-concealed in the pulvini (arrowhead); Bar in panels (A), (B), (D), (E), (G), and (H) = 5 cm; Bar in panels (C), (F), and (I) = 5 mm.
Flow cytometric analysis revealed that the fluorescence peak of the seedling plant obtained from the cross between diploid A. kolomikta and hexaploid A. deliciosa did not fit the estimated fluorescence intensity of allotetraploidy in a hybrid plant, and it was assumed to be aneuploidy (Fig. 3A; Table 5). Other young plants obtained from the crosses with diploid A. arguta var. hypoleuca, A. polygama, and A. rufa were estimated to be amphihaploid (Figs. 3B, 3C, and 3D, respectively; Table 5).
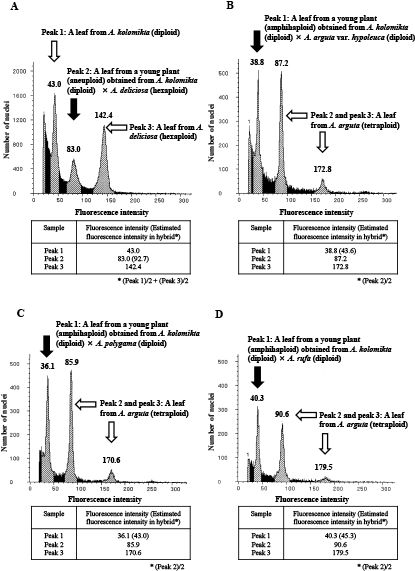
Estimation of ploidy levels by flow cytometry in young plants obtained from interspecific crosses between A. kolomikta and other native or cultivated species grown in Japan. Relative fluorescence intensities of DAPI-stained nuclei were measured. Values above the peaks show mean fluorescence intensities. (A) results for a young plant obtained from the interspecific cross between A. kolomikta and A. deliciosa. The white arrows indicate the leaves from diploid A. kolomikta and hexaploid A. deliciosa, respectively, which acted as internal controls; the black arrow indicate a leaf from an aneuploid young plant obtained from the interspecific cross between A. kolomikta and A. deliciosa. (B) results for a young plant obtained from the interspecific cross between diploid A. kolomikta and diploid A. arguta var. hypoleuca. The white arrows indicate the tetraploid A. arguta leaves, which were used as an internal control; peak 3 indicates G2 cells; the black arrow indicates a leaf from an amphihaploid young plant obtained from the interspecific cross between A. kolomikta and A. arguta var. hypoleuca. (C) results for a young plant obtained from the interspecific cross between diploid A. kolomikta and diploid A. polygama. The white arrows indicate a leaf from tetraploid A. arguta, which was used as an internal control; peak 3 indicates G2 cells; the black arrow indicates a leaf from an amphihaploid young plant obtained from the interspecific cross between A. kolomikta and A. polygama. (D) results for a young plant obtained from the interspecific cross between diploid A. kolomikta and diploid A. rufa. The white arrows indicate a leaf of tetraploid A. arguta, which was used as an internal control; peak 3 indicates G2 cells; the black arrow indicates a leaf of an amphihaploid young plant obtained from the interspecific cross between A. kolomikta and A. rufa. More than 5000 cells were analyzed for each measurement.
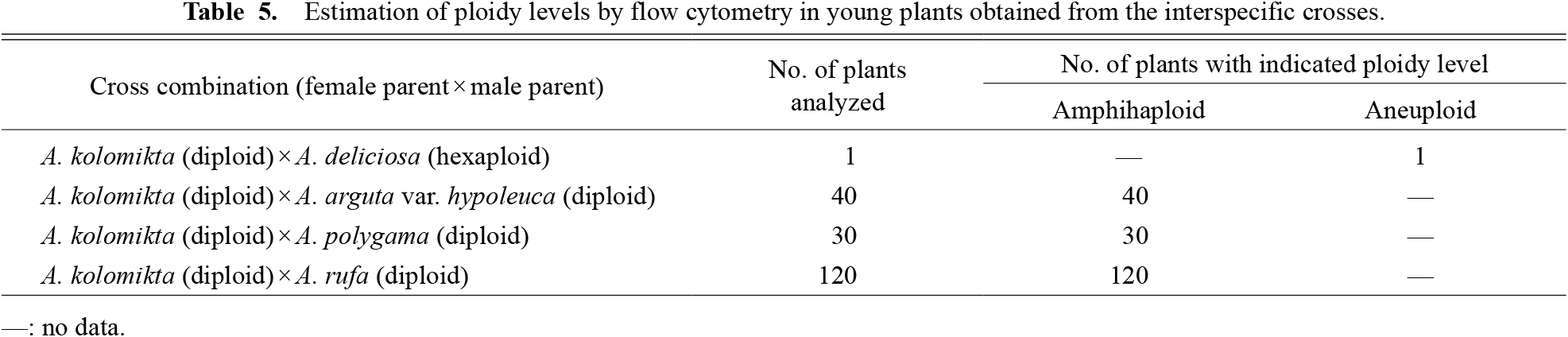
Estimation of ploidy levels by flow cytometry in young plants obtained from the interspecific crosses.
We evaluated the interspecific cross compatibility of the Japanese indigenous Actinidia species, A. kolomikta, with other native or cultivated species in Japan. In the tested crosses using A. kolomikta as the male parent (A. arguta × A. kolomikta and A. polygama × A. kolomikta) all the ovaries had fallen off within two weeks after pollination. Similar results were recorded for the control treatments (no pollination). This suggested that normal fertilization processes did not occur in these crosses. For example, pollen germination did not take place, there was insufficient fertilization by growing pollen tubes, and abortion of hybrid embryos occurred during the early stages of development after fertilization. However, further histological approaches are needed to clarify these results. In contrast, when A. kolomikta was used as the female parent, fruit setting reached 100% in all the cross combinations tested, which suggested that A. kolomikta could be highly receptive to the pollen of other species. The control treatment (A. kolomikta with no pollination) results indicated that the A. kolomikta accession used in this study is not parthenocarpic.
There was a considerable difference in the final number of viable seeds obtained from the A. kolomikta cross with tetraploid A. arguta and the cross with A. arguta var. hypoleuca, a diploid variety of A. arguta endemic to warm temperate regions in Japan. Most of the seeds obtained in the cross with tetraploid A. arguta did not appear to contain embryos or endosperm, and few viable seeds were obtained. However, we collected many viable seeds from the cross with diploid A. arguta var. hypoleuca. These results, together with the results of the cross experiment with hexaploid A. deliciosa, support the hypothesis that different ploidy levels in the parents could be one of the factors that lead to the failure of interspecific hybridization in the genus Actinidia (Ferguson et al., 1997). In Actinidia, as in many other genera, crosses between different ploidy levels often lead to fewer viable plantlets than crosses using species with the same ploidy level (Hirsch et al., 2001). In commercially grown kiwifruit, ploidy varies from diploid and tetraploid in A. chinensis to hexaploid in A. deliciosa. In this study, we did not use A. deliciosa and A. chinensis as a seed parent because these plant materials could not be prepared for pollination experiments. The hybridization between A. kolomikta and kiwifruit (A. deliciosa or A. chinensis) has been reported in previous studies (Chen et al., 2006; Guthrie et al., 2007; Hirsch et al., 2001; Xiao et al., 2004). Hirsch et al. (2001) obtained hybrid plants by an embryo rescue technique in the cross combinations of A. kolomikta × A. chinensis (diploid) and A. kolomikta (diploid) × A. deliciosa (hexaploid). Hybrid plants between A. kolomikta and A. chinensis were obtained by ovule culture (Chen et al., 2006) and protoplast fusion (Xiao et al., 2004). Guthrie et al. (2007) obtained seeds from A. chinensis × A. kolomikta, A. kolomikta × A. chinensis, and A. kolomikta × A. deliciosa. However, there is no description of A. deliciosa as a seed parent in these previous studies. Thus, cross compatibility between A. kolomikta and kiwifruit species has been confirmed. Since A. chinensis has good fruit quality, and cultivation is also expanding, future breeding programs using hybridization between A. kolomikta and A. chinensis may be of interest. To facilitate the hybridization of A. kolomikta with other species, including kiwifruit with different ploidy levels such as hexaploid A. deliciosa, other approaches have been tested. These involved producing various ploidy series in A. kolomikta (Asakura and Hoshino, 2017a, b).
Compared with the control treatment (open pollination), the seed germination rate was relatively low in the cross with A. arguta var. hypoleuca and A. polygama, although there was no marked difference in the number of mature seeds per fruit. The mature seeds obtained from these two interspecific crosses were not different in appearance from those obtained from the control treatment (open pollination) in terms of size, shape, and surface texture. However, in these two crosses, there is a possibility that some kind of incongruity resulting from the interspecific cross such as abortion or insufficient growth of hybrid embryos occurred during the process of development after fertilization. In contrast, fairly good results were obtained in the cross between A. kolomikta and A. rufa in terms of fruit setting, the number of viable seeds finally obtained, and seed germination, all of which were almost comparable to the control treatment (open pollination), and many vigorous young plants were obtained. There has been controversy regarding the A. kolomikta and A. rufa phylogenetic relationships in the genus (Ferguson and Huang, 2007). Actinidia rufa was once thought to be related to A. arguta and was even treated as a variety of A. arguta in the section Leiocarpae, to which A. kolomikta and A. polygama were also placed (Li, 1952). However, the vegetative morphology, flowers, and fruit characteristics of A. rufa are quite distinct from A. arguta. Recent reports describing flavonoid composition (Webby et al., 1994), isozyme patterns (Testolin and Ferguson, 1997), chloroplast and ribosomal DNA sequences (Chat et al., 2004; Cipriani et al., 1998; Li et al., 2002), and Random Amplified Polymorphic DNA (RAPD) (Huang et al., 2002; Kokudo et al., 2003) analyses support the exclusion of A. rufa from the section Leiocarpae. Analyses of the isozyme patterns (Testolin and Ferguson, 1997) and SSR (simple sequence repeat) molecular markers (Korkovelos et al., 2008) suggest that there is a close relationship between A. rufa and A. hemsleyana in the section Strigosae. In addition, chloroplast and ribosomal DNA sequences (Chat et al., 2004; Li et al., 2002), and RAPD analyses (Huang et al., 2002) also suggest a close relationship between A. rufa and A. hemsleyana in the section Strigosae, and between A. persicina and A. zhejiangensis in the section Stellatae. In contrast, Jung et al. (2003) placed A. rufa close to A. kolomikta and A. melanandra in the section Leiocarpae because of similarities in the noncoding chloroplast DNA sequences. Recent research based on A. kolomikta, leaf flavonoid composition (Webby et al., 1994) and isozyme patterns (Testolin and Ferguson, 1997) indicated that it was quite distinct from any other species in the section Leiocarpae, although RAPD analysis supports retaining A. kolomikta in Leiocarpae (Huang et al., 2002). The results of the crossing experiments undertaken in this study suggest that there is a potentially close relationship between A. kolomikta and A. rufa, although it has been shown that many Actinidia species can be crossed under experimental conditions (Hirsch et al., 2001). Actinidia rufa is a very productive species (Ferguson and Seal, 2008). Therefore, the vigorous young plants obtained from the cross with A. rufa could improve productivity to a greater extent than A. kolomikta.
In conclusion, the interspecific cross compatibility of A. kolomikta with other native or cultivated species in Japan was categorized as follows: no fruit setting (A. arguta × A. kolomikta and A. polygama × A. kolomikta); fruit setting with few viable seeds (A. kolomikta × A. arguta and A. kolomikta × A. deliciosa); and normal fruit setting with viable seeds (A. kolomikta × A. arguta var. hypoleuca, A. kolomikta × A. polygama, and A. kolomikta × A. rufa). These results could inform future crop breeding programs using A. kolomikta and interspecific hybridization. Further studies are needed to confirm the hybridity of the young plants obtained in this study using molecular markers. Other cross combinations, including reciprocal crosses not tested in this study, need to be investigated.