2018 年 87 巻 4 号 p. 549-556
2018 年 87 巻 4 号 p. 549-556
Information about mechanical reinforcement of decorative organs could help development of a novel technique that would give flowers with robust floral organs and broadly contribute to postharvest flower preservation. Hydrangeas (Hydrangea spp.) exhibit remarkable characteristics in terms of mechanical reinforcement of decorative sepals. Although decorative sepals at the flowering stage shrink when they are desiccated, decorative sepals after flowering maintain their shape even after desiccation. In this study, the lignifications of the vein cells in decorative sepals were analyzed using phloroglucinol/HCl-staining. The microstructure of the cell wall was analyzed using transmission electron microscopy (TEM). The three-dimensional structure of vein cells was analyzed using serial block-face scanning electron microscopy (SBF-SEM). Tubular- and spindle-shaped dead cells with a lignified pitted secondary cell wall were observed around the vessel elements in decorative sepals after flowering. These cells were observed as living cells without a secondary cell wall in the veins of decorative sepals at flowering and in fully expanded leaves. Further, 10 hydrangea cultivars were analyzed for development of mechanical reinforcement in vein cells, and some of them were compared by desiccation testing. Decorative sepals of a cultivar lacking those cells exhibited shrinkage after flowering when they were desiccated. In conclusion, dead cells with a lignified pitted secondary cell wall contribute to the reinforcement of veins in decorative sepals of hydrangeas and become sclerified parenchyma cells. Axial parenchyma sclerifying in veins after flowering is essential for robust hydrangea floral organs and represent a new type of mechanical reinforcement tissue in plant decorative floral organs.
Hydrangeas (Hydrangea spp.) are popular ornamental plants that exhibit remarkable characteristics in the floral organ architecture development after flowering (Kitamura et al., 2018; van Gelderen and van Gelderen, 2004). Hydrangeas have two types of florets: non-decorative florets, which bear tiny sepals, and decorative florets, which bear large decorative sepals (Uemachi and Nishio, 2005; Uemachi et al., 2006). The decorative sepals of hydrangeas exhibit a purple and/or pink color during flowering and change to green after flowering. They stay on the plant for several weeks after greening, and finally turn red (Fig. 1A). Although decorative sepals shrink before greening when they are desiccated, completely green-colored decorative sepals maintain their shape even after desiccation (Fig. 1B, C; Kitamura et al., 2018). In the flowering stage, the decorative sepals completely rot under periodic mist conditions, but the veins of completely green-colored decorative sepals remain under the same conditions (Fig. 1D), suggesting mechanical reinforcement. Several studies have been conducted on decorative organs maintained after flowering, and the three concerning hydrangeas include our previous studies (Kitamura et al., 2017, 2018; Melo et al., 1995; Salopek-Sondi et al., 2000, 2002; Schmitzer et al., 2013; Tavares et al., 1998; Yoshida et al., 2008). In these studies, photosynthesis, the relationship between decorative organ maintenance and fruit development, pigments, stomatal conductance, and vase life of cut flowers were all studied. However, none of them focused on mechanical reinforcement in maintained decorative organs.

Maturation stages and mechanical reinforcement in hydrangea decorative sepals. (A) Four stages of maturation in decorative sepals of hydrangeas. Stage 1, before greening of the decorative sepals at flowering; stage 2, initial greening of decorative sepals after flowering; stage 3, complete greening of decorative sepals; stage 4, complete red pigmentation after greening. (B) Desiccated decorative sepal at stage 1. (C) Desiccated decorative sepal at stage 3. (D) Remaining veins after rotting of a decorative sepal at stage 3 under periodic mist conditions. (B and C) After 6 days’ desiccation treatment. Scale bars = 1 cm.
The secondary cell wall consists of cellulose, hemicellulose, lignin, and other molecules and thickens on the inside of the primary cell wall (Campbell and Sederoff, 1996; Popper, 2008; Reiter, 2002). It is mainly the thickening in specialized parenchyma cells, sclerenchyma cells, and tracheary elements that is responsible for mechanical reinforcement in many kinds of plants (Raven et al., 2005; Reiter, 2002). On the other hand, most decorative organs consist of parenchyma cells and are soft and vulnerable, and the decorative sepals of hydrangeas before greening are no exception. Some examples of reinforced cells in floral organs have been reported in the literature. Nishikawa et al. (2008) reported that the scarious bract of Helichrysum bracteatum consists of dead cells with a secondary cell wall. Zhang et al. (2011, 2017) suggested that sclereids differentiate in the mesophyll, in which the secondary cell wall is thickened, and function as a mechanical reinforcement in Camellia petals during flowering. Taking into account our observations mentioned above (Fig. 1D), thickening of the secondary cell wall in vein cells may contribute to the mechanical reinforcement of the completely green-colored decorative sepals of hydrangeas. Though Nishikawa et al. (2008) and Zhang et al. (2011, 2017) reported that the cells contributing to mechanical reinforcement developed a secondary cell wall before flowering, the mechanical support that reinforces the decorative sepals of hydrangeas seems to develop after flowering. To the best of our knowledge, no research has been conducted on the development of mechanical reinforcement in decorative organs after flowering. Information about mechanical reinforcement of decorative organs could give direction for developing a novel technique to produce flowers with robust floral organs and could broadly contribute to postharvest flower preservation.
In the present study, we aimed to explain the mechanical reinforcement developed in decorative sepals of hydrangeas after flowering. The maturation stage of decorative sepals, in which reinforcement of the vein cells occurs, was studied. Lignification was studied using phloroglucinol/HCl-staining. The microstructure of the cell wall of vein cells was analyzed using transmission electron microscopy (TEM). Also, the three-dimensional structures of vein cells were investigated using serial block-face scanning electron microscopy (SBF-SEM; Denk and Horstmann, 2004). The differences in vein cells in various cultivars were also studied. For comparison, the same analyses were also conducted on vein cells of leaves. Finally, the tissues that contribute to the mechanical reinforcement of decorative sepals of hydrangeas after flowering are discussed.
Plants of Hydrangea spp. ‘Endless summer’, ‘Fairy Eye’, ‘Glowing Alps’, ‘Green Shadow’, ‘Grünherz’, ‘Jyogasaki’, ‘Magical Diamond’, ‘Masja’, ‘Renata’, ‘Sumida-no-Hanabi’, and ‘Xi’an’ were maintained as previously described (Kitamura et al., 2018). ‘Endless summer’, ‘Fairy Eye’, ‘Green Shadow’, ‘Jyogasaki’, ‘Sumida-no-Hanabi’, and ‘Xi’an’ were purchased from nurseries from 2009 to 2012. ‘Grünherz’, ‘Masja’, and ‘Renata’ were obtained as cuttings from the Kyoto University experimental farm in May 2010. ‘Glowing Alps’ and ‘Magical Diamond’ were obtained as cuttings from a farmer in 2010. Decorative florets with decorative sepals and leaves of these plants were used in the following analyses.
Maturation stages of decorative sepals and tissue samplingFour stages of hydrangea decorative sepal maturation were considered (Fig. 1A). Stage 1, complete cultivar-specific coloration of the decorative sepals at flowering; stage 2, initial greening of the decorative sepals after flowering; stage 3, complete greening of the decorative sepals; stage 4, complete bluish-red coloration of the decorative sepals. A razor blade was used to hand-section 5- and 2-mm square pieces of decorative sepal tissue, or of a fully expanded leaf containing the central vein, and sampled pieces of tissue were used for lignification analysis and electron microscopy, respectively (Fig. 2A, B).
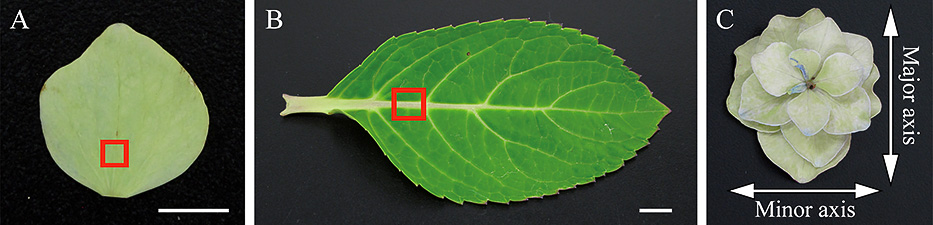
Sampling of tissue pieces of hydrangea decorative sepals and measurement of hydrangea decorative florets. (A and B) The position of the vein tissue pieces sampled for lignification analysis or electron microscopy is indicated by a red square. (C) Major and minor axes of a decorative floret measured for the desiccation test. Scale bars = 1 cm.
Lignification of the vein cells was visualized by phloroglucinol/HCl-staining (Weisner reaction; Gahan, 1984). Five-mm square tissue pieces sampled from decorative sepals or fully expanded leaves containing the central vein were sectioned at 80 μm thickness using a Plant Microtome (Nippon Medical & Chemical Instruments Co., Ltd., Osaka, Japan). The sections were stained for 10 min using phloroglucinol/HCl reagent, comprising 1% (w/v) phloroglucinol in 10.1 M hydrochloric acid-ethanol (25/75, v/v). Then, sections were covered with a coverslip, sealed with nail varnish and observed under a light microscope (BH2; Olympus, Tokyo, Japan).
Transmission electron microscopy (TEM)Two-mm square tissue pieces sampled from decorative sepals or fully expanded leaves containing the central vein were fixed in 3% glutaraldehyde in 0.05 mol·L−1 phosphate buffer (pH 7.4) overnight at 4°C. The tissue pieces were rinsed three times for 10 min in 0.05 mol·L−1 phosphate buffer. Then, they were post-fixed in 2% osmium tetroxide in 0.05 mol·L−1 phosphate buffer (Nisshin EM Co., Ltd., Tokyo, Japan) for 1 h at room temperature, and rinsed three times for 10 min in 0.05 mol·L−1 phosphate buffer. The tissue pieces were dehydrated by passing through a graded ethanol series: 30%, 50%, 70%, 80%, 90%, 95%, 95%, 95%, 100%, and 100% at 10 min for each step. The tissue pieces were then substituted with Plain Resin (Nisshin EM Co., Ltd., Tokyo, Japan) and QY-1 (Nisshin EM Co., Ltd., Tokyo, Japan) for 30 min (Plain Resin:QY-1 = 1:3), 1 h (Plain Resin:QY-1 = 1:1), and 2 h (Plain Resin:QY-1 = 3:1). Then, the tissue pieces were embedded in pure Plain Resin. Ultra-thin sections of the vein were cut using an ultramicrotome (Super Nova; Reichert-Jung, Vienna, Austria). These were stained with TI blue (Nisshin EM Co., Ltd., Tokyo, Japan) for 10 min and 2% (w/v) lead citrate for 5 min and observed under a transmission electron microscope (JEM 1400; JEOL, Tokyo, Japan).
Serial block-face scanning electron microscopy (SBF-SEM)The tissue pieces were fixed and embedded according to Nguyen et al. (2016). The observation position of the sample block was identified by serial block-face scanning electron microscopy (JSM-7800F; JEOL, Tokyo, Japan) at an acceleration voltage of 1 kV. A fresh surface for serial block-face imaging was exposed by an ultramicrotome incorporated in the SEM chamber. Serial block-face images were obtained with a backscattered electron detector at an acceleration voltage of 1 kV, and a working distance of 6 mm; 250 block-face images were obtained (field of view: 33.65×33.65 um2 or 59.83×59.83 um2, 100-nm interval). These images were converted to eight-bit grayscale tiff images (2048×2048 pixels) using an 8.0 μs dwell time (pixel size: 16.4 × 16.4 nm2 or 29.0 × 29.0 nm2). The contrast of the images was inverted. Three-dimensional reconstruction and segmentation of the vein cells was performed using 3D imaging software (Stack N Viz and COLORIST; SYSTEM IN FRONTIER Inc., Tokyo, Japan). The software was used to perform three-dimensional reconstruction from two-dimensional SBF-SEM datasets, which allows reconstructed image evaluation from various angles.
Desiccation testing of decorative florets with decorative sepalsIn order to evaluate the mechanical strength of the decorative sepals, decorative florets were subjected to a desiccation test. In the test, the major and minor axes of the decorative florets were measured just after harvesting (Fig. 2C), after which the decorative florets were desiccated in a room maintained at 25 ± 2°C and 50 ± 5% relative humidity. After a 6-day desiccation, the major and minor axes of the florets were measured again. The percentage decrease in the major and minor axes of decorative florets after desiccation compared to the sizes at harvest was calculated as an index for the shrinkage of the decorative sepals.
Maturation stage of decorative sepals in which vein cell reinforcement occursDecorative sepals of the cultivar ‘Endless Summer’ were analyzed at all four stages to determine the maturation stage in which vein cell reinforcement occurs (Fig. 1A). A fully expanded leaf was also analyzed for comparison (Fig. 2B). Lignification analysis and TEM analysis were performed on the samples. Phloroglucinol/HCl-stained cells were counted in 3–5 decorative sepals at each maturation stage while distinguishing between vessel elements and other cell types. The mean number of stained cells was analyzed by Tukey’s test. Decorative florets with decorative sepals at stages 1 and 2 were sampled during July 2015 for TEM analysis and were sampled for phloroglucinol/HCl-staining in July 2017. Those at stage 3 were sampled in August 2015 for TEM analysis and were sampled for phloroglucinol/HCl-staining in August 2017. Those at stage 4 were sampled in September 2015 for TEM analysis and were sampled for phloroglucinol/HCl-staining in September 2017. Leaves were sampled in August 2015 for TEM analysis and were sampled for phloroglucinol/HCl-staining in September 2017.
Three-dimensional structure of vein cellsDecorative sepals of ‘Endless Summer’ at stages 1 and 3 were analyzed using SBF-SEM, and the three-dimensional structures of vein cells were determined. A fully expanded leaf was also analyzed for comparison. Leaves and decorative florets with decorative sepals at stages 1 and 3 were sampled in August 2015.
Cultivar differences in vein cell reinforcementDecorative sepals of the cultivars ‘Glowing Alps’, ‘Green Shadow’, ‘Grünherz’, ‘Jyogasaki’, ‘Magical Diamond’, ‘Masja’, ‘Renata’, and ‘Xi’an’ at stages 1 and 3 were analyzed for differences in vein cell lignification (Fig. 3A–P). Leaves of those cultivars were also investigated for comparison. Decorative sepals of ‘Jyogasaki’ at stage 4 and those of ‘Fairy Eye’ and ‘Sumida-no-Hanabi’ at stage 3 were additionally investigated for lignification (Fig. 3Q–S). One decorative sepal was sampled randomly from 5 independent inflorescences for each cultivar or stage, and 5 sampled decorative sepals were used for the investigation. Five decorative florets with stage-3 decorative sepals of ‘Jyogasaki’ and ‘Fairy Eye’ were also used for a desiccation test and were compared in terms of the changes in shapes of florets. Decorative florets with decorative sepals at stage 1 were sampled in July 2017. Those at stage 3 were sampled in August 2017. Those at stage 4 were sampled in September 2017. Leaves were sampled in August 2017.

Hydrangea cultivars analyzed for differences in the vein cells of decorative sepals. (A and B) ‘Glowing Alps’; (C and D) ‘Green Shadow’; (E and F) ‘Grünherz’; (G and H) ‘Jyogasaki’; (I and J) ‘Magical Diamond’; (K and L) ‘Masja’; (M and N) ‘Renata’; (O and P) ‘Xi’an’; (A, C, E, G, I, K, M, and O) decorative florets with stage-1 decorative sepals; (B, D, F, H, J, L, N, and P) decorative florets with stage-3 decorative sepals; (Q) decorative floret of ‘Jyogasaki’ with stage-4 decorative sepals; (R and S) decorative florets of ‘Fairy Eye’ and ‘Sumida-no-Hanabi’ with stage-3 decorative sepals. Scale bars = 1 cm.
Numbers of phloroglucinol/HCl-stained cells differed among stages (Fig. 4). Many phloroglucinol/HCl-stained cells were observed among the vein cells of decorative sepals after stage 2 (Fig. 4B–D, F), and their numbers outside vessel elements increased significantly during the maturation of decorative sepals (ANOVA, F3,11 = 3.59, P < 0.001, Table 1). The number of vessel elements was almost identical among all stages. Only vessel elements were phloroglucinol/HCl-stained in decorative sepals at stage 1 and leaves (Fig. 4A, E). In the TEM images of vein cells at stages 2, 3, and 4, flattened and pitted secondary cell wall thickening was observed on the inside of the primary cell walls of the cells surrounding the vessel elements (Fig. 4H–J, L). They were living cells in stage 2, but were dead in stages 3 and 4. The observed pits had overarching walls that formed a bowl-shaped chamber and had the pit membrane formed from the original primary walls and intervening middle lamella, thus bordering pits (Fig. 4J). Several pit pairs were observed in stages 2, 3, and 4 (Fig. 4H–J). Secondary cell wall thickening was observed only in vessel elements in decorative sepals at stage 1 and in fully expanded leaves (Fig. 4G, K). In these samples, the cells surrounding the vessel elements were observed as living cells without a secondary cell wall, and some of them in the leaves contained starch particles (Fig. 4K).

Development of the vein cells in hydrangea decorative sepals at different maturation stages. (A–F) Light micrographs in a transverse vein section (adaxial uppermost) with phloroglucinol/HCl-stain: (A) stage-1 decorative sepal; (B) stage-2 decorative sepal; (C) stage-3 decorative sepal; (D) stage-4 decorative sepal; (E) leaf; (F) stage-3 decorative sepal, corresponding to the area indicated by the square in (C). (G–L) TEM images of vein cell transverse sections: (G) stage-1 decorative sepal; (H) stage-2 decorative sepal; (I) stage-3 decorative sepal; (J) stage-4 decorative sepal; (K) leaf; (L) a vein cell with secondary cell walls of a stage-2 decorative sepal. Abbreviations: CS, cell with secondary cell wall excluding vessel element; CYT, cytoplasm; PW, primary cell wall; SP, starch particle; SW, secondary cell wall; V, vacuole, VE, vessel element; arrowhead, middle lamella (intercellular layer); arrow, bordered pit. Scale bars: (A–D) = 50 μm; E = 100 μm; F = 25 μm; (G–L) = 5 μm.

Number of phloroglucinol/HCl-stained cells in veins of hydrangea decorative sepals at four different maturation stages.
The block-face images from SBF-SEM were identical to the TEM images of each sample (Fig. 5). A series of block-face images indicated that the cells surrounding the vessel elements were spindle-shaped in decorative sepals (Fig. 5A–P). Reconstructed three-dimensional images of the cells surrounding vessel elements in stages 1 and 3 also indicated that the cells were tubular- and spindle-shaped and that the stages differed in the thickening of their secondary cell walls inside the primary cell wall observed in stage 3 (Fig. 6A, B). The bordered pits on the secondary cell walls observed in the TEM images of decorative sepals after stage 2 could also be observed in the reconstructed three-dimensional images of vein cells in stage-3 decorative sepals and were arranged in rows (Fig. 6B). The reconstructed three-dimensional images of the cells surrounding the vessel elements in the leaves indicated that these cells had a primary cell wall and were tubular-shaped with flat ends (Fig. 6C).
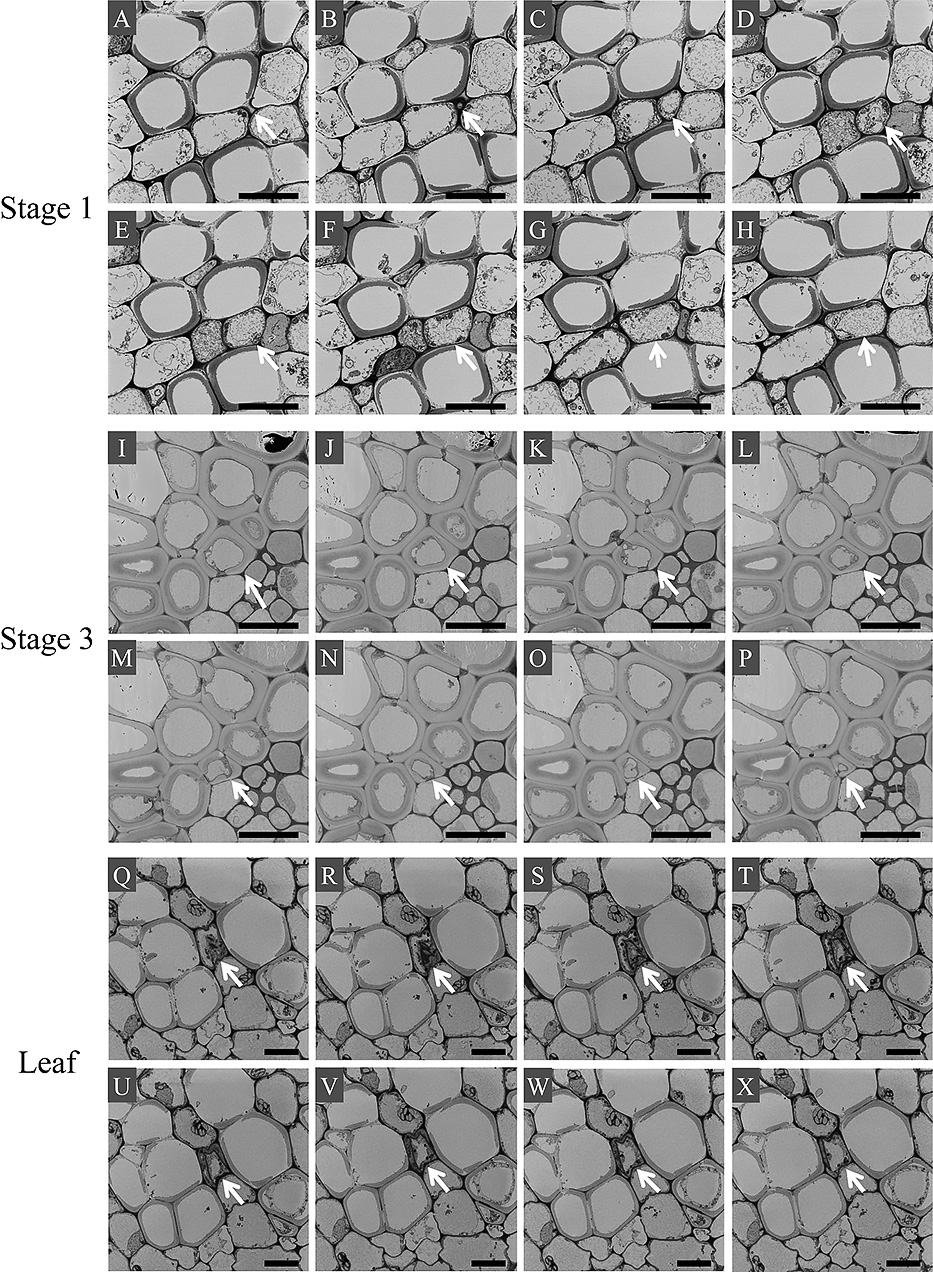
Serial block-face images of vein cells in hydrangea decorative sepals taken using SBF-SEM. (A–H) Vein cells of a stage-1 decorative sepal; (I–P) vein cells of a stage-3 decorative sepal; (Q–X) vein cells of a leaf. White arrow in each image indicates where a cell starts or ends. Images were taken at 2000 nm (A–H and I–P) or 700 nm (Q–X) intervals. Scale bars = 10 μm.

Reconstructed three-dimensional images of the cells surrounding vessel elements in hydrangea decorative sepals. (A) Vein cells of a stage-1 decorative sepal; (B) vein cells of a stage-3 decorative sepal; (C) vein cells of a leaf. Arrowhead denotes cell ends and arrow denotes a bordered pit. Pixels that had a brightness range similar to cell walls were extracted by software, and some cell contents are drawn in the figures.
In all studied cultivars except for ‘Jyogasaki’, all studied decorative sepals at stage 3 had phloroglucinol/HCl-stained cells around the vessel elements (Fig. 7). In all decorative sepals of ‘Jyogasaki’ at stage 3, all decorative sepals of all cultivars at stage 1, and all leaves, only vessel elements were stained by phloroglucinol/HCl except for leaves of ‘Glowing Alps’ and ‘Magical Diamond’. Leaf veins of ‘Glowing Alps’ and ‘Magical Diamond’ had phloroglucinol/HCl-stained cells other than the vessel elements, and these were observed on the adaxial side of vessel elements (Fig. 7Q, U). Since the maturation stage of decorative sepals was a possible factor in the unique result of ‘Jyogasaki’ at stage 3, 5 decorative sepals at stage 4 were additionally studied for the stainability of vein cells by phloroglucinol/HCl. However, we rarely observed any phloroglucinol/HCl-stained cells around the vessel elements (Fig. 8A). Also, ‘Jyogasaki’ was the only double-flowered cultivar among the cultivars studied, and it was possible that double-flowered cultivars had no mechanical reinforcement in their decorative sepals even after stage 3. Thus, using two double-flowered cultivars, ‘Fairy Eye’ and ‘Sumida-no-Hanabi’, decorative sepals at stage 3 were additionally investigated for stainability of their vein cells with phloroglucinol/HCl. In these samples, phloroglucinol/HCl-stained cells were observed as in stage-3 decorative sepals from single-flowered cultivars (Fig. 8B, C).

Difference in phloroglucinol/HCl-staining of transverse vein sections of different hydrangea cultivars (adaxial uppermost). (A–H) Stage-1 decorative sepals; (I–P) stage-3 decorative sepals; (Q–X) leaves. (A, I, and Q) ‘Glowing Alps’; (B, J, and R) ‘Green Shadow’; (C, K, and S) ‘Grünherz’; (D, L, and T) ‘Jyogasaki’; (E, M, and U) ‘Magical Diamond’; (F, N, and V) ‘Masja’; (G, O, and W) ‘Renata’; (H, P, and X) ‘Xi’an’. Yellow arrows in (Q) and (U) indicate phloroglucinol/HCl-stained cells observed in leaves. Each photo indicates a representative of 5 decorative sepals studied for each cultivar. Scale bars: (A–P) = 50 μm; (Q–X) = 100 μm.

Light micrographs in a transverse section of a phloroglucinol/HCl-stained vein (adaxial uppermost) sampled from decorative sepals of double-flowered hydrangea cultivars. (A) Stage-4 decorative sepal of ‘Jyogasaki’; (B and C) stage-3 decorative sepals of ‘Fairy Eye’ and ‘Sumida-no-Hanabi’. Each photo indicates a representative of 5 decorative sepals studied for each cultivar. Scale bars = 50 μm.
The size of the major and minor axes of desiccated ‘Fairy Eye’ decorative florets decreased to approximately 97.0% of those at harvest, whereas those of ‘Jyogasaki’ were approximately 75.1% and 66.8%, respectively (Table 2). The difference between the two cultivars was significant (t = 6.61, df = 4, P = 0.003 for major axes, t = 15.58, df = 4, P = 9.89E-05 for minor axes). Decorative florets of ‘Fairy Eye’ rarely showed shrinkage, even after complete desiccation, but for those of ‘Jyogasaki’, shrinkage was apparent under the same condition (Fig. 9).
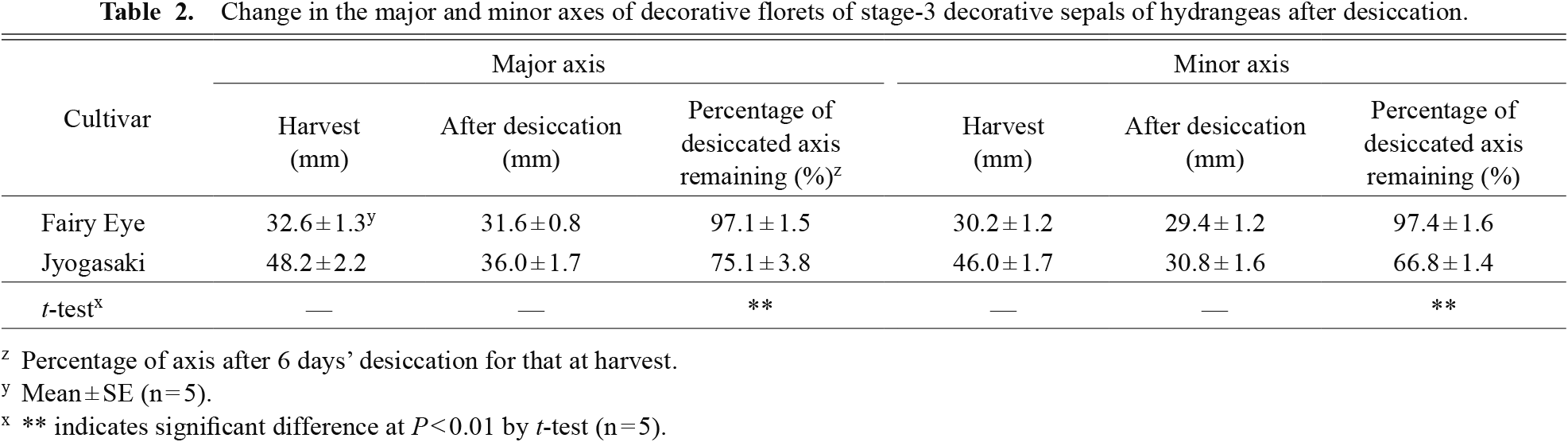
Change in the major and minor axes of decorative florets of stage-3 decorative sepals of hydrangeas after desiccation.
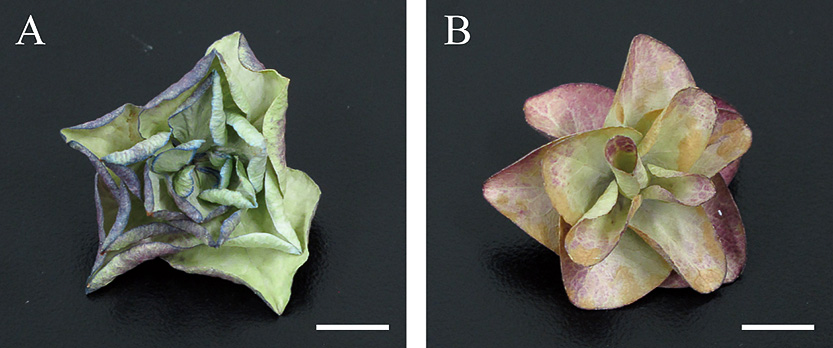
Desiccated hydrangea decorative florets of stage-3 decorative sepals. (A) ‘Jyogasaki’; (B) ‘Fairy Eye’. After 6 days’ desiccation. Scale bars = 1 cm.
Phloroglucinol/HCl (Weisner’s reagent) stains lignified cell walls red, so red staining of the cells surrounding the vessel elements indicated that the cell walls were highly lignified in the cells of decorative sepals of hydrangeas after stage 2 (Fig. 4B–D, F). Our results indicated that the lignification of those cells started just after flowering was completed before stage 3 (Table 1). We previously observed that decorative sepals of hydrangeas strengthened after stage 3 (Fig. 1C; Kitamura et al., 2018), and the present results were consistent with that observation. Since the number of stained vessel elements did not differ much between the studied maturation stages, this aspect of the differentiation in decorative sepals of hydrangeas seemed to be completed before stage 1 (Table 1).
Since the secondary cell walls contain a large amount of lignin (Evert, 2006), the strong stainability of the cells around the vessel elements with phloroglucinol/HCl is likely due to the development of secondary cell walls in those cells (Fig. 4). The observed characteristics of living cells without a secondary cell wall surrounding vessel elements in the leaves and stage-1 decorative sepals indicated that the cells were axial parenchyma cells, a type of xylem parenchyma cell (Figs. 4K, 5Q–X, and 6C; Carlquist, 2001). The observation of vein cells of stages 1–3 strongly suggested that the axial parenchyma cells observed in stage 1 developed secondary cell walls in stage 2 and finally maturated to dead cells in stage 3 (Fig. 4, Table 1). The characteristics of those maturated dead cells, bearing a flattened secondary cell wall with bordered pits, and their positional relationship to the vessel elements in stages-3 and -4 decorative sepals indicated that the cells were sclerified parenchyma cells. Sclerified parenchyma cells have a pitted secondary cell wall on the inside of the primary cell wall and are often dead at maturation (Evert, 2006; Trigiano and Gray, 2004). Additionally, the sclerified parenchyma cells generally die after the cell death of vessel elements. For example, sclerified parenchyma cells die in the course of heartwood formation in many woods, and this occurs several months or years after the death of the vessel elements (Holbrook and Zwieniecki, 2005). In the decorative sepals of hydrangeas, the differentiation by dying of cells with a secondary cell wall surrounding the vessel elements occurred after the death of the vessel elements (Table 1). Since sclerified parenchyma cells are generally contained in wood and contribute to the mechanical strength of the tissue, reinforcement of the decorative sepals after flowering is likely due to the differentiation of axial parenchyma cells in the veins to sclerified parenchyma cells. As stated above, differentiation of the vessel elements is completed before stage 1, so vessel elements contribute little to the development of mechanical reinforcement in decorative sepals after flowering.
In our previous study, transpiration from completely green-colored decorative sepals increased in the light period and decreased in the dark period (Kitamura et al., 2017), suggesting that the physiological state of completely green-colored decorative sepals was similar to that of the leaves. Thus, we hypothesized that the reinforcement of the vein cells occurring in the decorative sepals of hydrangeas could also be observed in leaf veins. However, except for vessel elements, lignified cells were rarely observed in the veins of leaves in most hydrangea cultivars studied (Figs. 4–7). Thus, among the organs originating from leaves, sclerified parenchyma differentiation specifically occurs in the veins of decorative sepals after flowering, and this may be a characteristic of hydrangeas.
An example of reinforced cells in decorative floral organs was given by Nishikawa et al. (2008); they reported that the scarious bracts of Helichrysum bracteatum consisted of dead cells with a secondary cell wall. However, those cells with secondary cell walls were not sclerified parenchyma. Moreover, the scarious bracts of H. bracteatum were composed of dead cells, whereas the decorative sepals of hydrangeas after flowering were composed of living cells except for the sclerified parenchyma and vessel elements. Mechanical reinforcement in floral organs composed of living cells was reported for Camellia petals (Zhang et al., 2011, 2017). The report suggested that sclereids in the mesophyll functioned as a mechanical reinforcement in Camellia petals during flowering. However, the sclereids reported in their study differed from the sclerified parenchyma observed in the present study in their location. Hence, the differentiation of sclerified parenchyma in the decorative sepals of hydrangeas may be a new type of mechanical reinforcement of decorative floral organs.
Analysis for the difference between cultivars revealed that the double-flowered hydrangea cultivar ‘Jyogasaki’ was a unique cultivar in which sclerified parenchyma rarely differentiated in the veins of decorative sepals, even after stage 3. Additional investigation suggested that this characteristic is not common in double-flowered hydrangea cultivars, but is unique to ‘Jyogasaki’. The results of a desiccation test including ‘Jyogasaki’ florets indicated that the lack of sclerified parenchyma cells in the vein led to a decrease in the mechanical strength of decorative sepals. Although the mechanisms underlying the lack of sclerified parenchyma cells in the veins of ‘Jyogasaki’ decorative sepals have yet to be studied, a comparison of ‘Jyogasaki’ and other cultivars would help to elucidate the mechanism of their differentiation in the veins of hydrangeas’ decorative sepals.
In conclusion, dead tubular-shaped sclerified parenchyma cells with a pitted secondary cell wall contribute to the reinforcement of veins in the decorative sepals of hydrangeas after flowering. Sclerified parenchyma cells differentiate in the decorative sepals after flowering, and this may be a new type of mechanical reinforcement in decorative floral organs of plants. These findings will support development of novel technique that gives flowers with robust floral organs and would broadly contribute to postharvest flower preservation.