2019 年 88 巻 4 号 p. 499-506
2019 年 88 巻 4 号 p. 499-506
Plants produce a variety of crystals with different shapes and sizes. Based on their appearance, calcium oxalate crystals, especially raphides, have been suggested to play a key role in the defense mechanism against insect attack and microbial infections. Colocasia esculenta, a tropical plant primarily grown for its edible corms contains a large number of cells (idioblasts) with needle-like crystals of calcium oxalate (i.e., raphides). The concentration of raphides in the plant varies with the ploidy level, cultivar, organ, and micro environment. The objective of this study was to evaluate the physiological, organic and inorganic biochemical changes in the differentiated leaves of elephant ear (Colocasia esculenta var. aquatilis) and examine the rate of release of these compounds in water soluble forms. Regarding photosynthetic functions, the net photosynthetic rate (Pn) was positively related to light intensity, especially in fully expanded and old leaves. However, the Pn, transpiration rate (E), and stomatal conductance (gs) in young leaves were lower than those in fully expanded and older leaves, resulting in low levels of total soluble sugar content in both the petioles and leaf blades of young leaves. In contrast, oxalic acid and calcium in both petioles and leaf blades peaked at > 2.0 mg·g−1 FW and ~185 mg·g−1 FW, respectively. A large number of idioblasts (~5.5 idioblasts per observed microscopic field) were observed in young leaves. Oxalic acid and calcium ions extracted from the leaf tissues were rapidly dissolved in hot water (85°C) for 10–15 min, leading to a decline in the number of idioblasts. Based on these results, petioles and leaf organs of elephant ear may be eaten safely after boiling in hot water for 15 min to dissolve CaOx.
Over the past decade, crop species collected from the wild have been grown as a source of food, feed stock, clothes, wood chip, fuel and herbal medicines (Finley and Seiber, 2014). Therefore, consumption of poisonous plant species is a risk as they contain various compounds toxic to human health (Agra et al., 2007; Botha and Penrith, 2008). For example, calcium oxalate crystals (CaOx) (i.e., raphides) are the main toxic substance in several members of the Araceae family (namely Anthurium, Arum, Dieffenbachia, Monstera, Philodendron, and Spathiphyllum), and are capable of producing allergenic symptoms in the skin, mouth, oral cavity, and stomach upon contact/ingestion (Bruni et al., 2010; Edeoga and Ogbebor, 1999). In addition, daily CaOx intake carries a risk of developing calcium oxalate kidney stones (Butterweck and Khan, 2009; Gettman et al., 2005; McHarg et al., 2003). As a result, the recommended daily consumption of CaOx is in the range of 44–352 mg·day−1, especially in hyperoxaluria (only 132 mg·day−1) (Holmes and Kennedy, 2000; Siener et al., 2003). Therefore, it is of the utmost importance to reduce the amount of CaOx in food during preparation using various processes such as blanching, boiling, and steaming before human consumption or feeding to animals (Chai and Liebman, 2005; Judprasong et al., 2006; Savage et al., 2000).
In higher plants, CaOx has been reported in 215 families including Araceae, Cataceae, Malvaceae, Orchidaceae, and Rubiaceae, in which it has more specialized functions such as acting as a physical deterrent to herbivore feeding (Korth et al., 2006; Molano-Flores, 2001; Nakata, 2012; Raman et al., 2014; Ward et al., 1997). There is large diversity in the type of plant crystals including raphides, styloids, and druses, and they are present in intravacuolar crystal chambers and special organelles known as idioblasts (Nakata and McConn, 2000; Prychid and Rudall, 1999; Webb, 1999; Webb et al., 1995). Raphides, a major form of CaOx, are reportedly the main type of crystals in idioblasts, especially in the Araceae family (Cote, 2009). Previously, several key factors in terms of genetics, plant organelles, exogenous calcium regulation, light intensity, biotic/abiotic stresses, and microenvironment, have been identified to play major roles in CaOx regulation of raphide crystals in higher plants (He et al., 2014; Kuo-Huang et al., 2007; Radex and Savage, 2008). Colocasia esculenta or ‘taro’ is an edible plant species with a rich source of carbohydrates in the storage corm. It is also well known to accumulate a high level of CaOx in its organs such as leaf blades, petioles, and corm (Franceschi and Nakata, 2005; Murakami and Ueda, 2007; Tanaka et al., 2003a, b). Previous investigations suggested that ascorbic acid is a precursor to oxalic acid biosynthesis (Keates et al., 2000; Kostman et al., 2001). Exogenous application of calcium, shading, and soil moisture content directly regulate the level of CaOx in ‘taro’ organs (Islam et al., 2015; Tanaka et al., 2003c). Unfortunately, there is little data available on the potentially edible candidate species Colocasia esculenta var. aquatilis in terms of the presence of organic and inorganic substances, number of idioblast crystals, and morphological and physiological changes in differentiated leaves. Although, the corm of taro is the main source of its nutritional value, leaf blades and petioles are also consumed and provide mineral nutrients and a high level of fiber (Ejoh et al., 1996). Wild type taro (C. esculenta var. aquatilis) is normally distributed in swamp habitats (Fig. 1A) across the Asean region in countries including Myanmar, Thailand, Vietnam, and the Philippines, and is as good source of Ca2+ and ascorbic acid, especially in the petioles and leaves when prepared in traditional cooking (Fig. 1B) such as boiling and curry cooking methods (Matsuda and Nawata, 2002; Matthews, 2004; Matthews and Naing, 2005; Matthews et al., 2012; O’Hair et al., 1982). Therefore, the objective of this study was to evaluate the physiological, organic and inorganic biochemical changes in the differentiated leaves of elephant ear (Colocasia esculenta var. aquatilis). Also, the rate of release of organic and inorganic substances in hot water was determined with the aim of reducing the amounts of CaOx during food preparation (Oscarsson and Savage, 2007).

Natural habitat (A), traditional food products from petioles and leaf blades of Colocasia esculenta var aquatillis (B) and the relationship between stomatal conductance (gs) and transpiration rate (E) of C. esculenta Schott var. aquatilis Hassk. with differentiated leaves.
Corms of elephant ear (Colocasia esculenta var. aquatillis) were collected from a natural swamp habitat near a fresh water canal (Fig. 1A) in the Den-chai district, Phrae Province, Northern Thailand. They were planted in plastic bags containing 2 kg mixed soil (50% sand, 30% silt, 20% clay; EC = 2.687 dS·m−1; pH = 5.5; organic matter = 10.36%; total nitrogen = 0.17%; total phosphorus = 0.07%; total potassium = 1.19%). Corms were watered daily, and grown under the following conditions: 32 ± 2°C temperature, 70 ± 5% relative humidity, and 40% solar transmission shading (by plastic shade netting). After approximately two months, young leaves (leaf not fully-expanded or one week after emergence), fully-expanded leaves (two weeks after leaf emergence), and old leaves (the oldest leaf or fifth leaf, counting from the youngest leaf) were harvested (Fig. S1). Photosynthetic rates, calcium, magnesium, oxalic acid, ascorbic acid, total soluble sugar, morphological characteristics, and number of idioblasts in the leaf blade and leaf stalk samples were measured. In addition, the release rates of oxalic acid, ascorbic acid, calcium, and magnesium from leaf blade tissues (1 g dry weight in 10 mL deionized water) in a hot water bath at 85°C in were evaluated after 0, 5, 10, 15, and 30 min.
Photosynthetic functionsNet photosynthetic rate (Pn), transpiration rate (E), intracellular CO2 (Ci), and stomatal conductance (gs), of young leaf blades, fully-expanded leaf blades, and old leaf blades (n = 5) were measured by a portable photosynthesis system connected to an infra-red gas analyzer (Model LI 6400XT, LI-COR Inc., Lincoln, NE, USA) following the method of Cha-um et al. (2007). The air-flow rate of the sample chamber was 500 μmol CO2·s−1 at 25°C and the light intensity was 1,000 μmol·m−2·s−1 photosynthetic photon flux density (PPFD) from a 6400-02B red-blue light-emitting diode (LED) light source.
Biochemical assayTotal soluble sugars (sucrose, glucose, and fructose) in differentiated stages of leaves (i.e., leaf blades and petioles) were analyzed according to a modified method of Karkacier et al. (2003). Petiole and leaf blade samples (n = 5) were freeze-dried. In a pre-cooled mortar, 100 mg tissue was ground with liquid nitrogen, extracted with 1 mL of deionized water, vigorously shaken for 15 s, sonicated for 15 min and then centrifuged at 12,000 × g for 15 min. The supernatant was filtered through a 0.45 μm membrane filter (VertiPure; Vertical Chromatography Co., Ltd., Bangkok, Thailand) and stored at −20°C prior to the measurement of total soluble sugars content by high-pressure liquid chromatography (HPLC; Model Water 2690, Water Associates, Millford, MA, USA). A 20 μL volume of a crude extract was injected into a Waters HPLC fitted with a Waters 600 pump using a MetaCarb 87°C column (Agilent Technologies, Santa Clara, CA, USA) equipped with a guard column. Deionized water was used as the mobile phase at a flow rate of 0.5 mL·min−1. Online detection was performed using a Waters 410 differential refractrometer detector, and the data was analyzed by Empower® software (Waters). Sucrose, glucose, and fructose (Sigma-Aldrich, USA) were used as standards.
Oxalic acid (OA) and ascorbic acid (ASA) were assayed using an HPLC method. In brief, petiole and leaf blade samples (n = 5) were cut into small pieces (2 mm2) and dried in an oven at 60°C for 3 d. One hundred milligrams of each sample were ground into a powder in a mortar with liquid nitrogen, subsequently adding distilled water (1 mL) and boiling at 85°C in a water bath for 15 min. Contents were centrifuged at 5,000 × g for 15 min, and the supernatant was collected and then filtrated through a 0.45 μm membrane filter (VertiPure). Oxalic acid (Savage et al., 2000) and ASA (Pérez et al., 1997) in a water soluble form were determined using HPLC (Model Water 2690) with a column (VertiSep OA 8 μm [7.8 × 300 mm]; Vertical Chromatography Co., Ltd.) coupled with a Water 996 Photodiode Array Detector (Water Associates, Millford, MA, USA) at 210 nm (Fig. S2) and 245 nm (Fig. S3). For the mobile phase, a solution of 0.002 M H2SO4 in deionized water was used at a flow rate of 0.5 mL·min−1. Oxalic acid and ascorbic acid (Sigma, St. Louis, USA) were used as standards.
Calcium ions and Mg2+ were assayed following the modified method of Tanaka et al. (1999) and Hossain et al. (2006). In brief, petioles and leaf blade tissues of elephant ear at different developmental stages were collected (n = 5). Then, the samples were washed with deionized water to remove any contaminating surface ions. The tissues were ground into a powder in liquid nitrogen, extracted with boiling distilled water, and centrifuged at 10,000 × g for 10 min. The supernatant was filtered through a 0.45 μm membrane filter (VertiPure). Cellular Ca2+ and Mg2+concentrations were determined using an HPLC column (Model Water 2690) coupled with a conductivity detector (Model 432 IC detector, Water Associates, Millford, MA, USA) and ion-exclusion column (WATER IC-PACKTM, Waters Associates). For the mobile phase, a mixed solution of 0.012 μM nitric acid and 71.73 μM Na-EDTA (ethylene diamine tetraacetic acid disodium salt dehydrate) in deionized water was used at a 0.6 mL·min−1 flow rate. Calcium ions and Mg2+ (Sigma) were used as standards (Fig. S4).
Idioblast morphological characteristics and densityPetioles and leaf blades were collected (n = 5), cut into small pieces (2 mm2) using a razor blade and subsequently put on glass slides. Five-hundred μL of 1 mM NaOH were added and tissues were incubated at room temperature for 10 min, rinsed twice with distilled water, crushed, covered by a cover slip and observed (0.0625 mm2) under a compound 100 × light microscope (model Axiostar plus, Carl Zeiss NY, USA) connected to a Motic microscope camera (Motic®, Hong Kong, China). The number of idioblasts under five observed fields in each sample was recorded (Fig. S5) (Brown et al., 2013).
Experiment design and statistical analysisThe experiment was arranged in a completely randomized design (CRD) with five replicates (n = 5). The mean values obtained in each developmental leaf stage were compared using Tukey’s HSD test and analyzed with SPSS software (version 11.5; IBM, Chicago, IL, USA).
The net photosynthetic rate (Pn) in young leaf blades of elephant ear was 2.5 fold lower than in fully expanded leaf blades and 2.3 fold lower than in old leaf blades (Table 1). The transpiration rate (E), intracellular CO2 (Ci), and stomatal conductance (gs) in the young leaf blades were lower compared with fully expanded and old leaf blades. Moreover, a positive, linear relationship between gs and E in elephant ear leaves was also demonstrated (Fig. 1C).
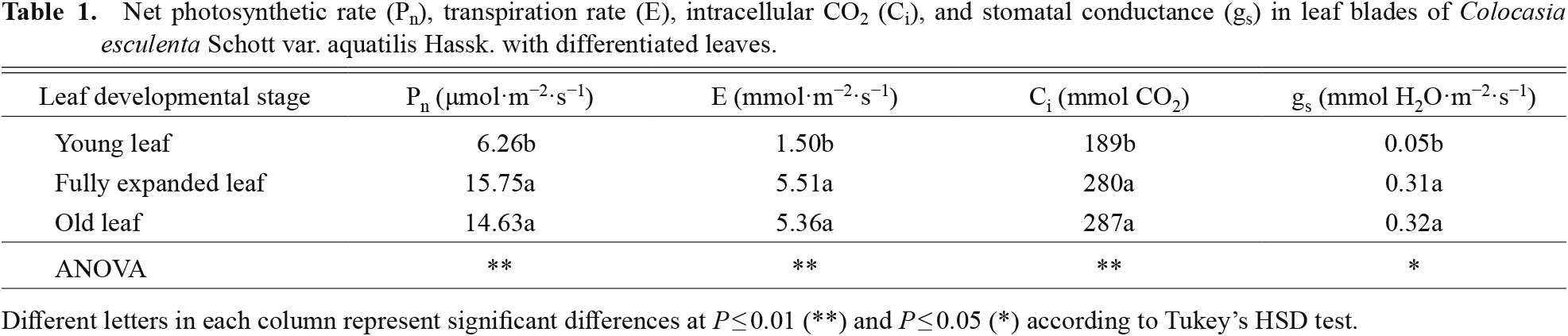
Net photosynthetic rate (Pn), transpiration rate (E), intracellular CO2 (Ci), and stomatal conductance (gs) in leaf blades of Colocasia esculenta Schott var. aquatilis Hassk. with differentiated leaves.
Glucose (Gluc) and fructose (Fruc) (monosaccharide sugars) were the dominant sugars in the petioles of all differentiated leaf stages with very low concentrations of sucrose (Suc) (disaccharide sugar) (Table 2). Glucose and Fruc concentrations in the petioles of fully-expanded leaves were 2.2 and 1.7 fold greater than those in the petioles of young leaves, respectively. Interestingly, Suc accumulation in the young leaf blades was higher than that in the young petioles by 100–300-fold, whereas Gluc and Fruc were lower. Sucrose, Gluc, Fruc, and TS concentrations in fully expanded leaf blades were greater by 3.8, 2.5, 1.6, and 2.5 fold, respectively, compared with those in young leaves. Sucrose and Gluc levels in the old leaves were significantly reduced compared with those in fully expanded leaves, while Fruc and TS were unaffected by the age of the leaf blades.

Glucose (Gluc; mg·g−1 DW), fructose (Fruc; mg·g−1 DW), sucrose (Suc; mg·g−1 DW), and total soluble sugar (TS; mg·g−1 DW) contents in the petioles and leaf blades of Colocasia esculenta Schott var. aquatilis Hassk. with differentiated leaves.
The oxalic acid content in petioles and leaf blades of young leaves was greater by 4.1 and 1.3-fold compared to that in old leaves (Table 3). In our study, ascorbic acid in the petioles was unchanged with the age of the leaves, but it increased in both fully expanded (2.5-fold over young leaves) and old leaves (2.2 fold over young leaves). The numbers of calcium ions in the petioles of young leaves were 2.2 and 4.0-fold higher than fully expanded and old leaves, respectively. Likewise, Ca2+ in young leaf blades was 1.7 times higher than that in old leaf blades (Table 4). The numbers of magnesium ions in the petioles of young leaves were 3.0 and 9.4-fold higher than those in the fully-expanded and old leaves. In addition, Mg2+ in the leaf blades of young leaves was 1.8 fold higher than that in old leaves. In our study, the morphological characteristics of idioblasts at various developmental stages in both the petioles and leaf blade organs were observed (Fig. 2A). The number of idioblasts in the petioles of young leaves was maximized at 5.4 idioblasts per observed field, which was higher than the fully expanded (4.2 idioblasts per observed field) and old leaves (4.2 idioblasts per observed field). Similarly, the number of idioblasts in young leaf blades (6.5 idioblasts per observed field) was higher than that in fully-expanded (4.2 idioblasts per microscopic observed field) and old leaves (4.0 idioblasts per observed field) by 1.6 and 1.6-fold, respectively (Fig. 2B).

Oxalic acid and ascorbic acid contents in the petioles and leaf blades of Colocasia esculenta Schott var. aquatilis Hassk. with differentiated leaves.

Calcium and magnesium contents in the petioles and leaf blades of Colocasia esculenta Schott var. aquatilis Hassk. with differentiated leaves.

Morphological characteristics (100 ×) in young, fully expanded and old leaves (A) and number of idioblasts in the petioles and leaf blades (B) of Colocasia esculenta Schott var. aquatilis Hassk. Errors in each bar represent ± SE. Different letters in each bar indicate a significant difference at P ≤ 0.01 according to Tukey’s HSD test.
The oxalic acid and ascorbic acid contents in leaf samples decreased (Fig. 3A, C) as the incubation time in hot water (85°C) increased. Oxalic acid and ascorbic acid were observed to quickly dissolve in hot water (Fig. 3B, D), and their concentrations in the water decreased as the incubation time increased. In our study, Ca2+ and Mg2+ in the leaf samples were rapidly dissolved by hot water (Fig. 3E–H), and their concentrations decreased as incubation time increased. Concentrations of Ca2+ and Mg2+ in hot water increased as incubation time increased, and we observed that the number of idioblasts decreased following incubation in hot water for a short period (5–15 min incubation time) (Fig. 4A, B).
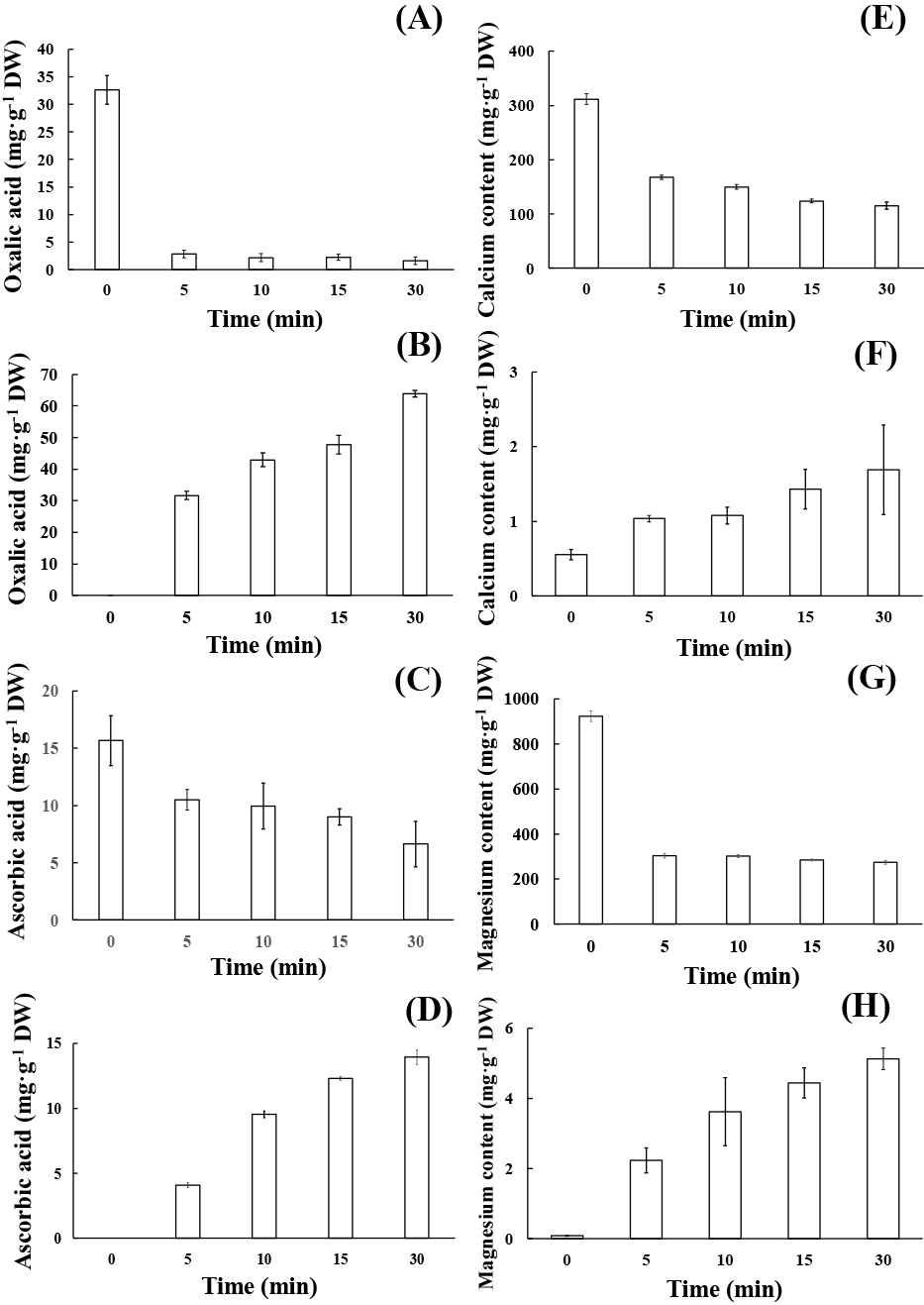
Oxalic acid content in leaf samples (A), the solution (B), ascorbic acid in a fully expanded leaf sample (C), the solution (D), calcium ion concentration in a leaf sample (E), the solution (F), magnesium ion concentration in a fully expanded leaf sample (G) and the solution (H), after incubation in boiling water (85°C) for 0, 5, 10, 15, and 30 min. Errors in each bar represent ± SE.

Idioblast morphological characteristics (A) and number of idioblasts (B) in a fully expanded leaf sample after incubation in boiling water (85°C) for 0, 5, 10, 15, and 30 min. Errors in each bar represent ± SE.
In the present study, Pn, E, Ci, and gs were higher in the first fully expanded leaf blades compared to the young leaf blades, similar to previous reports on taro (Schaffer and O’Hair, 1987). During taro leaf development, both Pn and gs increased in relation to leaf age (Schaffer and O’Hair, 1987). In taro, the CO2 assimilation rate in fully expanded leaves (two weeks after emergence) was greater compared to younger or older leaves (Schaffer and O’Hair, 1987). The net photosynthetic rate, gs and Ci followed a curve in response to leaf age, with a peak at the center for fully expanded leaf blades (Miyazawa and Terashima, 2001; Schaffer and O’Hair, 1987). A positive correlation between gs and E in the leaves of elephant ear has been found, as observed in most higher plants (Jarvis and McNaughton, 1986; Jones, 1998; Tuzet et al., 2003). In C. esculenta (wild type taro), stomatal conductance plays a major role in the control of the transpiration rate in different water regimes (Cha-um et al., 2018), confirming the key results of the present study.
Sugars are primary products of photosynthesis in leaves and they play a key role in plant metabolism (Rolland et al., 2002; Smeekens, 2000). In higher plants, Suc is the major form of sugar that accumulates in leaf blades (O’Hair et al., 1982). Sucrose, Gluc, Fruc, and TS in mature leaves were enriched by 1.25, 1.08, 1.08, and 1.14-fold compared to immature leaves (Patakas and Noitsakis, 2001). Likewise, soluble sugar levels in the 5 + 6 leaf positions (fully expanded leaves) of spinach (Spinach oleracea) peaked at a higher level (3.4 mg·g−1 FW) compared to younger (9 + 10 leaf position) and older (1 + 2 leaf position) leaves by 1.55 and 2.14-fold, respectively (Dietz and Heilos, 1990).
The soluble oxalate content in the petioles of ‘endo’ plants (Colocasia esculenta (L.) Schott var. antiquorum Hubbard & Rehder) was higher than those in the leaf blades or corm (Islam et al., 2015). It was previously reported in taro that soluble oxalate in young leaves was 1.32-fold higher compared to old leaves (Oscarsson and Savage, 2007). In another study, Ca2+ and Mg2+ contents in the differentiated leaves of Lagos spinach were unchanged (Adediran et al., 2015). In taro, raphide idioblasts per microscopic field were higher in petioles and corm (storage organ), than the leaf tissues (Saadi and Mondal, 2013). Likewise, the total raphides (number of idioblasts per cm3) in taro leaves peaked at 3.2 × 103 idioblasts in the first leaf, which was higher than the second leaf by 5.71-fold (Tanaka et al., 2003a). Correspondingly, the number of idioblasts per microscopic field in the leaves of the Chinese evergreen (Aglaonema commutatum) (Saadi and Mondal, 2012a) and arrowhead plant (Syngonium podophyllum) (Saadi and Mondal, 2012b) were found in the order: young > medium > mature leaves. Also, the total crystal idioblasts (number of idioblasts per cm3) in Colocasia species (C. esculenta and C. gigantea) were observed to depend on the cultivar and organ (Tanaka et al., 2003b).
The recovery percentage of ascorbic acid in different cultivars of cauliflower boiled at 70°C for 10 min depended on the organ and was maximized in cauliflower florets (Volden et al., 2009a) and there was a high rate of ascorbic acid dissolution in hot water (Volden et al., 2009b). In contrast, there was a high degree of oscillation in the amount of total calcium and soluble oxalate contents in four different ‘taro’ cultivars, So Tarang, Trom, Tia and Chum (Thanh et al., 2017). In taro, the number of idioblasts (calcium oxalate raphides) and level of oxalate were found to vary with the variety and type of tissue being studied (Murakami and Ueda, 2007). In several leafy vegetables, soluble oxalate was quickly dissolved within 2–6 min (> 30% loss of oxalate) (Judprasong et al., 2006). In addition, soluble oxalate loss was detected in samples of spinach and carrot after boiling them in water during cooking (Chai and Liebman, 2005). The release rate of water soluble oxalate from spinach explants varies along with temperature and incubation time (Kusuma et al., 2016).
In conclusion, the present study revealed that the photosynthetic efficiency and subsequent availability of soluble sugars corresponds to age, with fully-expanded leaves being more efficient than old and young leaves. Moreover, variations in the content and rate of dissolution of organic (ascorbic acid and oxalic acid), and inorganic substances (calcium and magnesium) in hot water and the number of calcium oxalate raphide idioblasts, contribute to significant changes in the leaves at various developmental stages. For consumption, the leaves and petioles of elephant ear may be safe to eat after boiling at 85°C for 15 min to dissolve CaOx.
The authors would like to thank the National Science and Technology Development Agency (NSTDA), Thailand for facilities and equipment.