2019 年 88 巻 4 号 p. 514-520
2019 年 88 巻 4 号 p. 514-520
Modern molecular biology techniques have enabled the generation of novel flower colors. Standard cultivated varieties of delphinium have blue flowers as a result of the biosynthesis and accumulation of delphinidin-based anthocyanins. Some cultivars have pink flowers due to the biosynthesis and accumulation of pelargonidin-based anthocyanins. The biosynthetic pathway of the latter becomes active due to the inactivation of flavonoid 3',5'-hydroxylase. Cyanidin-based red-purple flowers have not been identified to date in delphiniums because these species do not express the flavonoid 3'-hydroxylase gene. However, in our previous work, we identified expression of the flavonoid 3'-hydroxylase gene in a wild delphinium (Delphinium zalil) that accumulates quercetin 3-glycoside. D. zalil lacks the anthocyanidin synthase, the key enzyme to produce anthocyanins, so the flowers do not contain any anthocyanins. Here, we report the use of conventional breeding to introduce cyanidin biosynthesis into delphiniums. We introduced the flavonoid 3'-hydroxylase gene of D. zalil into D. cardinale by hybridization breeding, causing accumulation of cyanidin-based anthocyanin. In the hybrid plants, flavonoid 3'-hydroxylase was transcribed and a cyanidin-based anthocyanin was biosynthesized, generating novel purple-red flowers. Greater understanding of the anthocyanin biosynthetic genes expressed in wild species will benefit the development of breeding strategies to generate novel flower colors in cultivars of high horticultural value.
Recent advances in molecular biology have accelerated the plant hybridization process by reducing the time and space required for breeding. Next-generation sequencing (NGS) platforms now provide one of the most potent tools in molecular biology and whole-genome sequencing of many horticultural crops has been undertaken (Hirakawa et al., 2014; Hoshino et al., 2016; Yagi et al., 2014). In particular, NGS is capable of providing genetic markers for ornamental plant breeding, and marker-assisted selection can then accelerate the molecular breeding process to produce diverse flower colors and shapes, disease resistance, extended vase life, and other traits (Matsubara et al., 2006; Nakatsuka and Koishi, 2018; Nakatsuka et al., 2011; Nishihara et al., 2018; Yagi, 2013, 2018).
Conventional cross-breeding programs make use of variants that have been developed spontaneously or induced artificially after treatment with mutagens such as gamma rays and heavy-ion beams. However, this approach requires the cultivation of a large number of seedlings and the availability of large fields for planting, growth, and improvement of novel cultivars (Okamura et al., 2013; Yamaguchi et al., 2008, 2009, 2010). Breeders must then wait until the plants bloom to identify novel flower-color traits. Therefore, the process of defining a novel mutant and establishing a new cultivar for commercial production has many spatial and temporal requirements. Further, the success of such an approach depends on the experience of the breeder (Anderson, 2006; Onozaki et al., 2018; Shibata, 2008). However, the application of molecular biological methods can overcome these difficulties. For example, obtaining gene expression information by RT-PCR in potential parental lines before hybridization can provide vital information regarding the genetic backgrounds of the prospective parents and enable breeding plans to be developed that will achieve the desired objective. As a result, the use of RT-PCR can eliminate laborious and ineffectual crossing processes and reduce the time and space required for breeding.
The genus Delphinium (Ranunculaceae) comprises over 400 species (http://www.theplantlist.org/browse/A/Ranunculaceae/Delphinium/). Only a few species of Delphinium, such as D. elatum L., D. grandiflorum L., and Delphinium × belladonna (a hybrid between D. elatum × D. grandiflorum ex Bergmans) are grown worldwide. In Japan, approximately 30 million flowers are produced annually and they are used as cut flowers. Considerable effort has been devoted to expanding the variety of flower colors available, resulting in the generation of blue, light blue, violet, purple, lavender, white, and pink flowers (Hashimoto et al., 2002; Katoh et al., 2004; Legro, 1961; Miyagawa et al., 2014).
The anthocyanin pigments that produce flower colors vary in terms of the hydroxylation pattern of the of anthocyanidin B ring (anthocyanidin is an aglycone of anthocyanin). For example, pelargonidin has a hydroxyl residue at the 4' position, cyanidin has two hydroxyl residues at the 3' and 4' positions, and delphinidin has three hydroxyl residues at the 3',4', and 5' positions (Davies, 2009; Tanaka and Brugliera, 2013). Studies of anthocyanin structure in delphinium have shown that blue, blue-violet, and pink sepals are generated by the aglycones delphinidin and pelargonidin (Hashimoto et al., 2000, 2002; Honda et al., 1999; Kondo et al., 1990, 1991; Miyagawa et al., 2015). Based on the results of anthocyanin structural analysis, molecular and biochemical analyses of the flavonoid and anthocyanin biosynthesis pathways in delphinium have confirmed these biosynthetic enzymes produce the anthocyanin structures. These analyses also elucidated characteristics of the anthocyanin molecules associated with particular flower color traits (Fig. 1; Ishii et al., 2017; Matsuba et al., 2010; Miyagawa et al., 2014, 2015; Miyahara et al., 2016; Nishizaki et al., 2013, 2014). However, delphinium cultivars do not bear cyanidin-based red-purple flowers due to the absence of flavonoid 3'-hydroxylase (F3'H), which is required to generate cyanidin derivatives; this enzyme catalyzes hydroxylation at the 3' position of the B ring (Fig. 1).

Schematic pathways of anthocyanin and flavonol biosynthesis in delphiniums.
Our previous study showed that D. zalil Aitch. & Hemsi. has F3'H activity, but defective anthocyanidin synthase (ANS) expression. Therefore, D. zalil does not produce anthocyanin, but rather accumulates flavonol glycosides in its sepals. The recombinant D. zalil F3'H enzyme protein expressed in yeast showed hydroxylation activity to convert naringenin, apigenin, dihydrokaempferol, and kaempferol to eriodictyol, luteolin, dihydroquercetin, and quercetin, respectively. Although kaempferol and quercetin glycosides are accumulated in D. zalil sepals, the recombinant enzyme activity showed a preference for dihydrokaempferol over other flavonoids (Fig. 1; Miyahara et al., 2016). In addition, we have also shown that D. cardinale Hook. accumulates large amounts of a single pelargonidin-based anthocyanin, which leads to the production of vivid red flowers (Miyagawa et al., 2015). However, neither flavonoid 3',5'-hydroxylase (F3'5'H) activity nor accumulation of F3'5'H reaction products has been detected in wild-type D. zalil or D. cardinale.
In this study, we introduced the F3'H gene from D. zalil into D. cardinale with the expectation that this would enable generation of a new cultivar that would bear red-purple flowers as a result of cyanidin biosynthesis.
Seedlings of D. zalil and D. cardinale were obtained from Miyoshi & Co., Ltd. and cultivated in a greenhouse at the Research and Development Center of Miyoshi & Co., Ltd. Immature anthers were removed from the maternal parent (D. zalil) before the flower opened. After ripening, the pistils were hand-pollinated with freshly collected mature pollen from D. cardinale. Then, ~40 days after pollination, the fruit capsules were harvested and the seeds inside were collected to develop breeding lines of D. zalil × D. cardinale. We used D. zalil and D. grandiflorum to confirm the genomic flavonoid 3'-hydroxylase (F3'H) nucleotide sequences.
Flower color assessmentThe sepal colors of D. zalil, D. cardinale, and the F1 hybrid lines of D. zalil × D. cardinale were evaluated using the Royal Horticultural Society Colour Chart (RHS CC; 5th edition, 2007).
Anthocyanin profiles of D. zalil × D. cardinale hybrid linesApproximately 200 mg of sepals from fully-opened flowers were frozen in liquid nitrogen and then ground into powder using a mortar and pestle. Subsequently, the powder was mixed in 100 μL of 80% methanol containing 0.1% trifluoroacetic acid (TFA). Cell debris was removed by centrifugation at 15,000 × g for 10 min and the supernatant was analyzed by high performance liquid chromatography (HPLC) using an LaChrom Elite HPLC System (pump L-2130, column oven L-2300 at 30°C, diode array detector L-2450 [Hitachi High-Technologies, Tokyo, Japan] with a Handy octadecylsilyl column [internal diameter 4.6 mm × length 250 mm, Wako Pure Chemical Industries, Osaka, Japan]). The injected sample was separated by linear gradient elution at a flow rate of 1 mL·min−1 in 20%–80% methanol/1.5% aqueous phosphoric acid for 20 min and detected at 520 nm.
Analysis of anthocyanin aglycones in the sepals of F1 hybridsThe sepal extracts in 80% methanol/0.1% TFA were allowed to dry, the residue was dissolved in 100 μL of water. Then, 100 μL of 12 N HCl was added, and hydrolysis was performed at 80°C for 1 h. The aglycones in the crude hydrolysis solution were extracted by the addition of 200 μL of ethyl acetate. The organic layer was recovered and dried, then dissolved in 50 μL of 0.1% TFA, and a 10 μL of an aliquot of this solution, containing the hydrolyzed aglycones, was analyzed using HPLC. The equipment used was the same as described above, and the elution conditions were identical to those described by Miyagawa et al. (2014). The aglycones delphinidin, cyanidin, and pelargonidin were purchased from Extrasynthese Co., Genay, France, for use as standards.
Genomic PCR and RT-PCR analysisTotal RNA was extracted from fully-opened sepals of D. zalil, D. cardinale, and F1 hybrids, and first strand cDNAs were synthesized from 1 μg of total RNA, the same as described previously (Ishii et al., 2017). The first strand cDNA reaction mixture was diluted 3-fold, then 1 μL of the cDNA mixture was used in PCR as a template. Full-length cDNA corresponding to the F3'H gene was amplified by PCR using the primers F3'H-F (5'-ATGCCTTCTCTATACTTTCTA-3') and F3'H-R (5'-CTATACTTGATAGACACTTGG-3'). The reference gene Actin was amplified using the primer sets described previously (Miyahara et al., 2016). PCR was performed using PrimeStar GXL DNA polymerase (Takara Bio Inc., Shiga, Japan) under the following conditions: denaturation for 2 min at 94°C, followed by 30 cycles of 5 s at 98°C, 15 s at 55°C, and 20 s at 68°C. The amplified PCR products were then analyzed by agarose gel electrophoresis. Genomic sequence analysis was also performed as described previously (Ishii et al., 2017). F3'H genomic sequences in D. zalil and D. grandiflorum were confirmed using the same primer set used for RT-PCR. The genomic sequence of F3'H from D. grandiflorum was registered under accession number LC441150 in the GenBank/EMBL/DDBJ databases.
Initially, we attempted to use D. cardinale as the seed parent and D. zalil as the pollen parent; however, pollen development in D. zalil flowers failed because of deficient stamen development under the prevailing cultivation conditions. Therefore, we chose to use D. zalil as the seed parent and D. cardinale as the pollen parent. Legro (1961) reported that seeds could be obtained by this type of cross and that over 80% of the seeds are capable of germination. We collected more than 1,000 seeds from cross-pollinated D. zalil flowers, sowed 765 in a 406-hole tray, and achieved a germination rate of 43.5%. All the resulting seedlings were planted, of which 126 developed to the flowering stage. All the hybrid plants had a single-flower structure, as in the parental plants, but the hybrid flowers displayed characteristics that were intermediate in comparison to the parental types. The growth period from sowing to flowering was shorter in the hybrid plants than in the parental varieties, and all the hybrid plants had red-purple sepals (Fig. 2).

Flower phenotypes of the parental lines (D. zalil and D. cardinale) and representative F1 hybrids.
We selected four representative hybrids (numbered 1–4), assessed their sepal colors against the RHS CC, and compared the colors with those of the parental D. zalil and D. cardinale (Table 1). Miyahara et al. (2016) reported that D. zalil could not biosynthesize anthocyanin, and its RHS CC value was in the green-yellow group (1C). Delphinium cardinale accumulates pelargonidin glycosides (Miyagawa et al., 2015), and its RHS CC value is in the red group (42B). The sepal colors of the four hybrids fell into the red-purple group (72A–72D), and the purple group (N78A; Table 1). The sepal colors in the hybrids showed little individual variation, but no plant demonstrated a red value (42B) secondary to the accumulation of pelargonidin derivatives.

Flower colors of the parental lines D. zalil and D. cardinale, and the F1 hybrid lines 1–4.
Next, we performed HPLC analysis to elucidate the composition of the anthocyanin molecules in the sepals of F1 hybrids (Fig. 3). All the anthocyanin retention times were consistent in each F1 hybrid. Three of the four breeding lines had one major anthocyanin molecule and four or more minor anthocyanin molecules. The exception was line No. 3, which was assessed as 72B using the RHS CC assessment, but had a different major anthocyanin molecule. This major anthocyanin molecule was the second largest peak in other lines. Although the detailed chemical structures of the anthocyanins in the hybrids’ sepals were not identified, the backbone structure of these anthocyanins, aglycone, was analyzed after hydrolysis of the anthocyanins (Fig. 4). The major anthocyanidin molecule was cyanidin in these hybrids, while pelargonidin, the major aglycone in the parental line D. cardinale (Miyagawa et al., 2015), was only present in small quantities. Miyagawa et al. (2015) reported that D. cardinale accumulates pelargonidin 3-(6-malonyl)-glucoside-7-(6-(4-(6-(p-hydroxybenzoyl)-glucosyl)-oxybenzoyl)-glucoside) (Supplemental Fig. 1). We speculate that F1 hybrids may accumulate cyanidin-type anthocyanin molecules with the same modification as in D. cardinale [cyanidin 3-(6-malonyl)-glucoside-7-(6-(4-(6-(p-hydroxybenzoyl)-glucosyl)-oxybenzoyl)-glucoside)], and that the other minor anthocyanin molecules are perhaps precursors of the major anthocyanin molecules. A previous study showed that the flowers resulting from hybridization breeding of D. cardinale × D. grandiflorum (which accumulates delphinidin-based anthocyanin) and D. nudicaule Torrey & A. Gray (which accumulates pelargonidin-based anthocyanin) × D. grandiflorum accumulate delphinidin-based anthocyanins, but not pelargonidin-based anthocyanins. This led to the conclusion that delphinidin is dominant over pelargonidin in delphiniums (Honda et al., 1999). Our data show that cyanidin is also dominant over pelargonidin in delphiniums.

HPLC profile demonstrating the anthocyanins in the sepals of individual F1 hybrids. Traces for four hybrids are shown, numbered 1–4.

Aglycone analysis of the anthocyanins in the sepals of representative F1 D. zalil and D. cardinale hybrids. Standard numbers are 1, delphinidin; 2, cyanidin; 3, pelargonidin.
Delphinium zalil has an active F3'H that catalyzes the conversion of the aglycone flavonoids naringenin, apigenin, dihydrokaempferol, and kaempferol to eriodictyol, luteolin, dihydroquercetin, and quercetin, respectively (Fig. 1; Miyahara et al., 2016). Anthocyanidin profiling of the F1 hybrids showed that they all accumulate cyanidin compounds, implying the inheritance of an active F3'H gene from D. zalil. Qualitative RT-PCR analysis demonstrated the expression of F3'H in D. zalil and the F1 hybrids, but it was undetectable in D. cardinale sepals (Fig. 5). Our previous studies showed that ANS gene expression cannot be detected in D. zalil, although DFR can be detected and has been sequenced. These results indicate that a defect in ANS gene expression is responsible for the lack of anthocyanin biosynthesis in D. zalil (Miyahara et al., 2016). In D. cardinale, the main anthocyanin has been structurally identified as a pelargonidin-based anthocyanin. This fact indicates that both DFR and ANS must be expressed in D. cardinale. These findings suggest that the F3'H gene introduced into D. cardinale by hybridization with D. zalil is expressed in the hybrids and can catalyze the conversion of dihydrokaempferol to dihydroquercetin as part of the cyanidin biosynthesis pathway (Fig. 1). The fact that the ANS gene was defective in D. zalil implies that all the hybrids had obtained active ANS genes from D. cardinale, while the DFR gene could have come from either parental line and become active in the F1 hybrids. This indicates that the substrate specificities of DFR and ANS have little effect on anthocyanidin structures such as pelargonidin and cyanidin in delphiniums.
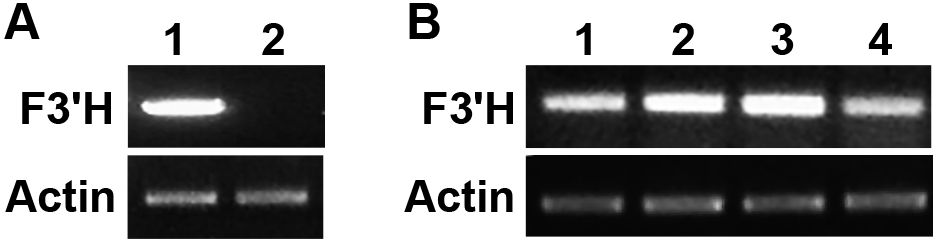
Qualitative RT-PCR analysis of F3'H expression. A: F3'H gene (upper) and Actin gene (reference, lower) expression in the sepals of the parental lines D. zalil (1) and D. cardinale (2). B: F3'H and Actin expression in F1 hybrids, numbered 1–4.
A previous report described the expression of F3'H in D. zalil, but not in D. grandiflorum. The genomic structure of the F3'H coding region in D. zalil is the same as that of the cDNA structure; there are no intronic sequences and it is directly connected to exons (Supplemental Fig. 2). The genomic structure is similar to that of F3'5'H in D. nudicaule (Miyagawa et al., 2014). However, F3'5'H is not expressed in D. nudicaule. In D. grandiflorum, the F3'H genomic sequence comprises three exons and two introns. Moreover, each exonic sequence in D. grandiflorum has > 90% identity with that of D. zalil (Supplemental Fig. 2). Therefore, although the F3'H coding region sequence of D. grandiflorum has been confirmed, the reason for its lack of expression has not been clarified. We speculate that there is a similar reason for the lack of expression of F3'H in both D. cardinale and D. grandiflorum.
Legro (1961) reported that hybridization of D. zalil and D. cardinale produced hybrids with wide color variations in their main sepals, including apple blossom-white, creamy-green, soft-pink, raspberry-red, and magenta. In contrast, the F1 hybrids produced here demonstrated a comparatively narrow range of red colors. Legro (1961) used D. zalil plants raised from seeds obtained from the Botanical Garden in Geneva, while the D. cardinale plants were raised from seeds collected from the Santa Monica Mountain, at the junction of Tuna Canyon and Saddle Park, California. At present, the genus Delphinium is cultivated worldwide, and D. zalil and D. cardinale are important species that are grown and distributed as the commercial brands “Zalil” and “Cardinal larkspur (Scarlet larkspur)”. It is possible that Legro (1961) used heterogeneous populations as hybridization parents in his study, whereas the parental plants used here were thought to be relatively homogeneous because they had been derived by self-hybridization from selected lines to provide stable phenotypes.
In this study, we used wild-type delphiniums as a gene resource to produce novel delphinium flower colors associated with the accumulation of cyanidin. These hybrid lines can be used to generate novel varieties colored by cyanidin, e.g., black (Dahlia variabilis: Deguchi et al., 2016), sky blue (Meconopsis grandis: Tanaka et al., 2001), blue (Centaurea cyanus: Takeda et al., 2005), and a rich variety of reds. Consequently, our progress in the generation of delphinium hybrids that accumulate cyanidin will undoubtedly lead to an expansion of the available flower color varieties of delphiniums.
ConclusionsThe F3'H gene of D. zalil was introduced into D. cardinale by conventional hybridization breeding. The F1 hybrids produced by this cross showed novel red-colored sepals containing cyanidin-based anthocyanin, which has not been found to date in delphiniums. Our results demonstrate the feasibility of a strategy based on the identification of the chemical structures of flower pigments and the screening of the genes for enzymes that can generate these structures. This approach can be applied to both horticultural and wild cultivars and has the potential to identify novel traits and under-utilized gene resources that could be used with conventional hybridization techniques to generate novel phenotypes.