2022 年 91 巻 1 号 p. 16-23
2022 年 91 巻 1 号 p. 16-23
Dwarf cherry tomatoes ‘Red Robin’ and ‘Tiny Tim Red’ were cultivated in two sections. The two sections were set as (1) section irradiated only by fluorescent lamps (main wavelengths 433, 543, and 610 nm; 230 μmol·m−2·s−1 of photosynthetic photon flux density) and non-treated with UV-A irradiation (hereafter, non-UVA), and (2) section irradiated with fluorescent lamps and treated with UV-A irradiation (maximum peak emission: 352 nm; 15.1 W·m−2 ultraviolet radiation intensity; hereafter, t-UVA). The fruit weight per plant and the number of fruits per plant were not significantly affected by cultivar or section. Regarding fruit quality, the fruit cracking rate was lower in the t-UVA than in non-UVA section. There were no anatomical or histochemical differences in fruit structure or distribution of pigments such as lycopene or β-carotene, but the shape of the pigment in the epidermal cells was needle-shaped in the non-UVA and unclearly-shaped in the t-UVA, so the pigment shape was different between the two sections. In addition, the number of layers of the hypodermis in the exocarp that accumulated the pigment was smaller in the t-UVA than in non-UVA section. Fruit components in t-UVA showed increased total soluble solids (TSS), titratable acidity (TA), and ascorbic acid content, but a decreased carotenoid content. Therefore, it was shown that UV-A irradiation had a positive effect on the TSS, TA and ascorbic acid content and had a negative effect on the size of the fruit per fruit and the carotenoid content. UV irradiation did not affect the yield per plant. Comparing ‘Red Robin’ and ‘Tiny Tim Red’, there was no significant difference in yield, but the fruit size of ‘Red Robin’ was larger, while and the fruit component of ‘Tiny Tim Red’ was higher than that of ‘Red Robin’. In conclusion, when cultivating tomato plants in a plant factory, further improvements in fruit size and composition are expected by appropriately adjusting the time and intensity of UV irradiation for each cultivar.
A plant factory with artificial light has an environmental parameter control mechanism to adjust light, carbon dioxide, air temperature, and humidity, the nutrition solution and water, and a multi-layered hydroponic cultivation shelf (Kozai, 2013a; Benke and Tomkins, 2017). Plants can be cultivated regardless of the weather by isolating the cultivation shelves from the outside environment. Therefore, leafy vegetables or herbs with a short plant height that can be cultivated on multi-layered cultivation shelves are produced in plant factories and are mainly sold in fresh markets. On the other hand, few fruit vegetables are cultivated in plant factories, and strawberry fruits are produced in some plant factories (Shamshiri et al., 2018). Therefore, the cultivation of fruit vegetables in plant factories could contribute to the diversification of cultivated production. In one study on tomatoes in a plant factory with artificial light, the plant factory was used to produce tomato seedlings. Results for light quality and water efficiency, CO2 and light energy use to produce higher quality tomato seedlings to improve productivity in greenhouses have been reported (Kozai, 2013b). Kato et al. (2010, 2011) bred a line that crossed a transgenic tomato line that expresses the miraculin gene driven by the CaMV 35S promoter and a dwarf cultivated tomato, while the plant productivity of recombinant proteins in a plant factory have also been reported. Ohashi-Kaneko et al. (2017) conducted research using wild tomato species and dwarf mutant lines, and reported that lines with increased content of soluble solids and vitamin C in the fruit during a 24-hour photoperiod under low photosynthetic photon flux density (PPFD, 180 μmol·m−2·s−1) are useful germplasm resources. However, few studies have investigated tomato cultivars suitable for use in plant factories with artificial light.
In a plant factory where light quality can be adjusted, it is possible to use ultraviolet (hereinafter, UV) irradiation when cultivating vegetables. It has been reported that UV irradiation increased the phytochemical contents such as flavonoids, carotenoids, antioxidants and phenolic compounds in vegetables (Lee et al., 2014; He et al., 2020). In tomatoes, there are many studies showing that irradiation with UV radiation of fruits post-harvest increases antioxidants, phenolic compounds, flavonoids, carotenoids, and soluble solids content (Castagna et al., 2013, 2014; Kasim and Kasim, 2015; Mariz-Ponte et al., 2019). Therefore, beneficial effects of irradiating tomato fruits with ultraviolet radiation are expected, and it is possible to obtain fruits of better quality, but there are few studies using UV irradiation in plant factories. In this study, we investigated (1) cultivation using dwarf cultivars and (2) the effect on tomato fruit quality under UV-A irradiation conditions in a plant factory.
The dwarf cherry tomato cultivars (Solanum lycopersicum) ‘Red Robin’ and ‘Tiny Tim Red’ were used in this study (Fig. 1). These cultivars were selected because of their varietal characteristics as shown below. ‘Red Robin’ has a growth habit determinate, dwarf plant size (23–30 cm tall) and all fruits ripen at the time. ‘Tiny Tim Red’ has a growth habit determinate, is self-pruning (sp) and is a dwarf (d) plant (15–30 cm tall).

Plants of cultivated cherry tomatoes used in this study (left side: ‘Red Robin’, right side: ‘Tiny Tim Red’). When the fruits of these cultivars were fully ripened, they turned red.
Seeds of these cultivars were sown on urethane mats (23 mm × 23 mm × 27 mm; width × length × height) soaked in water and allowed to stand in a dark place at 25°C for 24 hours. On the 10th day after sowing, the germinated seedlings with true leaves were transplanted into a container (376 mm × 602 mm × 76 mm), and the container was placed under a white fluorescent lamp (hereinafter, FL) with main wavelengths of 433, 543, and 610 nm (FHF32EX-N-H; TOSHIBA LIGHTING & TECHNOLOGY Corp., Kanagawa, Japan; Supplemental Fig. S1) for 30 days. The environment for raising seedlings was a 16 h/8 h photoperiod (light/dark), 120 μmol·m−2·s−1 of PPFD, 25 ± 3°C air temperature, 65 ± 5% relative humidity, and CO2 concentration was adjusted so that it was maintained at 1,000 ppm. As the culture solution, OAT-house A solution (OAT Agrio Co., Ltd., Tokyo, Japan), maintained at electrical conductivity (EC) of 1.2 dS·m−1 and pH 6.5 was used as a nutrient solution. On the thirtieth day after germination, four seedlings were transplanted into a container (423 mm × 684 mm × 100 mm) which was a non-circulating type deep-flow technique hydroponic apparatus (DFT) and cultivated in a section on a multi-layered cultivation shelf under the following environmental conditions (Fig. 2).

Multi-layer cultivation shelves (A) installed in the cultivation room (8274 mm × 13636 mm × 3000 mm). A container with transplanted seedlings was placed in one cultivation layer (1050 mm × 5010 mm × 500 mm), and a cultivating space in one cultivation layer (B) and planting distance in a container (C).
Each section was set with a shelf was divided into two parts based on the type of light quality. The two sections were set as (1) section irradiated only fluorescent lamps and non-treated with UV-A irradiation, and (2) section irradiated with fluorescent lamps and treated with UV-A irradiation. The two cultivation sections are indicated by (1) section: non-UVA and (2) section: t-UVA in this study. The cultivation conditions were maintained with a white fluorescent lamp (230 μmol·m−2·s−1 of PPFD), a fluorescent blacklight lamp with a maximum peak emission at 352 nm (15.1 W·m−2 UV radiation intensity; FHF32BLB-T; TOSHIBA LIGHTING & TECHNOLOGY; Supplemental Fig. S2) used in UV-A irradiation, and a photoperiod of 8 h/16 h (light/dark) on the top side of the plant. Air temperature, relative humidity and CO2 concentration were adjusted to maintain levels of 25 ± 2°C, 60 ± 10% and 1,000 ppm, respectively. A culture solution was used in OAT-house A solution (maintained in EC: 1.2 dS·m−1 and pH 6.5). The culture solution was changed once a week. Pollination was accomplished by flicking all flowers at the flowering stage. Three months after transplanting, ripe fruits were harvested. For the fruits, the yield per plant and the rate of cracking fruits out of the total number of harvested fruits were calculated. For the size of the fruit, the fruit diameter and fruit weight were measured with electronic digital calipers (mm) and a digital scale (g), respectively. Then, the fruits were used for anatomical and histochemical investigations of the exocarp and fruit composition analysis.
Anatomical and histochemical investigation of fruitsThe external appearance of the fruit structure was investigated by observing tissue pieces prepared by peeling the pericarp of ripe red-colored fruit. In addition, 30 μm fresh cross-sections were cut by an automatic plant microtome (MT-3; Nippon Medical & Chemical Instruments Co., Ltd., Osaka, Japan). Then, each cross-section was used for histochemical detection of cuticle substances in the exocarp. Fresh sections were washed with 50% ethanol for three minutes and then immersed in 0.02% Sudan IV 70% ethanol for 20 minutes. Then, they were washed with 50% ethanol for one minute (Jensen, 1962). For anatomical and histochemical investigation, a light microscope (Olympus BX51; Olympus Corp., Tokyo, Japan) was used for observations.
Analysis of fruit componentsAll ripe fruits harvested from four plants from each of the two sections were randomly sorted so that the number of fruits was divided into five groups for replication. The number of fruits per group in the non-UVA and t-UVA section for each cultivar was as follows: 15–16 fruits and 16 fruits of ‘Red Robin’, 19–20 fruits and nine fruits of ‘Tiny Tim Red’. A paste was made from each ripe fruit, and the required volume was weighed from the paste for to measure the ascorbic acid and carotenoid content. Next, a juice extract was made with the remaining paste. Total soluble solids (TSS) in 1 mL of juice extract were measured using a digital refractometer (PAL-1; ATAGO Co., Ltd., Tokyo, Japan) and expressed as Brix (%). Titratable acidity (TA) in 25 mL juice extract filled up to 50 mL with distilled water was determined by titrating with 0.1 N NaOH, and the result was expressed in terms of citric acid. The TSS to TA ratio was also calculated. The content of ascorbic acid in the 1.0 g paste was measured by using a Reflectoquant Ascorbic Acid Test with a reflectometer (RQ flex plus 10; Merck Corp., Darmstadt, Germany). The carotenoid content in the 1.0 g paste was measured by using a spectrophotometer (UV-1200; Shimadzu Corp., Kyoto, Japan) with a measurement method based on Nagata and Yamashita (1992). Component analysis was repeated 5 times (derived from the 5 divisions of the number of ripe fruits), and the results are shown as the mean ± standard error.
Statistical analysisStatistical analysis was performed using EZR software (EZR software version 1.5.3; Kanda, 2013). Data were analyzed by two-way analysis of variance (ANOVA). When the interaction of two-way ANOVA was significant, the data were analyzed by Tukey’s test.
The fruit weight per plant showed no significant difference in terms of cultivar or section by two-way ANOVA, and there was no effect for the interaction between cultivar and section (C × S) (Table 1). This result was also observed for the difference in the number of fruits per plant. The fruit weight per fruit was significantly heavier for ‘Red Robin’ than ‘Tiny Tim Red’ at P < 0.001 and a significant difference in the effect of UV-A irradiation treatment was observed at P < 0.01, but no significant difference was found in the interaction (Table 1). The fruit diameter per fruit was significantly different between cultivars at P < 0.001 and the fruit diameter per fruit of ‘Red Robin’ was larger than that of ‘Tiny Tim Red’, but there was no significance in the sections or the (C × S) (Table 1).

Effects of ultraviolet radiation on yield and fruit size of ‘Red Robin’ and ‘Tiny Tim Red’.
Bacci et al. (1999) showed that tomato plants UV-B radiated by using Q-PANEL UV-B 313 FL (maximum peak emission: 315 nm; UV radiation intensity: 0.11 W·m−2) for seven hours per day resulted in reduction in fruit weight per plant and a similar or greater number of fruits per plant. Mariz-Ponte et al. (2019) reported that UV-A for 1 h per day using fluorescent blacklight (368 nm; 0.8 W·m−2) and UV-B for 2 min per day using 8W lamps TFP-M/WL (312 nm; 2.94 W·m−2) for irradiation significantly increased the number of fruits per plant. In our study, the fruit weight per plant and number of fruits per plant were not affected by cultivar or section because there was no significant difference in the measured values of the fruit weight or the number of fruits, which was inconsistent with Bacci et al. (1999) and Mariz-Ponte et al. (2019). On the other hand, UV-A irradiation was accompanied by a decrease in fruit weight and fruit diameter per fruit (Table 1). These results were the same as Bacci et al. (1999) and Mariz-Ponte et al. (2019). Mariz-Ponte et al. (2019) pointed out that a decrease in fruit size may be accompanied by an increase in the number of fruits. However, in our results, the number of fruits of ‘Red Robin’ and ‘Tiny Tim Red’ did not increase significantly. Therefore, UV irradiation of tomato plants affected the size of fruits, especially the fruit weight per fruit.
External appearance of the fruit, and exocarp pigment distributionFruit cracking was observed in the harvested ripe fruits (Fig. 3). The number of cracked fruits to harvested fruits showed no significant difference in cultivars or sections by two-way ANOVA (Table 1). There was no effect for the interaction between cultivar and section (C × S). This result was also observed for the rate of cracked fruits. Fruit cracking occurred in all cultivation areas. When the pericarp was observed from the outside, a yellowish epidermis was observed in both cultivars, and a needle-shaped red pigment like lycopene was accumulated in the epidermal cells (Fig. 4). The pigment had a needle-shape in the non-UVA section, but was an unclear shape in t-UVA, and a difference in the pigment shape was observed (Fig. 4: black arrow). In particular, the epidermal cells of t-UVA in ‘Tiny Tim Red’ were prominently observed and contained an unclear shaped red pigment.

Fruit cracking symptoms were observed in the harvested ripe fruits (left: normal fruit, right: cracked fruit). Clear crevices were found on the surface of some fruits (black arrows).

Epidermal structure and pigment distribution of the pericarp in ‘Red Robin’ and ‘Tiny Tim Red’. Indicates left: non-UVA, right: t-UVA. Black arrows indicate pigment in the epidermal cells. Different pigment shapes were observed between non-UVA (needle-shape) and t-UVA (unclear shape). The black scale bar is 50 μm.
Regarding fresh cross-sections (Fig. 5), the exocarp was composed of a one layer epidermis and 3 to 4 layers of hypodermis, and the mesocarp consisting of thin-walled parenchymatous cells was observed just below the hypodermis. The pericarp constituent cells contained a red-colored pigment. Although there was no difference in the pericarp structure in the cultivation area, the number of hypodermis layers in which pigment distribution was observed was 1 to 2 layers in t-UVA and 3 to 4 layers in non-UVA, and there were fewer layers in t-UVA than non-UVA. The epidermis of the exocarp was most strongly stained with Sudan IV in both cultivars, and it was found that the cuticle substance was localized (Fig. 6). In addition, no difference was observed in the localization of the cuticle substance in two sections. According to Peet (1992), tomato fruit cracking occurs when fruit ripens or other factors reduce the strength and elasticity of the pericarp, while there is a rapid net influx of water and solute into the fruit. In another report, it was shown that when excessive water is supplied to greenhouse tomatoes grown in bags, fruit cracking increases as the tomato plant receives more water (Peet and Willits, 1995). In hydroponics, fruit cracking has been observed in both open and closed systems (Maboko et al., 2011). The anatomical characteristics of cultivars susceptible to fruit cracking are: 1) large fruit size, 2) low skin tensile strength and/or low skin extensibility when turning to the pink stage of maturity, 3) thin skin, 4) thin pericarp, 5) shallow cutin penetration, 6) fewer fruits per plant, 7) fruit not covered by foliage (Peet, 1992). It has been reported that UV-A irradiation increases the firmness of fruit (Mariz-Ponte et al., 2019) and that it does not damage the epidermis (Maneerat et al., 2003). In our study, too, because a DFT system similar to a hydroponic cultivation system was adopted, no difference in fruit cracking rate was observed in the sections and no anatomical or histochemical differences were observed between the cultivation areas in terms of epidermal structure; therefore, it was considered that fruit cracking occurred due to the cultivation environment in which the tomato plants received more water.

Exocarp structure and pigment distribution in fresh cross-sections of ‘Red Robin’ and ‘Tiny Tim Red’. Indicates left: non-UVA, right: t-UVA. Symbols in the figure indicate e: epidermis, h: hypodermis and m: mesocarp. The black scale bar is 50 μm.

Detection of cuticle substances in the exocarp. The cuticle substances were stained orange by Sudan IV. Indicates left: non-UVA, right: t-UVA. Symbols in the figure indicate e: epidermis, h: hypodermis and white arrow: cuticle layer. The black scale bar is 50 μm.
Maboko and Du Plooy (2013a, b) reported that when several tomato cultivars were grown at different plant densities, less fruit cracking was observed at higher plant densities than at lower ones. This paper suggested that a high planting density resulted from high canopy coverage and reduced the exposure of fruits to direct sunlight and high temperatures. Therefore, this study could not clarify whether planting density affected the occurrence of fruit splitting, which is an important issue as well as the irrigation status of the plant body in plant factories. Matsuo et al. (2012) suggested that cytokinins (CK) are involved in cell division during tomato fruit development by applying a synthetic CK, forchlorfenuron (1-(2-chloro-4-pyridyl)-3-phenylurea, CPPU), to unpollinated tomato ovaries to induce parthenocarpic fruit development. A single spraying of CPPU on tomato fruit clusters (fruit size: 3.0–4.9 cm diameter) reduced fruit cracking as a result of an increase in the number of cells near the epidermis (Sano et al., 2018). In addition, the effectiveness of UV-cut film (UV protection film) has been reported to reduce tomato fruit cracking in open field cultivation (Kimura et al., 2012; Uetani et al., 2014; Suzuki, 2019). It is possible to use this method based on the intensity and duration of UV irradiation. Therefore, it could be incorporated into tomato cultivation in plant factories that control the environment, and we plan to further investigate the appropriate conditions needed to suppress tomato fruit cracking.
Effects of ultraviolet radiation on fruit componentsThe TSS of ripe tomato fruit was significantly higher in t-UVA than non-UVA at P < 0.001 by two-way ANOVA, but there was no significant difference by cultivar (Table 2). A difference with the UV-A irradiation treatment was observed and a significant difference was observed in the interaction between cultivar and section (C × S) at P < 0.05. The t-UVA in ‘Tiny Tim Red’ had the highest TSS compared to the other fruits by Tukey’s test at P < 0.05 (Table 2). A difference in the TA was observed in the cultivars, sections and (C × S) this was significant at P < 0.001 (Table 2). The non-UVA of ‘Tiny Tim Red’ was significantly higher than other TAs by Tukey’s test at P < 0.05. The TSS to TA ratio was FL: 19.9, UV-A: 16.2 in ‘Red Robin’ and FL: 8.8, UV-A: 10.6. Previous studies reported that harvested tomato fruit with irradiated UV-B for 8 min (2.35 W·m−2) increased the TSS content (Kasim and Kasim, 2015). On the other hand, Mariz-Ponte et al. (2019) reported that tomato plants with irradiated UV-A (0.8 W·m−2) for 1 h and 4 h or UV-B (2.9 W·m−2) for 2 min and 5 min had reduced TSS content. In our study, it was shown that the TSS content increased under the influence of UV irradiation, as in Kasim and Kasim (2015), even though the harvested fruits were not UV-irradiated. Cote et al. (2013) reported that irradiation of post-harvest fruits with UV-C radiation for 2 min (peak emission: 254 nm; 33 W·m−2) slightly reduced the TA and Dyshlyuk et al. (2020) reported that the TA was hardly affected by UV-A radiation (UV intensity: 353 nm, 365 nm and 400 nm; UV irradiated continuously for 10 min, 180 min, and 360 min at the following doses: 0.33, 0.28 and 0.28 W·m−2, respectively for UV intensity types). It was considered that the increase in the TA was related to the irradiation time of UV-A and the irradiation intensity because it was different from the results of this study, in which the TA increased when irradiated with UV-A.
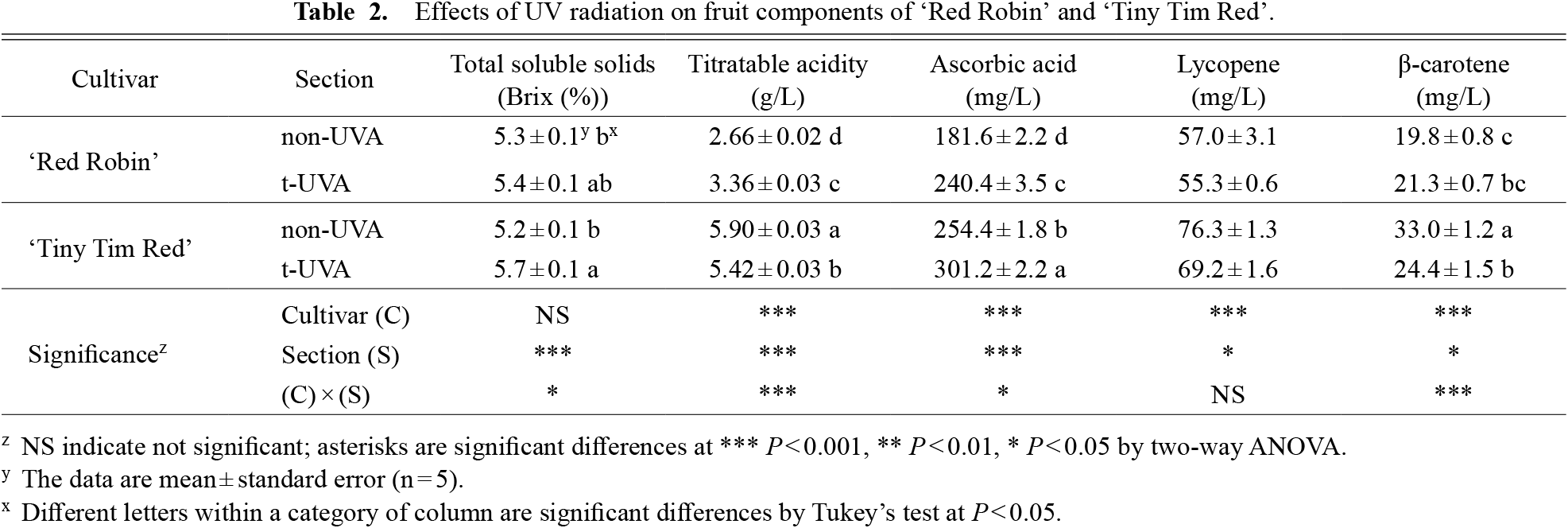
Effects of UV radiation on fruit components of ‘Red Robin’ and ‘Tiny Tim Red’.
The ascorbic acid content of ‘Tiny Tim Red’ was significantly higher than that of ‘Red Robin’ at P < 0.001, and the UV-A irradiation treatment showed a significant effect on the ascorbic acid content in the fruit at P < 0.001 (Table 2). There was a significant difference in the (C × S) at P < 0.05 and, the t-UVA of ‘Tiny Tim Red’ had significantly the highest ascorbic acid content compared to the other fruits by Tukey’s test at P < 0.05 (Table 2). Previous papers reported an increase in the ascorbic acid with UV-B (1.69 W·m−2) or UV-C irradiation of post-harvest fruits (Jagadeesh et al., 2011; Castagna et al., 2013), and it is considered that the ascorbic acid also increased due to the effect of the UV-A irradiation in this study.
The lycopene content of ‘Tiny Tim Red’ and non-UVA was significantly higher than that of ‘Red Robin’ at P < 0.001, and the UV-A irradiation treatment reduced the lycopene content of the fruit at P < 0.05 (Table 2). There was no significant difference in the (C × S) by two-way ANOVA. The β-carotene content was significantly different in the cultivars and sections at P < 0.001 and at P < 0.05, respectively. There was a significant difference in the interaction at P < 0.001, and the non-UVA of ‘Tiny Tim Red’ had the highest β-carotene and lycopene content of all sections by Tukey’s test at P < 0.05 (Table 2). Giuntini et al. (2005) showed that tomato plants grown in a greenhouse with UV-B had a higher β-carotene and lycopene content than tomato plants grown without UV-B. Castagna et al. (2013, 2014) and Dyshlyuk et al. (2020) also reported that post-harvest UV-B or UV-A radiation increased the content of β-carotene and lycopene. In these reports, UV-B radiation of tomato plants or fruits promoted further carotenoid accumulation. In our study, the carotenoid content showed a decrease, except for β-carotene in the UV-A of ‘Red Robin’, which was different from these reports. In Perez et al. (2009), UV-B irradiation (0.075 Wh·m−2, 22 h) of pre-harvest tomatoes ‘Liberto’ showed a maximum increase in β-carotene and lycopene content, but a reduction in carotenoid content was observed depending on the ultraviolet irradiation intensity and irradiation time. Therefore, in this study, too, it was considered that the β-carotene and lycopene content decreased due to UV-A irradiation at a higher irradiation intensity than in other reports during the cultivation period until the harvest date.
In conclusion, we examined the cultivation of the dwarf cherry tomatoes ‘Red Robin’ and ‘Tiny Tim Red’ for use in a plant factory with artificial light and investigated the effect of ultraviolet radiation. Our research showed that UV irradiation had a positive effect on the TSS, TA and ascorbic acid, and a negative effect on fruit size per fruit and carotenoid content. UV irradiation did not affect the yield per plant. Comparing ‘Red Robin’ and ‘Tiny Tim Red’, there was no significant difference in yield, but the fruit size of ‘Red Robin’ was larger than the other fruits, and the fruit component of ‘Tiny Tim Red’ was higher than that of ‘Red Robin’. Therefore, when cultivating tomato plants in a plant factory, further improvements in fruit size and fruit composition are expected by appropriately adjusting the time and intensity of UV irradiation for each cultivar.
We wish to thank Tamagawa University Research Institute Biosystems & Biofunctions Research Center for the use of the plant factory with artificial light and the researchers at the center for supplying kind advice. We thank students and all our colleagues for their assistance in the research for this paper.