2022 年 91 巻 1 号 p. 94-103
2022 年 91 巻 1 号 p. 94-103
Roses are among the most widely produced cut flowers, with important ornamental and economic value in the flower market. In order to investigate how to regulate flowering periods, the negative regulator of GA biosynthesis RcSPY was cloned from Rosa chinensis and ectopically expressed in tobacco. The overexpression of RcSPY in SPY4 transgenic tobacco significantly increased plant height. Antisense interference with the conserved SPY domain of RcSPY led to GA accumulation in SPY5 transgenic tobacco plants, and antisense interference with the conserved TPR repeats of RcSPY resulted in late flowering in SPY6 transgenic tobacco plants. This result suggests that RcSPY is a negative regulator of GA biosynthesis, since the knock-down of RcSPY increased GA content. However, other phenotypes displayed by transgenic tobacco plants may not be caused by changes of GA content, but rather be the direct effects of RcSPY. Therefore, RcSPY not only participates in GA biosynthesis, but also plays a role in other vegetative and reproductive plant behaviors not related to GA. This result provides theoretical support for the molecular breeding of new cultivars of rose plants.
Gibberellic acid (GA), noted for its crucial role in the regulation of seed germination (Jacobsen et al., 1996; Ge and Steber, 2018), was later found to participate in many steps of plant development processes, such as cell elongation, shoot extension, flower induction, and seed development (Blázquez et al., 1998; Singh et al., 2002; Gomi and Matsuoka, 2003). A GA inhibitor, paclobutrazol, inhibited germination and induced dwarfing (Silverstone et al., 2007), further confirming the significant influence of GA on plant development.
As tetracyclic diterpenoid plant growth regulators, GAs are well-known for their biosynthesis pathway in plant. This large family includes more than 100 active members, of which GA1, GA3, GA4, and GA7 are biologically active in higher plants. The precursor of GAs is geranylgeranyl diphosphate (GGPP), which is converted by ent-copalyl diphosphate synthase (CPS) to form copalyl diphosphate (CDP). Then, CDP is converted into GA9 and GA19 by several enzymatic steps. The final step of the formation of bioactive GA4 and GA1 is catalyzed by GA 3-oxidase (GA3ox), while GA 2-oxidase (GA2ox) can deactivate GA1 and GA4 (Olszewski et al., 2002; Israelsson et al., 2005).
Many studies on GA-related mutants and exogenous treatment with GAs or paclobutrazol showed that the GA-GID1-DELLA module may be a pivotal regulatory hub in GA signal transduction (Sun, 2010). GID1 (GA INSENSITIVE DWARF1) is considered to be a GA receptor (Ge and Steber, 2018). DELLA proteins are identified by their DELLA motifs near the N terminus, including REPRESSOR OF ga1-3 (RGA), GA INSENSITIVE (GAI), RGA LIKE1 (RGL1), and RGL2 (Silverstone et al., 2007), which were considered as a molecular switch for GA signaling (Gomi and Matsuoka, 2003; Salanenka et al., 2018).
In addition to the negative effects of DELLA proteins in GA regulation, another negative regulator, SPINDLY, was also found to participate in GA signal transduction (Izhaki et al., 2001). The SPINDLY (SPY) protein was first reported in animals. Its homologous protein, O-linked GlcNAc transferase (OGT), can modify other proteins by adding a single GlcNAc in an O-linkage to Ser and/or Thr (Hart, 1997). These homologous proteins have a tetratricopeptide repeat (TPR) domain and an OGT catalytic domain. Many studies on biochemistry structure and functional characterization of SPY have found that the TRP domain is critical for the function of SPY protein, acting as scaffolds for the assembly of multiprotein complexes (Jacobsen et al., 1996; Tseng et al., 2001; Silverstone et al., 2007). These reports also showed that SPY protein in plants generally acts as a negative regulator of gibberellin signaling, as it inhibited various GA-regulated processes (Swain et al., 2002). According to genetic analyses of GA-related mutants, SPY protein may control the suppressive function of the DELLA protein to regulate GA-response genes (Gomi and Matsuoka, 2003; Shimada et al., 2006).
SPY can delay flowering by inhibiting the induction of flowers, acting as a negative regulator of the GA pathway (Tseng et al., 2004). The petunia SPY protein reduced sensitivity to GA and induced a similar phenotype to that of paclobutrazol treatment. Additionally, the over expression of PhSPY delayed the flowering time of Petunia hybrida by 6–7 weeks compared to the wild type (Izhaki et al., 2001). The Arabidopsis O-GlcNAc transferases, SECRET AGENT (SEC) and SPY, both have unique and overlapping functions in leaf production and carpel development (Hartweck et al., 2006). The spindly mutant of Arabidopsis has a premature middle cortex phenotype in root, suggesting that inhibition of histone deacetylase activity caused by SPY may regulate middle cortex formation through an epigenetic mechanism (Cui and Benfey, 2009). In studies on the exact function of the SPY protein, some results indicated that it plays a role in GA signaling, but some also indicated that the phenotype caused by SPY was not directly related to GA (Izhaki et al., 2001; Swain et al., 2001; Maymon et al., 2009).
Rose, as one of four cut flowers worldwide, has great economic value. In order to elongate the vase life of rose, RcSPY involved in the GA signaling pathway was investigated in this study. The RcSPY gene was found to be highly expressed in the sepals and lowest in the blooming flowers of Rosa chinensis. We then transformed tobacco with the RcSPY gene, mainly to investigate its influence on the flowering periods. RcSPY was mainly located in the cytoplasm of infiltrated tobacco leaf cells. Furthermore, different regions of the RcSPY gene appear to play different roles in plant growth. Antisense interference with the conserved SPY family sequence of RcSPY may increase the GA content in transgenic tobacco plants, but antisense interference with the conserved TPR repeats in RcSPY delayed the flowering period with invariable GA content. However, the overexpression of RcSPY significantly increased the height of transgenic tobacco plants with invariable GA content. This result suggests that RcSPY may participate in various processes of plant development, not all of which are related to GA signaling.
Rosa chinensis ‘Slater’s Crimson China’ was bought from Fenghua Landscaping Co., Ltd., Wuhan, China and planted in the greenhouse of Wuhan University of Bioengineering, Wuhan, China (114.5°E, 30.7°N) under natural light conditions. Nicotiana tabacum was kindly provided by associate professor Wenjun Huang (Wuhan Botanical Garden, Chinese Academy of Sciences). Transgenic and wild-type tobacco plants were grown in the greenhouse under a 16 h light/8 h dark cycle at 25°C and 60% humidity.
RNA extraction and cloning of the RcSPY gene from rosesThe fresh young leaves, stems, pedicels, sepals, petals in three flowering stages (early flowering stage, blooming stage and withered stage) of Rosa chinensis were collected and ground into powder with liquid nitrogen. The total RNA was isolated using the TaKaRa MiniBEST Plant RNA Extraction Kit (Takara, China), following the manufacturer’s instructions. The RNA quality was assessed by agarose gel electrophoresis and determined using a Nano Drop 2000 instrument (Thermo Fisher Scientific, USA). The cDNA was synthesized using the PrimeScriptTMRT reagent Kit (Takara) and its integrity was confirmed by PCR with β-actin of a Rosa hybrida cultivar as the positive control (GeneBank: AB239794). The PCR conditions were 94°C, 3 min; followed by 35 cycles at 94°C, 30 s, 52°C, 30 s, 72°C, 30 s; and a final extension at 72°C for 8 min. The primers used to amplify the RcSPY gene were designed based on the sequence in NCBI (GeneBank: XM_024331721). The full-length sequence of RcSPY was cloned from the cDNA of the blooming flower sample. The PCR program was as follows: 94°C for 5 min, followed by 32 cycles of 94°C for 30 s, 53°C for 45 s, and 72°C for 1 min, 72°C for 10 min and hold at 16°C. The primer sequences are listed in Table S1.
Bioinformatic analysis of RcSPY and construction of the phylogenetic tree of SPY proteinsThe RcSPY coding region was sequenced by Sangon Biotech (Shanghai, China). The sequence was analyzed using ProtParam to evaluate the molecular weight and isoelectric point of RcSPY protein <http://web.expasy.org/protparam/>. The subcellular localization of RcSPY was predicted using CELLO v.2.5 <http://cello.life.nctu.edu.tw/>. The conserved domain of RcSPY was predicted with CDD in NCBI <http://www.ncbi.nlm.nih.gov/cdd>. The homologous SPY sequences from other species were downloaded from the NCBI database, including Medicago truncatula (XM_013612504.2), Cajanus cajan (XM_020370987), Vigna radiata (XM_014640348.2), Glycine max (XM_003553556.4), Lupinus angustifolius (XM_019588687.1), Rosa wichurana (FM999800.1), Prunus avium (XM_021963819.1), Pyrus × bretschneideri (XM_009374756.2), Cucurbita moschata (XM_023070391.1), Momordica charantia (XM_022276946.1), Malus domestica (XM_008388816.3), Spinacia oleracea (XM_022002642.1), Citrus sinensis (XM_025099695.1), Brassica napus (XM_022708218.1), Thellungiella halophila (AK353060.1), Eustoma grandiflorum (AB080739.1), Setaria italica (XM_022827578.1), Sorghum bicolor (XM_021465034.1), Oryza sativa (XM_015794260.1), Phyllostachys praecox (DQ013806.1), Hordeum vulgare (AF035820.1), Ananas comosus (XM_020232630.1), Dendrobium catenatum (XM_020846913.2), Phalaenopsis equestris (XM_020721381.1), Asparagus officinalis (XM_020391548.1), Helianthus annuus (XM_022114509.1), Sinningia speciosa (EU878416.1), Petunia hybrida (Y17720.1), Nicotiana attenuate (XM_019389486.1), Durio zibethinus (XM_022911292.1) and Populus euphratica (XM_011006393.1). The phylogenetic tree was constructed using MEGA5.1 software at the default settings.
Quantitative RT-PCR assayThe cDNA samples derived from the different tissues and different flowering stages were used as templates to detect the spatio-temporal expression patterns of the RcSPY gene. The β-actin gene was used as the internal control with the amplification primers listed in Table S1. The total RNA was extracted from transgenic tobacco plants and reverse-transcribed into the cDNA. The GA-related genes in tobacco were downloaded from the NCBI database, including NtSPY (XM_016597203.1), NtGA2-ox (JQ413249.1), NtGA2-ox2 (AB125233.1), NtGA2-ox5 (EF471118.1), and NtGA3-ox2 (EF471116.1). In order to investigate the involvement of RcSPY in the determination of flowering time, variation in the expression levels of the NtFT-10 gene (KY306478.1) was also measured. The primers used for qRT-PCR analysis are listed in Table S1, namely qNtSPY-F1/R1, qNtGA2ox-F1/R1, qNtGA2ox2-F1/R1, qNtGA2ox5-F1/R1, qNtGA3ox2-F1/R1, and qNtFT10-F1/R1. The Tubulin gene from tobacco was used as the internal control. The real-time PCR assay was conducted using ABI7500 (Thermo Fisher Scientific). The reagents were mixed in a 10 μL system containing 5 μL 2×SYBR Premix (Takara, China), and 0.25 μL each of up- and downstream primers (10 μM). The PCR procedure followed the default two-step settings: 95°C for 10 min, followed by two steps of 95°C for 15 s and 60°C for 1 min. The fluorescent signal of the SYBR green dye was measured and the PCR procedure used the default settings as reference. The relative expression value was calculated using the 2−△△CT (Livak) method: △△CT = (CT, gene- CT, actin) treatment- (CT, gene- CT, actin) control. The dissociation curves were used to detect primer dimers and other non-specific by-products. Samples were analyzed in biological triplicates.
The subcellular localization of RcSPY in tobacco leaf cellsThe CDS of RcSPY was subcloned into the pCambia1300-35S-GFP vector with the primers of RcSPY-F3/R3 listed in Table S1 between the KpnI and XbaI sites before the GFP ORF. The recombinant plasmid was introduced into Agrobacterium tumefaciens GV3101, which was then used to infiltrate the abaxial sides of leaves of N. benthamiana transiently, with pBWA(V)HS-ccdb-GLosGFP as the positive control. Then, the transfected tobacco leaves were observed under a confocal laser-scanning microscope (C2-ER; Nikon, Japan).
Construction of the overexpression vector and antisense interference vector of RcSPY and the Agrobacteria-mediated transformation of tobaccoThe CDS of RcSPY was subcloned into the pCambia1300-35S vector using the primers of RcSPY-F4/R4 listed in Table S1. According to the conserved region prediction in bioinformatic analysis, two typical regions homology with O-linked N-acetylglucosamine transferase and the TPR superfamily, corresponding to the specific fragments of the RcSPY gene 980–1409 bp and 21–462 bp, were chosen as the antisense fragments amplified using the primers RcSPY-F5/R5 and RcSPY-F6/R6 listed in Table S1, and were also ligated into the pCambia1300-35S vector between the XbaI and KpnI sites. Then, the plasmids were introduced into Agrobacterium tumefaciens GV3101, which was then used in tobacco transformation by the leaf disc methods described by Huang et al. (2016). The pCambia1300-35S-GFP vector was transformed into the tobacco as wild type (WT) control. Then the transgenic plants were selected on Murashige and Skoog (MS) medium supplemented with 30 mg/L hygromycin (neoBioFroxx GmbH, Germany).
Measurement of the GA1 and GA4 levels in tobacco transformantsThe stems of transgenic tobacco plants were shock-frozen in liquid nitrogen and approximately 0.5 g was homogenized in 5 mL of acetonitrile overnight. Then, the supernatant was centrifuged at 4°C, 12000 g for 5 min. The precipitate was extracted again with 4 mL of acetonitrile, and the two supernatants were combined. The collected supernatant was purified with 50 mg C18, 50 mg GCB, and 500 mg MgSO4, under vigorous vortexing. After centrifugation, the supernatant was dried under nitrogen gas and resuspended in 200 μL methanol. Each sample was purified with a 0.22 μm Millipore filter (Sigma-Aldrich, St. Louis, MO, USA) and stored at −20°C until measurement. The samples were separated on an Agilent 1290 series HPLC system (Agilent Technologies, Palo Alto, CA, USA) equipped with a C18 column (2.1 m × 150 mm, 2.7 μm), UV-Vis detector, an auto-sampler and a SCIEX-6500 Qtra MS system (Applied Biosystems, Foster City, CA, USA). The analysis was performed under the following conditions: detection wavelength, 280 nm; flow rate, 0.3 mL/min; injection volume, 2 μL; and column temperature, 30°C. The mobile phase was composed of a mixture of (A) methanol/0.1% formic acid, and (B) water/0.1% formic acid. The gradient program was set as follows: 0–1.0 min, 20% A; 1–3 min, 20–50% A; 3–9 min, 50–80% A; 9–10.5 min, 80% A; 10.5–10.6 min, 80–20% A; 10.6–13.5 min, 20% A. The GA contents (GA1 and GA4) were determined according to the retention time and MS analysis, followed by a calculation using the equivalents of the external standard chemicals. The authentic reference standards of GA1 (TRC, USA) and GA4 (Sigma-Aldrich) were diluted to 1 μg·mL−1 in methanol for storage and further diluted to yield a gradient of 0.1 ng·mL−1, 0.2 ng·mL−1, 0.5 ng·mL−1, 2 ng·mL−1, 5 ng·mL−1, 20 ng·mL−1, 50 ng·mL−1, and 200 ng·mL−1 for the standard curves. Each sample was analyzed in biological triplicates.
Phenotype analysis of tobacco transformantsThe plant height and transition time from the vegetative to reproductive phase of the transformed tobacco plants were quantified and compared to the wild type, at least three individual transgenic tobacco plants harboring corresponding vectors were included in the statistics. The abaxial epidermis of the transformed tobacco plant was removed and observed under an optical microscope (Tech Instrument, Beijing, China), and photographed using a camera (WV-CP500L; Panasonic, Japan).
Statistical analysisData on the relative expression levels of RcSPY in rose and GA-related genes in transformed tobacco plants were presented as the means ± SE from n = XY parallel experiments. The statistics of these data were subjected to analysis of variance using SigmaPlot 12.5 software. Data on the plant heights, flower initiation time and GA contents were presented as the means ± SD and multiple groups of data were compared via one-way ANOVA and Duncan’s test, with P < 0.05 considered significant.
The total RNA was collected from rose tissues and the cDNA was used to amplify the full-length sequence of the RcSPY gene. A fragment of about 2700 bp was amplified (Fig. S1). The sequencing result showed that RcSPY encompasses 2751 bp, encoding a protein of 916 amino acids with a predicted molecular mass of 101.7 kDa and pI of 5.59. The RcSPY protein was predicted to be located in the cytoplasm according to CELLO v.2.5. Based on the CDD prediction in the NCBI database, the region between 216 and 1421 nt shares homology with the TPR superfamily, and the region between 1002 and 2600 nt is predicted to be analogous with O-linked N-acetylglucosamine transferase (Fig. S1).
A total of 31 SPY protein sequences were downloaded from the NCBI database to construct a homology tree with RcSPY using MEGA5.1 software (Fig. S2). RcSPY shared the closest homology with RwSPY derived from Rosa wichurana. The phylogenetic tree suggested that the functions of SPY proteins among higher plants are highly conserved.
The RcSPY gene was generally highly expressed in vegetative organs and showed low expression in flowers at the blooming stageThe spatio-temporal expression pattern of the RcSPY gene in roses was analyzed in leaves, stems, petals, pedicels, flower buds, and petals at blooming stage and withered stage by qRT-PCR, with the endogenous β-actin gene as internal control. As shown in Figure 1, the RcSPY gene was highly expressed in sepals, stems and petals at the withered stage, while the expression was significantly lower in the flower buds, pedicels, and flowers at blooming stage. The SPY protein is well known as a negative regulator of GA signaling, and the expression pattern of RcSPY in roses suggests that the lowly expression of RcSPY in petals at blooming stage may increase GA accumulation in this stage, which could promote flowering and prevent flower abscission.
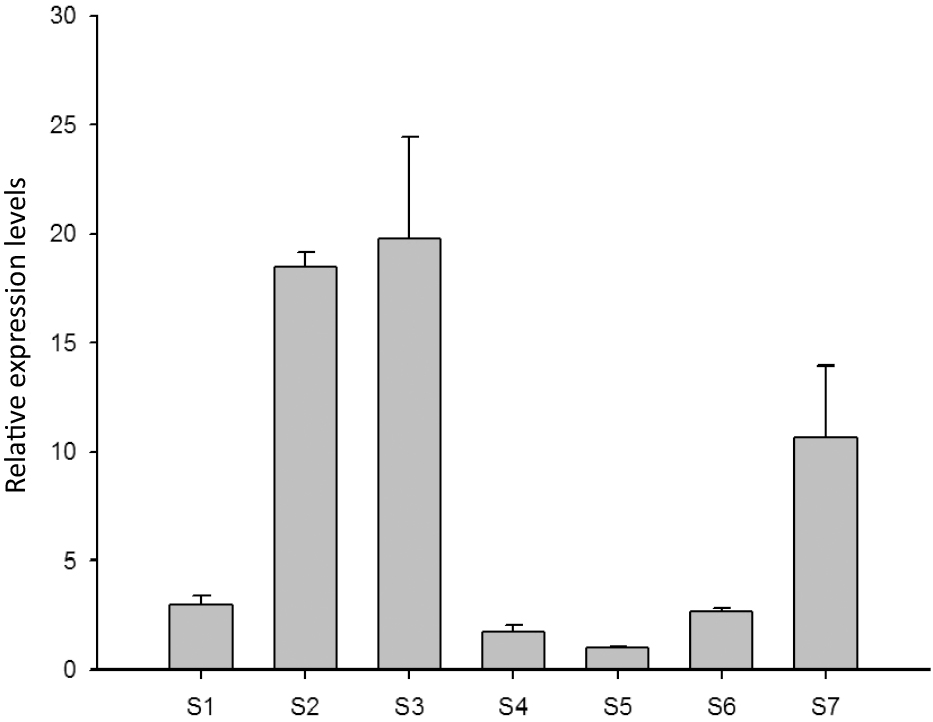
The expression pattern of RcSPY in Rosa chinensis. S1: leaf; S2: young stem; S3: sepal; S4: pedicle; S5: flowers bud; S6: flower at the blooming stage; S7: flower at the withered stage.
To assess the subcellular localization of RcSPY, the pC1300-35S-RcSPY-GFP plasmid was infiltrated into the young leaves of tobacco plants, and the fluorescence of the fusion protein was visualized by confocal microscopy. As shown in Figure 2, RcSPY was present mainly in the cytoplasm, although the fluorescence signal was not very strong. RcSPY was not present in the chloroplasts, which have red auto-fluorescence.
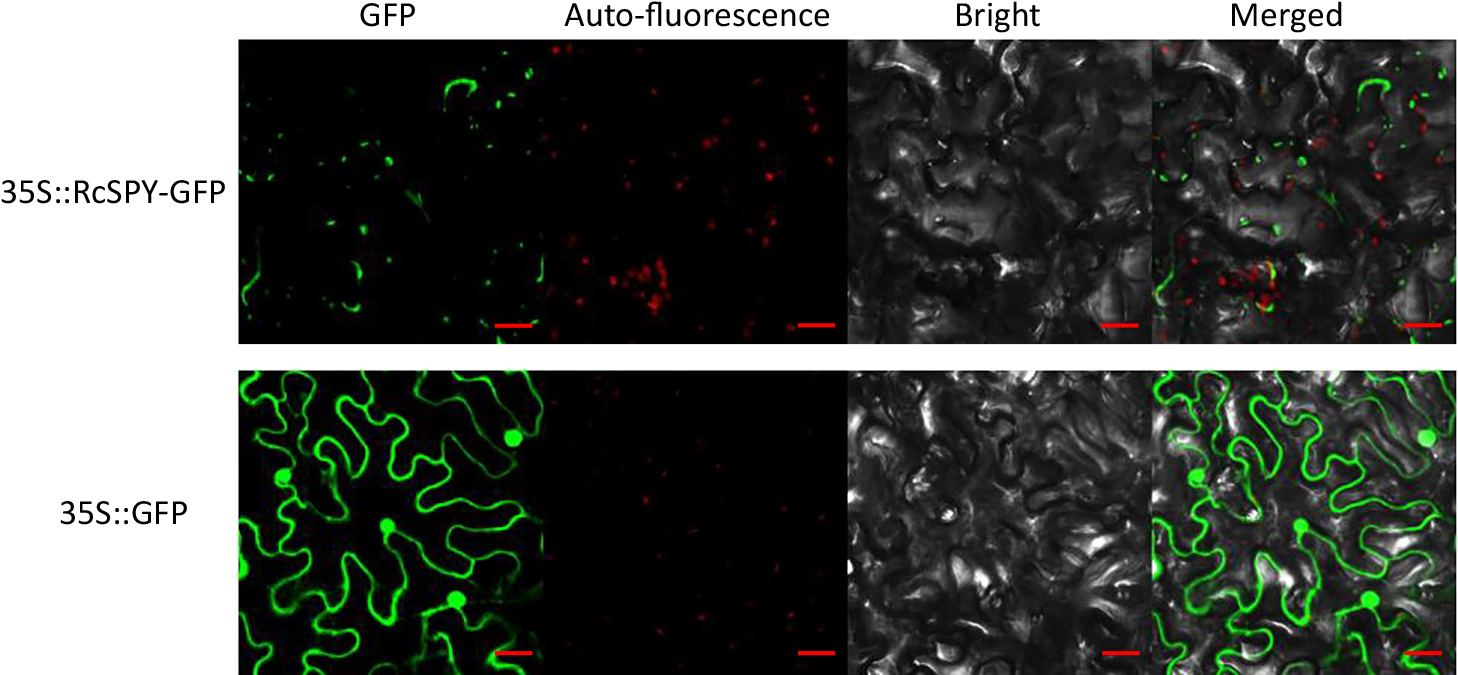
Subcellular localization of RcSPY in tobacco leaves. RcSPY and GFP positive control were transiently expressed in tobacco epidermal cells. The upper lane shows the 35S: RcSPY-GFP and the bottom lane shows the 35S: GFP. Images from left to right show the auto-fluorescence, GFP, bright-field, and overlay of the illuminations. Bars, 20 μm.
To identify the function of RcSPY, the pC1300-35S-RcSPY and the pC1300-35S-antiRcSPY plasmids were introduced into tobacco plants via Agrobacterium transfection. About 15 transgenic tobacco plants per construct were identified by PCR amplification (data not shown). The transformed tobacco plants were named SPY4 with pC1300-35S-RcSPY (4-1, 4-2, and 4-5), SPY5 with pC1300-35S-antiRcSPY980-1409 (5-1, 5-5) and SPY6 with pC1300-35S-antiRcSPY21-462 (6-1) were selected for further research. Compared to the wild type, the leaves of the SPY 6-1 transgenic plant showed a deep green color, while the leaves were pale green in the SPY 4-1 transgenic plant (Fig. 3a). The leaves of the SPY 4-1 transgenic plant appeared more brittle and easier to break than those of the SPY 6-1 transgenic plant when we transplanted the transformed tobacco plants into soil. At flower initiation time, the height of the whole plants was significantly different between transgenic plants and WT as shown in Table 1 and Figure 3b. The average height of SPY4, SPY5, and SPY6 transgenic plants was respectively 181 ± 7.5, 95 ± 5.0, and 86 ± 4.5 cm, compared to 144 ± 7.9 cm in the wild type. The SPY4 transgenic plants were significantly taller than the WT, but the plant heights of SPY5 and SPY6 transgenic plants were significantly lower, especially in SPY6 transgenic plants (Duncan’s test, with P < 0.05). The flower initiation time was 145 ± 6.2 d in SPY4 transgenic plants, 135 ± 6.1 d in SPY5 transgenic plants, 192 ± 7.2 d in SPY6 transgenic plants and 129 ± 3.6 d in the wild type as shown in Table 1. Therefore, SPY6 transgenic plants showed a significant delay in flowering compared to other tobacco plants (Duncan’s test, with P < 0.05). The flowering stage lasted almost the same time among the different tobacco plants (data not shown).
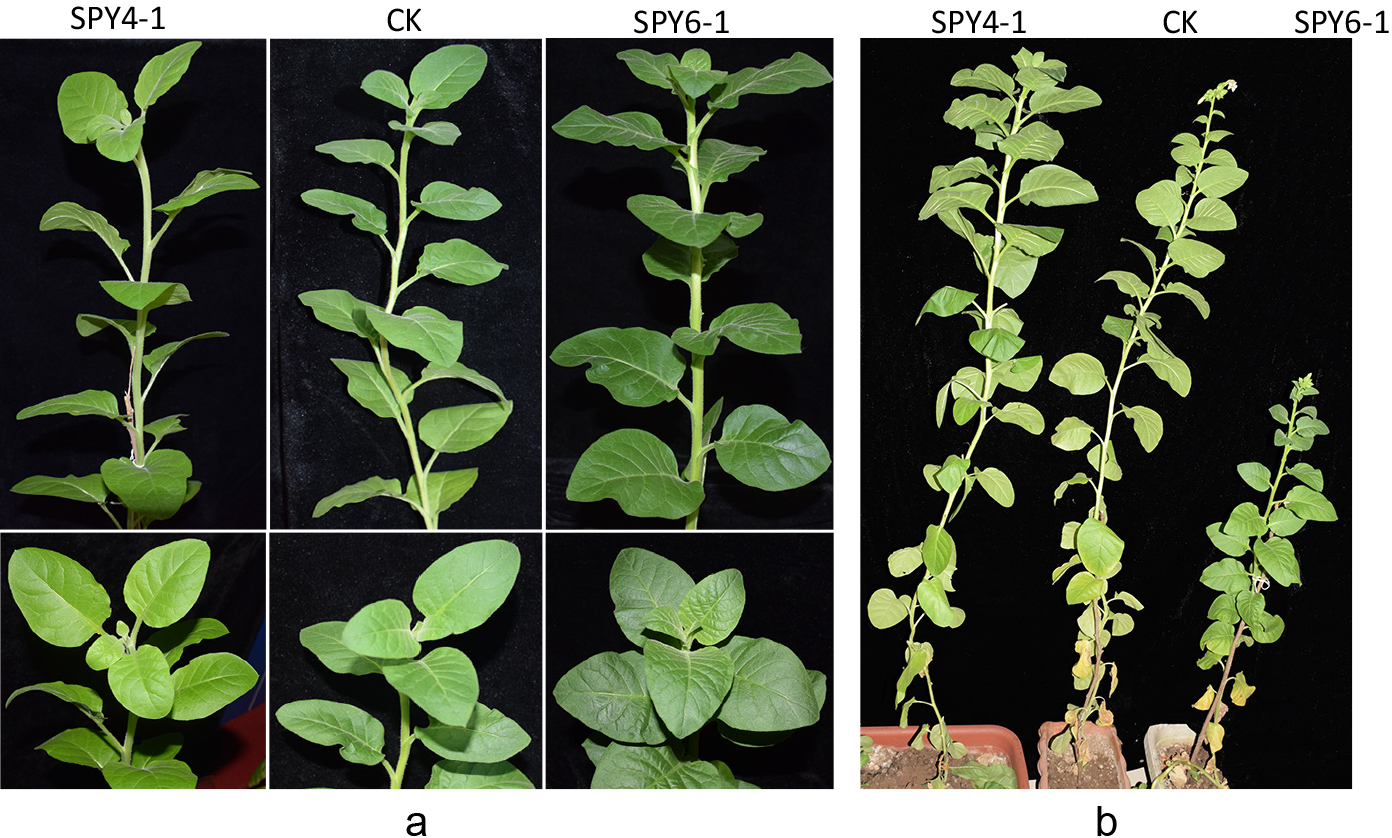
Phenotype of the transgenic tobacco plants with RcSPY gene. a. Transgenic tobacco plants in the vegetative phase. The upper lane was photographed from front to back, and the bottom lane was photographed from top to bottom. b. Transgenic tobacco plants in the flowering phase.

The plant height and vegetative phase of the transgenic tobacco plants and wild type control. The data are presented as the means ± SDs from three biological replicates. The letters indicate significant differences according to Duncan’s multiple range test (P < 0.05).
In order to investigate the differences of leaf color and plant height in transgenic tobacco plants, we observed the cells in the transgenic tobacco leaves. As shown in Figure 4, compared to the wild type, the cells were more condensed in SPY6-1 transgenic plant, but sparse in SPY4-1 transgenic plant, especially the guard cells. However, there was no obvious difference between SPY4-1 and SPY5-1 transgenic plants. These observations suggest that the dwarf phenotype and deep green color of SPY6 transgenic plants may be the result of condensed cell growth.

Cell density of transgenic tobacco leaves. Images from left to right show the wild type control, SPY4-1, SPY5-1, and SPY6-1, respectively. The magnification is 10 × 10 with an optical microscope.
To directly test the relationship between RcSPY and GA accumulation, the GA levels of the transgenic tobacco plants and WT were measured by HPLC-MS (Fig. S3). As shown in Table 2, the GA1 content was 0.22 ± 0.03 ng·g−1 in SPY4 transgenic plants, 0.49 ± 0.01 ng·g−1 in SPY5 transgenic plants, 0.21 ± 0.003 ng·g−1 in SPY6 transgenic plants, and 0.25 ± 0.02 ng·g−1 in the wild type. The GA4 content was 0.10 ± 0.007 ng·g−1 in SPY4 transgenic plants, 0.52 ± 0.02 ng·g−1 in SPY5 transgenic plants, 0.20 ± 0.03 ng·g−1 in SPY6 transgenic plants, and 0.09 ± 0.01 ng·g−1 in the wild type. Thus, the GA content in SPY5 transgenic plants was significantly higher than in the wild type, but was almost invariable in SPY4 and SPY6 transgenic plants compared to the wild type (Duncan’s test, with P < 0.05).
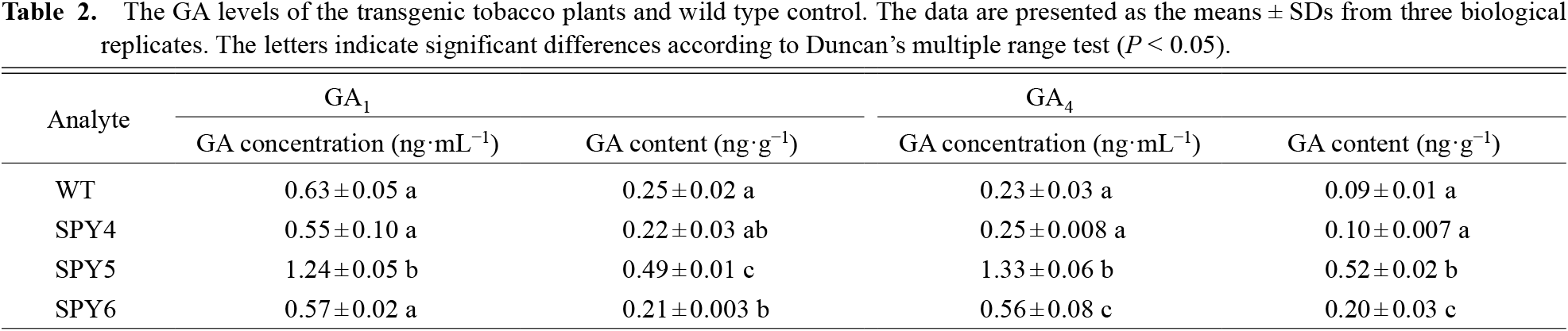
The GA levels of the transgenic tobacco plants and wild type control. The data are presented as the means ± SDs from three biological replicates. The letters indicate significant differences according to Duncan’s multiple range test (P < 0.05).
The expression levels of GA-related genes were measured by qRT-PCR. As shown in Figure 5, the expression of the endogenous NtSPY gene was decreased in SPY4 transgenic plants but increased in SPY5 and SPY6 transgenic plants, suggesting that the ectopically expressed RcSPY could partially compensate for the function of NtSPY in transgenic tobacco. The expression of the GA 3-oxidase genes responsible for GA formation and GA 2-oxidase genes responsible for GA deactivation were also detected. Interestingly, all the GA 3-oxidase and GA 2-oxidase genes were down-regulated in SPY4 transgenic plants, but up-regulated in SPY5 transgenic plants. In fact, the GA 3-oxidase gene was significantly increased in SPY5 transgenic plants, which may have contributed to the increased GA accumulation in SPY5 transgenic plants compared to the wild type and SPY4 transgenic plants. Despite the RcSPY antisense interference in SPY6 transgenic plants, its GA content was similar to the wild type, and all of the GA 3-oxidase and GA 2-oxidase genes in SPY6 transgenic plants were significantly down-regulated, even lower than that of SPY4 transgenic plants. This result suggests that different conserved regions in RcSPY may exert different effects on GA biosynthesis in tobacco plants.

The expression levels of GA-related genes in the transgenic tobacco plants. The error bars present the means of three biological replicates ± SEs. NtSPY: NtSPINDLY; FT-10: NtFT-10; GA2-ox: Nt GA2-oxidase; GA2-ox2: Nt GA2-oxidase 2; GA2-ox5: Nt GA2-oxidase 5; GA3-ox2: Nt GA3-oxidase 2.
Because the flowering time was delayed in the SPY6 transgenic tobacco plants compared to other plants, we also measured the expression of the NtFT gene (FLOWERING LOCUS T), in charge of floral development in transgenic tobacco plants. As shown in Figure 5, the NtFT gene was significantly up-regulated in SPY5 transgenic plants but down-regulated in SPY6 transgenic plants, although both were subjected to the antisense interference of RcSPY.
GA is widely present in plants and plays crucial roles in vegetative and reproductive growth. Moreover, the GA pathway appears to be a key regulator of flowering in roses (Remay et al., 2009). Elucidating the still unknown molecular mechanisms of flower longevity in roses would have great commercial value and significantly improve rose breeding. Accordingly, extensive studies have been performed to prolong the flowering period of this important horticultural crop. Arabidopsis AT-HOOK MOTIF NUCLEAR LOCALIZED 15 (AHL15) suppressed axillary meristems (AM) maturation and extend the plant’s lifespan mediating upstream of the flowering-promoting hormone gibberellic acid (Karami et al., 2020). External GA3 pre-treatment increased flower longevity in Iranian Anemone accessions (Yari et al., 2021). The senescence-associated genes (SAGs) in rose petals of two cultivars with different longevities were isolated based on cDNA-AFLP (Hajizadeh et al., 2014). In this study, a negative regulator gene of GA signaling, RcSPY, was cloned from rose plants and its function was primarily characterized. In order to investigate the function of RcSPY in the regulation of flowering and its contribution to prolonging flower longevity, especially long vase life, the RcSPY gene was ectopically expressed into tobacco by Agrobacterium-based transformation. According to HPLC-MS analysis, GA was accumulated in the SPY5 transgenic plants compared to the wild type, but not in SPY4 transgenic plants or SPY6 transgenic plants, suggesting that at least the repression of RcSPY in SPY5 transgenic plants could increase the GA contents in tobacco. RcSPY protein may therefore be a negative regulator of the GA biosynthesis pathway, which is consistent with previous research (Jacobsen et al., 1996; Izhaki et al., 2001; Shimada et al., 2006). DELLA proteins are known as repressors of GA signaling, and can be activated by SPINDLY (Hynes et al., 2003; Silverstone et al., 2007). The TPR repeats in the N-terminus of RcSPY, which were subjected to antisense interference in the SPY5 transgenic tobacco plants, are very important in contributing to plant development, and RcSPY may be a member of multiple complex networks of GA signaling.
In order to investigate how the RcSPY gene exerts its function in transgenic tobacco plants, the expression levels of GA-related genes were detected by qRT-PCR analysis. The final step of the formation of bioactive GA4 and GA1 is catalyzed by GA 3-oxidase (GA3ox), while GA 2-oxidase (GA2ox) can deactivate GA1 and GA4 (Israelsson et al., 2005; Zhong et al., 2014). As shown in Figure 5, the GA 3-oxidase and GA 2-oxidase genes showed the same expression trend in the transgenic tobacco plants, without apparent trade-offs for accumulation of active or inactive GAs. It seems that GA content in the plant is carefully balanced by the formation and deactivation mechanisms. The phenotype of the SPY 6-1 transgenic plants, consistent with the results reported in other plants, like Prunus salicina (El-Sharkawy et al., 2012), Poa pratensis (Tan et al., 2018), Nicotiana tabacum (Gargul et al., 2013), Camellia lipoensis (Xiao et al., 2016) and Brassica napus (Yan et al., 2017), included dwarfism, short stem internodes, late flowering and increased chlorophyll accumulation. However, the GA content is almost invariable compared to the wild type. The maize DELLA proteins dwarf plant8 and dwarf plant9 showed that the delay of flowering appears to be linked to dwarfing, which is the opposite of the Arabidopsis results (Lawit et al., 2010). Combined with the results in this study, it suggests that there are possible differences between monocots and dicots in GA signaling mechanisms.
It was reported that the TPR domain at the N terminus of SPY is responsible for the SPY-SPY and SPY-GI (GIGANTEA) interactions (Vaistij et al., 2000; Tseng et al., 2001). Furthermore, GI acts in the long-day flowering pathway upstream of CONSTANS (CO) and FLOWERING LOCUS T (FT) (Onouchi et al., 2000; Samach et al., 2000; Suárez-López et al., 2001). FT is related to the activation of flowering and is a primary candidate for encoding florigen (Zeevaart, 2008). In this study, the flowering time of tobacco plants was rarely related to RcSPY, but was closely related to the expression levels of the NtFT gene. The overexpression of RcSPY (SPY4 transgenic tobaccos) and antisense interference with RcSPY between 980 and 1409 nt (SPY5 transgenic tobaccos) both increased the expression of NtFT, but antisense interference with RcSPY between 21 and 462 nt (SPY6 transgenic tobaccos) decreased the expression of NtFT, which led to late flowering in SPY6 transgenic tobaccos compared to the wild type. Another reason for the different flowering times in SPY5 and SPY6 transgenic tobaccos may be the conserved TPR repeat domain of SPY protein, since SPY6 transgenic tobaccos were subjected to the antisense interference with the RcSPY fragment in the TPR repeat domain (21–462 nt), while SPY5 transgenic tobaccos were subjected to antisense interference with the RcSPY fragment adjacent to the conserved SPY domain (980–1409 nt). RcSPY protein contains 10 TPR repeats at the N-terminus, while NtSPY contains 9 TPR repeats but mostly lacks the conserved SPY superfamily domain.
Most of the SPY protein molecules are localized in the nucleus, consistent with the nuclear localization of other components of the GA signaling mechanism (Swain et al., 2002). However, in this study, fluorescence-based analysis of the subcellular localization of RcSPY in tobacco leaves showed that the majority of the SPY protein is located in the cytoplasm (Fig. 2), and was less abundant in the nucleus. This is consistent with the predicted lack of nuclear localization signals (NLS) in RcSPY. Currently, how it is transported into the nucleus to function together with other components of the GA signaling pathway is still unclear. It is possible that some other proteins with a NLS interact with RcSPY to guide it into the nucleus, with the help of the TPR repeats at the N terminus.
In fact, we would like to demonstrate the involvement of RcSPY in the regulation of flowering time in rose, but the latter result showed that RcSPY also has an effect on the vegetative phenotype of plants, including plant height, cell density and plant toughness, which is obviously reflected in the SPY4 transgenic plants overexpressing RcSPY. Plant hormone pathways are generally interlinked with each other and execute multiple functions in many different processes. Evidence for their contribution to the regulation of flowering time can be found in SPY6 transgenic plants, whose flowering was significantly delayed compared to the other plants, but without a change in GA content. This result indicates that RcSPY may not only influence the GA signaling pathway in plants, but also other phenotypes not related to GA. Moreover, the effects of SPY-mediated GA signaling on plant development are often interlinked with other plant hormones, like brassinosteroid (BR) (Shimada et al., 2006), cytokinin (CK) (Maekawa et al., 2009; Maymon et al., 2009), and abscisic acid (ABA) (Robertson, 2003).
ConclusionIn this study, the RcSPY gene derived from Rosa chinensis was ectopically transformed into tobacco to investigate its function in flowering development, especially in lengthening of flowering periods. Although the overexpression of RcSPY increased plant height in transgenic tobacco without invariable GA content, the antisense of RcSPY increased GA content in transgenic tobacco with dwarfism, short stem internodes and other traits. Therefore, it seems that different conserved domains in RcSPY play different roles in transgenic tobacco plants. Above all, these results are helpful for explaining the role of the RcSPY gene both in plant architecture and development.