2022 年 91 巻 4 号 p. 522-530
2022 年 91 巻 4 号 p. 522-530
Two chili pepper (Capsicum annuum L.) cultivars ‘Super Hot’ and ‘Num Khao’ grown under field conditions in Thailand were sprayed with 0 (distilled water, control), 25, 50, 100, and 200 ppm chitosan after one week from transplanting and weekly thereafter for five weeks. Chitosan spray improved vegetative growth of both cultivars as measured by increased canopy size. It also increased the plant height of the ‘Super Hot’ cultivar and reduced leaf curl incidence in the ‘Num Khao’ cultivar. Leaf size and chlorophyll content were not significantly affected. Fruit were harvested from cultivars at the commercial maturity stage; that is, the red-ripe stage for ‘Super Hot’ and light green stage for ‘Num Khao’. From the four harvests at weekly intervals, chitosan treatment increased the ‘Super Hot’ yield because of greater fruit production and increased fruit size and there was a higher number of fruit per plant produced by the ‘Num Khao’ cultivar. Fruit color (L*, a*, b*, and sensory color) was not affected, but overall acceptability increased in response to chitosan spray due to improved size and quality of the ‘Super Hot’ cultivar and improved appearance, size and glossiness of the ‘Num Khao’ cultivar. Chitosan at 50 ppm appeared to be the optimum concentration to induce the above effects in both cultivars.
Chili pepper (Capsicum annuum L.) is a major domestic and export fruit-vegetable in Thailand and is widely consumed worldwide as fresh and dried products mainly as a spice and ingredient in various food preparations. The fruit is also a source of many nutrients, such as vitamin A and C, essential oils, antioxidants, capsaicin, flavonoids, steroid saponin, and capsicol (Esyanti et al., 2019). Due to increasing demand in domestic and foreign markets, there is a need to increase chili pepper productivity. Boosting chili production has been conventionally approached by increasing chemical fertilizer and pesticide application. This practice poses a serious threat to human health and the environment. Crop productivity is also threatened by abiotic stresses such as drought and heat stress, which have become more frequent and unpredictable due to climate change. In recent years, sustainable agricultural practices using ecologically friendly technologies have become a focus in improving crop productivity to meet the rising demand for high quality and safe food that promotes human health, environmental protection, and climate resilience.
Chitosan is increasingly used in sustainable agriculture as it is a natural product with nontoxic, biodegradable and biocompatible properties (Malerba and Cerana, 2018; Sharif et al., 2018). Chitosan is a cationic polysaccharide consisting of (1,4)-linked-2amino-deoxy-b-D-glucan and is produced by alkaline deacetylation of chitin, which is a polysaccharide present in the exoskeleton of crustaceans (a byproduct of the seafood industry), many other invertebrates, the cell walls of most fungi, and some algae (Muzzarelli et al., 2012; Rhazi et al., 2000). Chitosan is the second most abundant biopolymer found in nature after cellulose (de Oliveira et al., 2013) and resembles cellulose in chemical structure, but differs in the ratio of β (1,4)-linked monosaccharides and N-acetyl-D-glucosamine (Mellegard et al., 2011). Chitosan has an average molecular weight ranging from 3.8 to 20 kDa, and a degree of deacetylation from 66% to 95% (Agnihotri et al., 2004). Commercially available chitosan can be obtained in various forms including powder, paste, and film.
Chitosan can be used to control diseases and insect pests, induce abiotic stress tolerance, and improve the growth and yield performance of horticultural crops (Sharif et al., 2018). Since the discovery of the antifungal activity of chitosan four decades ago (Allan and Hadwiger, 1979), numerous research has shown the antimicrobial activity of chitosan against a wide range of fungi, bacteria, and crop viruses (Chirkov et al., 1994; Goy et al., 2009; Jia et al., 2016; Li et al., 2013; Reddy et al., 1998) including chili pepper (Esyanti et al., 2019). Chitosan has the following properties: It can destroy cell walls resulting in electrolyte leakage and death of a pathogen, penetrate a pathogen’s nuclei, inhibiting protein and mRNA synthesis; form an external film over a plant’s surface, limiting nutrient availability for microorganisms; alter fungal growth and toxin production; induce the host plant’s defense systems, including the activation of the salicylic acid signaling pathway. The insecticidal activity of chitosan and its derivatives has been demonstrated in several crops including tomato, cucumber, eggplant, and potato and it was concluded that chitosan could be an effective bio-insecticide for horticultural crops (Carletto et al., 2009; Rabea et al., 2005; Sobhy et al., 2015). Furthermore, chitosan has been found to induce resistance to drought and heat stress in a number of crops through increases in antioxidant activities, endogenous H2O2 content, endogenous chitosan activity, and abscisic acid activity (Choi et al., 2013; Jiao et al., 2012; Li et al., 2017). In addition, chitosan has been applied as a biofertilizer to improve crop growth and yield as it can mitigate the effect of pathogens, increase antioxidant activities, and improve nutrient uptake (Spiegel et al., 1988; O’Herlihy et al., 2003; Bakiyalakshmi et al., 2016). In fruit and vegetables, preharvest foliar spraying of chitosan resulted in increases in vegetative growth, antioxidant activities, number, weight, and size of fruit, soluble solids content, firmness, and postharvest life (Chandrkrachang, 2002; Dzung et al., 2017; Kim et al., 2005; Rahman et al., 2018; Sultana et al., 2017). In chili pepper, foliar application of chitosan on an Indonesian cultivar starting in the second week of growth was found to increase plant height, number of leaves, chlorophyll content, and resistance to Phytophthora infection, suggesting the promising role of chitosan as an alternative to conventional fertilizers and fungicides (Esyanti et al., 2019). Earlier, Dzung et al. (2017) showed the promotive effect of chitosan on plant growth and fruit yield of Vietnamese chili cultivars. The present study examined the effects of preharvest chitosan spraying on the growth, yield and quality of two major chili cultivars in Thailand.
This study was conducted from November 2018 to February 2019 at the experimental farm of the Department of Plant Production Technology, School of Agricultural Technology, King Mongkut’s Institute of Technology Ladkrabang (KMITL), Thailand, using the two most widely grown chili cultivars, ‘Super Hot’ and ‘Num Khao’. The ‘Super Hot’ chili is widely grown in Thailand; it demonstrates high productivity and meets the market demand for desirable color, taste and pungency, while ‘Num Khao’ or cayenne pepper, is a moderately hot chili cultivar, which is a tapered, 10–25 cm long, skinny, red-colored fruit with a curved tip and rippled skin. ‘Num Khao’ bears flowers and fruit earlier and has a shorter life cycle than ‘Super Hot’. Seeds of the two cultivars (East-West Seed Co., Ltd., Thailand) were sown in polystyrene transplant flats filled with peat moss. Thirty days after germination, seedlings with two to three true leaves were transplanted into black plastic pots (30 cm) filled with 3:1 garden soil:cow manure mixture. Before transplanting, the soil mix in pots was saturated with water and allowed to drain through small horizontal slits about 4 cm from the ground. One plant was maintained per pot. Ten replicate pots were maintained for each treatment. The pots were spaced at 0.5 m × 0.5 m as usually practiced. Fertilizer (Yara Co., Ltd., Thailand) was applied weekly, consisting of 5 g of 46-0-0 and 16-16-16 for the first three weeks from transplanting to promote vegetative growth and then 5 g of 13-13-21 starting on the fourth week (flowering and fruit setting stage) to promote fruit development. Other cultural management practices, such as watering, pesticide application, and weed control, were applied following commercial recommendations.
Experimental design and chitosan treatmentThe experiments were conducted in a completely randomized design with six treatments and ten replications. Low-molecular weight chitosan (≥75% deacetylated chitin, poly D-glucosamine) was sourced from Sigma-Aldrich (Saint Louis, MO, USA). It was dissolved in 0.01% acetic acid and the desired concentrations (25, 50, 100, and 200 ppm chitosan) were prepared using distilled water. The plants were sprayed to runoff (about 25 mL chitosan solution per plant) starting a week after transplanting and weekly thereafter for five weeks. Distilled water spray (0 ppm chitosan) served as a control.
Data gatheredPlant growth was monitored based on plant height, stem size, leaf size (length and width), canopy size (length and width), and chlorophyll content at weekly intervals for 15 weeks for the ‘Super Hot’ cultivar and 10 weeks for the ‘Num Khao’ cultivar. Plant height and size of the leaf and canopy were measured using a metric rule. Seven leaves at the middle part of the canopy were used for size measurement. The canopy of a chili plant is not circular, so the longer side was measured as the length and the shorter side as the width all throughout the observation period. The size of the stem (5 cm from the base) was measured using Vernier calipers. The chlorophyll content of three index leaves per plant was measured using a SPAD-502 chlorophyll meter (Minolta Camera Co., Japan). Leaf curl incidence was evaluated using a severity index of 0 to 4 with 0– no leaf curl symptoms, 1– 20% of leaves with leaf curl symptoms, 2– 21–50% of leaves with leaf curl symptoms, 3– 51–75% of leaves with leaf curl symptoms and 4– > 76% of leaves with leaf curl symptoms.
‘Super Hot’ fruit were harvested at the red-ripe stage while ‘Num Khao’ fruit were harvested at the light green color stage, as commercially practiced. For each cultivar, four harvests at weekly intervals were completed. Then, the total number of fruit were counted and the marketable fruit were weighed using a digital weighing scale (Adventurer, Ohaus Corp., USA). From each harvest, ten sample fruits from each treatment per replicate were used to measure the average weight per fruit using the digital weighing scale and fruit size (length and width at mid portion) using Vernier calipers. Fruit color was measured using a Hunterlab Colorimeter (ColorFlex, Hunter Lab., USA) taking the average of five readings of L* (lightness), a* (green [−] to red [+]), and b* (blue [−] to yellow [+]) values from each fruit sample. Sensory quality attributes (appearance, size, color, glossiness, and overall acceptability) were evaluated by 20 trained panelists using a rating scale of 5 (excellent) to 1 (poor) while defects were rated using a scale of 1 (no defect) to 5 (severe defects).
Statistical analysisThe results were analyzed by analysis of variance (ANOVA) and a treatment mean comparison using Tukey’s honest significance test (HSD) (P < 0.05) with Statistix 8 software (Analytical Software, Tallahassee, FL, USA). For treatment means in graphs, the standard error of the mean (SE) was included.
Plant height increased with advancing stages of growth in both cultivars (Fig. 1). Chitosan application increased the plant height of ‘Super Hot’ with 50 ppm concentration causing the highest increase after 10–14 weeks from transplanting relative to the control (0 ppm chitosan). At an earlier stage, plants applied with 25 ppm chitosan were the tallest, but were comparable to plants with 50 ppm chitosan applied after 3–8 weeks from transplanting. In ‘Num Khao’, chitosan also increased plant height, particularly at the highest concentration of 200 ppm after 3 and 8–10 weeks from transplanting. In terms of stem size, chitosan had generally no marked effect relative to that of the control except in ‘Num Khao’, for which 200 ppm chitosan resulted in the biggest stems 9–10 weeks after transplanting (Fig. 2). Similarly, chitosan did not promote leaf enlargement and even reduced the leaf length and width of ‘Num Khao’ plants after 3–10 weeks from transplanting (Fig. 3). However, the canopy size increased with chitosan treatment (Fig. 4). In ‘Super Hot’, 50 ppm chitosan was sufficient to increase both canopy length and width; these did not increase further at higher chitosan concentrations and this was evident after 13–15 weeks from transplanting. In ‘Num Khao’, a chitosan concentration of 200 ppm was needed to increase canopy size after 5–10 weeks from transplanting, but differences relative to the control were statistically significant only for canopy width. Leaf chlorophyll content was not significantly affected by chitosan in the ‘Super Hot’ cultivar while in the ‘Num Khao’ cultivar, significant differences were observed only after 8 and 10 weeks from transplanting, at which point plants with 100 ppm chitosan applied had the highest SPAD values among treatments (Fig. 5). Furthermore, the SPAD values differed between the two varieties. They showed a sharp increase in ‘Super Hot’ within two weeks after planting, then no change or a gradual decrease until six weeks, and then an increase again from seven to nine weeks. In ‘Num Khao’, SPAD values increased up to six weeks and there was no change thereafter. Leaf curl incidence due to insect damage from thrips was low (less than 1.0 or 1–20% of leaves with leaf curl symptoms) and this was not affected by chitosan treatment in ‘Super Hot’ (Table 1). In ‘Num Khao’, chitosan significantly decreased leaf curl incidence with increasing concentrations up to 50 ppm and the effect was statistically comparable to that of 100–200 ppm chitosan.
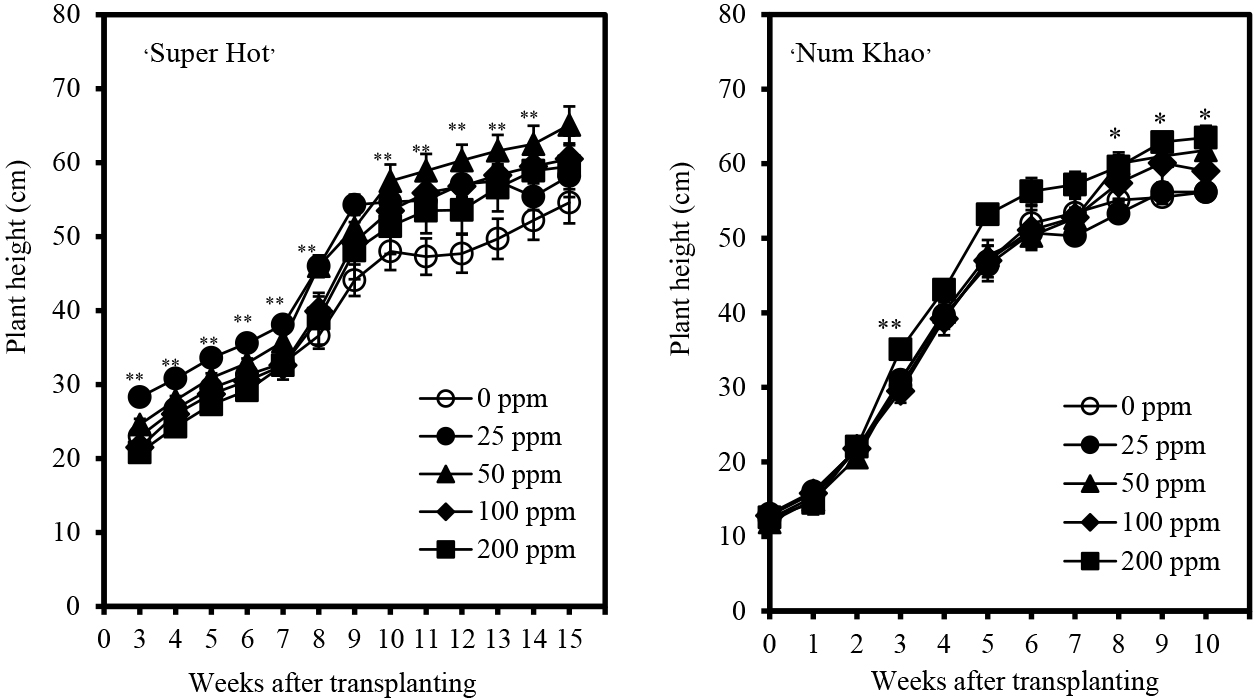
Plant height (± SE) of ‘Super Hot’ and ‘Num Khao’ chili peppers in response to preharvest chitosan spray (Treatment differences are significant at P = 0.01 indicated by ** and at P = 0.05 indicated by *; no asterisk indicates no significant differences among treatments).
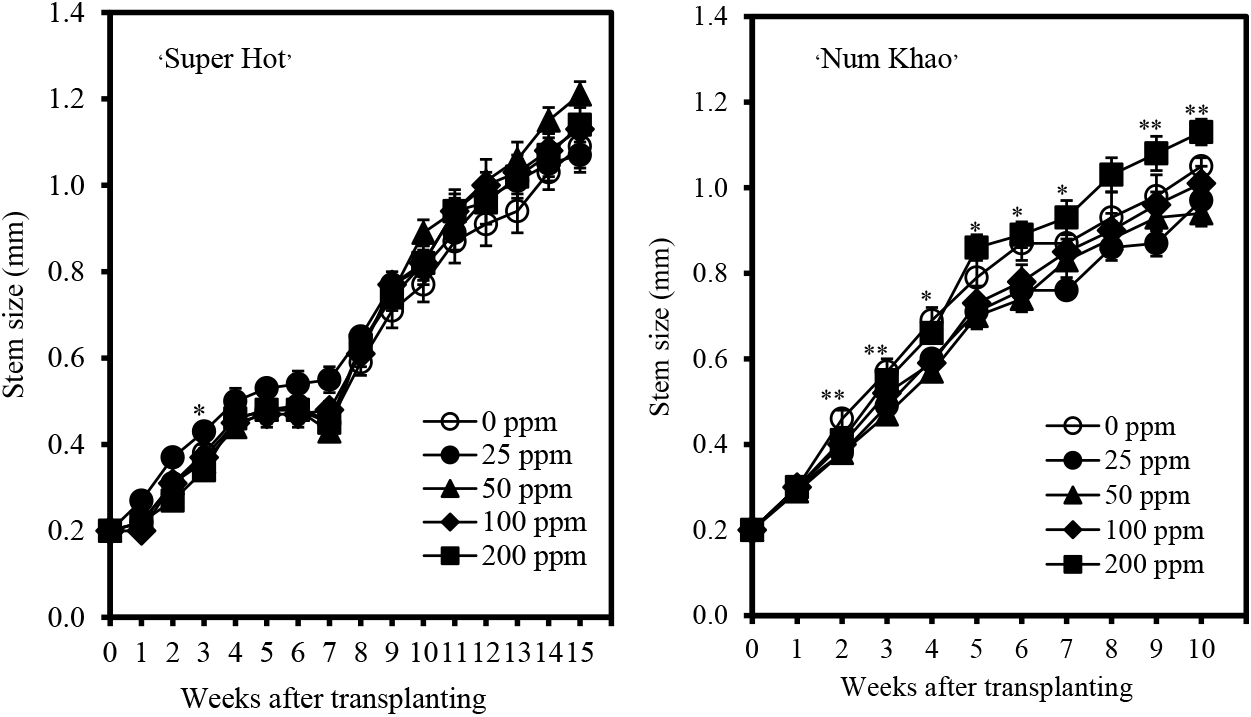
Stem size (± SE) of ‘Super Hot’ and ‘Num Khao’ chili peppers in response to preharvest chitosan spray (Treatment differences are significant at P = 0.01 indicated by ** and at P = 0.05 indicated by *; no asterisk indicates no significant differences among treatments).
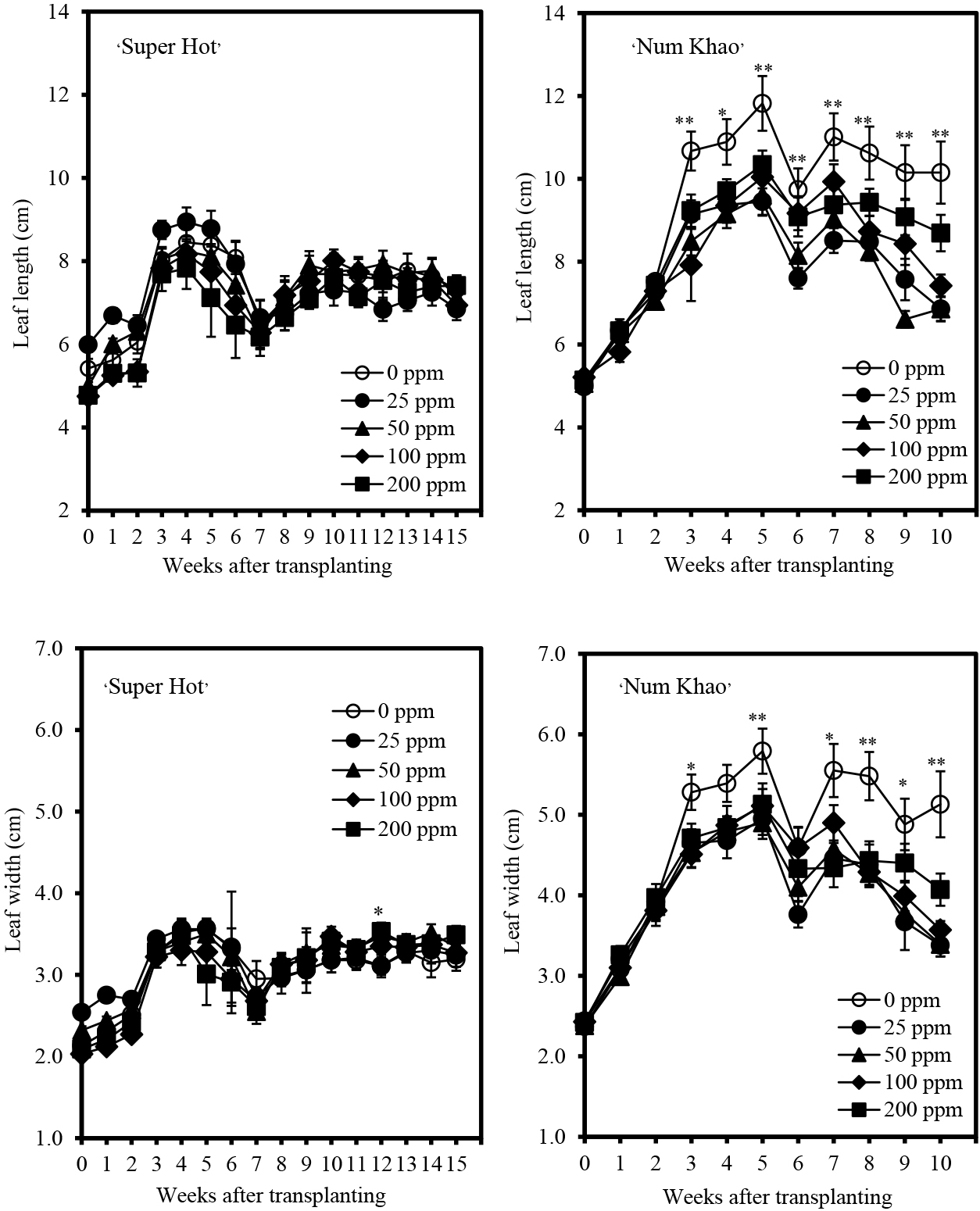
Leaf length and width (± SE) of ‘Super Hot’ and ‘Num Khao’ chili peppers in response to preharvest chitosan spray (Treatment differences are significant at P = 0.01 indicated by ** and at P = 0.05 indicated by *; no asterisk indicates no significant differences among treatments).
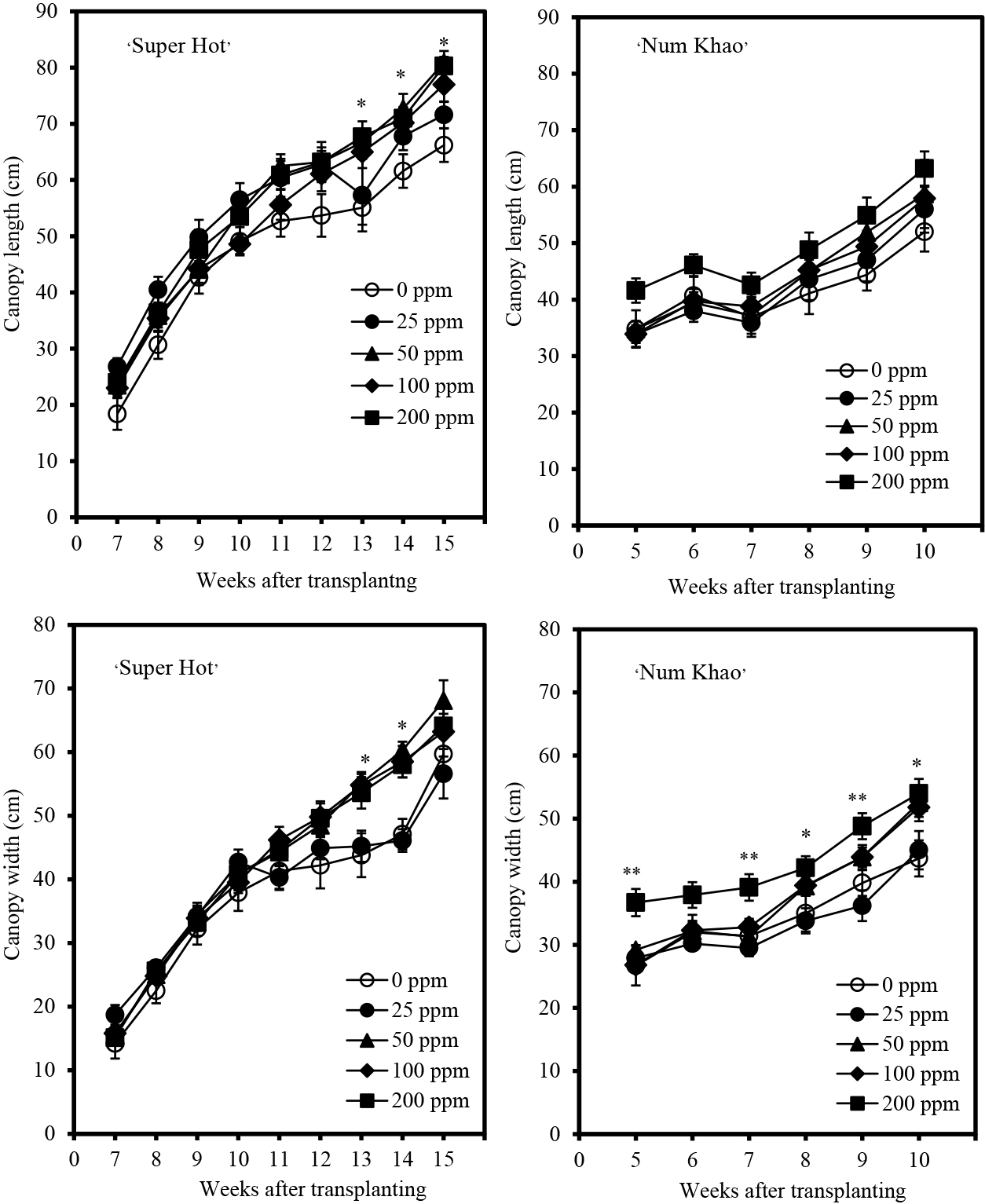
Canopy length and width (± SE) of ‘Super Hot’ and ‘Num Khao’ chili peppers in response to preharvest chitosan spray (Treatment differences are significant at P = 0.01 indicated by ** and at P = 0.05 indicated by *; no asterisk indicates no significant differences among treatments).
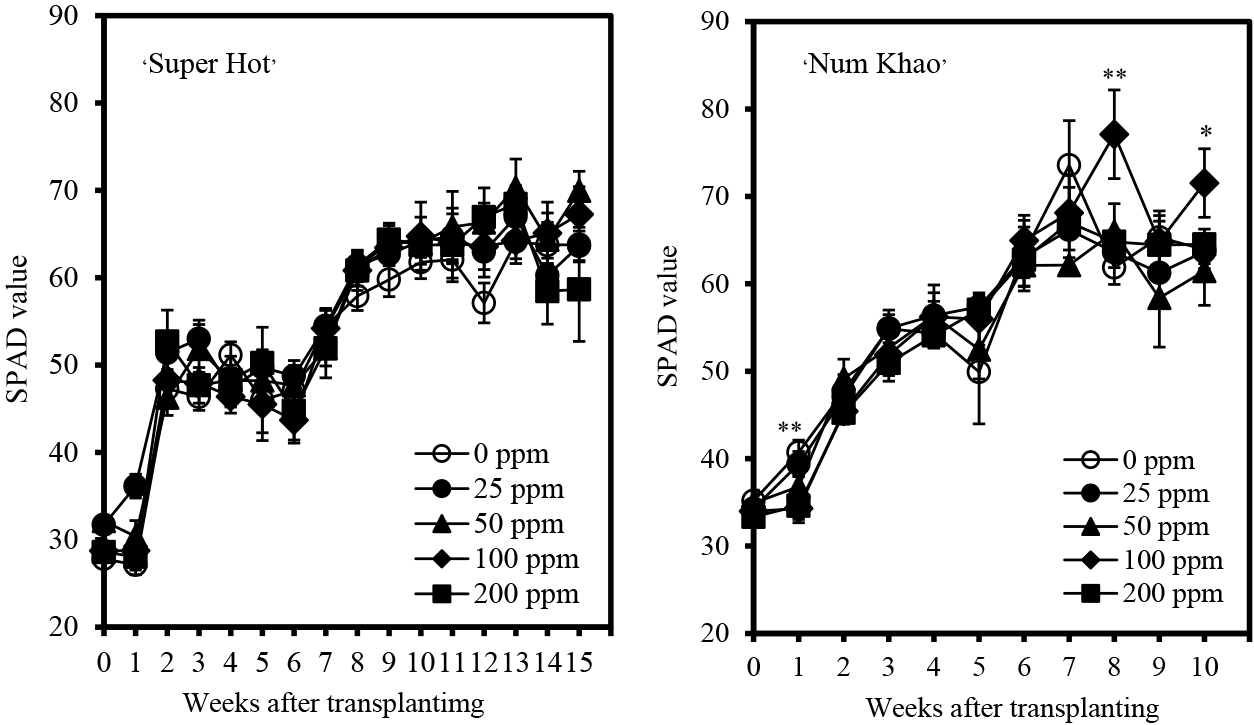
Chlorophyll content (SPAD value ± SE) of ‘Super Hot’ and ‘Num Khao’ chili peppers in response to preharvest chitosan spray (Treatment differences are significant at P = 0.01 indicated by ** and at P = 0.05 indicated by *; no asterisk indicates no significant differences among treatments).
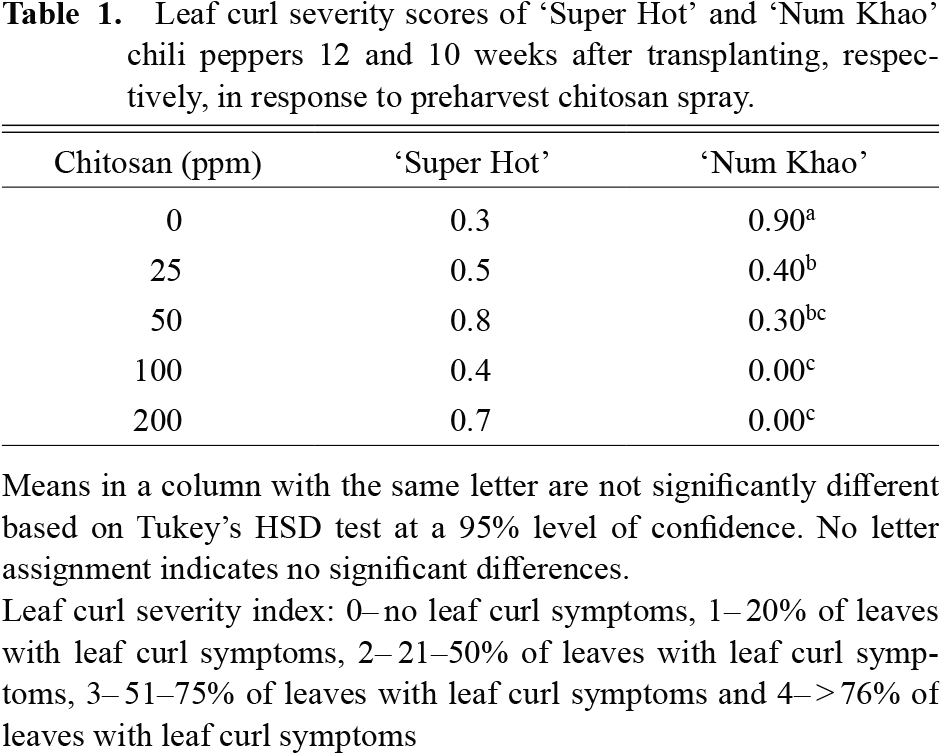
Leaf curl severity scores of ‘Super Hot’ and ‘Num Khao’ chili peppers 12 and 10 weeks after transplanting, respectively, in response to preharvest chitosan spray.
The results showed differing cultivar responses to chitosan, although leaf parameters (size and chlorophyll content) in both cultivars were not markedly affected. Chitosan increased the plant height of ‘Super Hot’ and reduced leaf curl incidence in ‘Num Khao’. However, in both cultivars, chitosan increased the canopy size, indicating greater production of vegetative parts. Chitosan at 50 ppm appeared to be the optimum concentration for promoting vegetative growth. Previous studies generally showed growth promotion in response to chitosan treatment. Esyanti et al. (2019) showed that in three chili peppers (Capsicum annuum L.), weekly spraying of 200–1000 ppm chitosan for three weeks resulted in healthier and taller plants than untreated plants, while Dzung et al. (2017) showed the stimulation of shoot development in hot chili (Capsicum frutescens L.) sprayed with 50 ppm low-molecular weight chitosan at 30, 40, and 50 days after sowing. Both studies found increases in leaf chlorophyll content which was not observed in the present study. Chitosan spray also promoted growth of other vegetable crops, such as sweet pepper (Ghoname et al., 2010), squash (Sabreen et al., 2015), cucumber (Shehata et al., 2012), cabbage (Hirano, 1988), Chinese cabbage (Chandrkrachang, 2002), tomato and eggplant (Mondal et al., 2012; Sultana et al., 2017), common bean (Abu-Muriefah, 2013), and radish (Farouk et al., 2011). Multiple mechanisms of chitosan-induced growth promotion have been reported. These include increased water and nutrient uptake through cell osmotic pressure adjustment, increased nitrogen and N-protein contents which are essential for chlorophyll synthesis and various metabolic enzymes, increased photosynthetic capacity due to increased leaf area, chlorophyll content and chloroplast size, increased synthesis of phytohormones auxin, cytokinins, and gibberellins, and increased antioxidant activity, thereby reducing lipid peroxidation and accumulation of harmful free radicals, overexpression of genes involved in photosynthesis, and changes in protein metabolism programming, thereby increasing various storage proteins, and hormone metabolism (El-Bassiony et al., 2014; Khan et al., 2002; Landi et al., 2017; Limpanavech et al., 2008; Malerba and Cerana, 2018; Pichyangkura and Chadchawan, 2015). Furthermore, chitosan has antimicrobial and insecticidal properties and increases plant tolerance to abiotic stress (Sharif et al., 2018). This could partly explain the reduced leaf curl incidence in the ‘Num Khao’ cultivar in the present study.
Fruit yieldChitosan did not significantly increase the fruit yield of the ‘Super Hot’ cultivar (Table 2). The total number of fruit and marketable yield of chitosan-treated plants increased only slightly to 64–75 and 136–158 g per plant, respectively, relative to that of the control (52 and 125 g per plant, respectively). However, all chitosan concentrations increased fruit length and width to 4.0–4.2 cm and 0.63–0.66 cm, respectively, and statistically differed from that of the control (3.2 cm and 0.5 cm, respectively) (Table 2). This increase in fruit size was apparently not sufficient to significantly influence fruit yield. In the ‘Num Khao’ cultivar, fruit yield significantly increased with chitosan treatment due to the production of more fruit per plant, rather than an increase in fruit size. Chitosan at 50 ppm increased the number of fruit and marketable fruit yield to about 33 and 180 g per plant from about 14 and 86 g per plant for the control, respectively. Higher chitosan levels of 100–200 ppm did not correspondingly increase the total number of fruit or marketable fruit yield per plant, while the effect of a lower chitosan concentration of 25 ppm was comparable to the control. Fruit length and width of the control and chitosan-treated plants were statistically similar and ranged from about 6.6–8.0 cm and 0.68–0.87 cm, respectively. Non-marketable fruit yield was not significantly affected by chitosan treatment in either cultivar and was slightly higher in ‘Super Hot’ (20–24 fruits/plant) than in ‘Num Khao’ cultivar (12–23 fruits/plant).
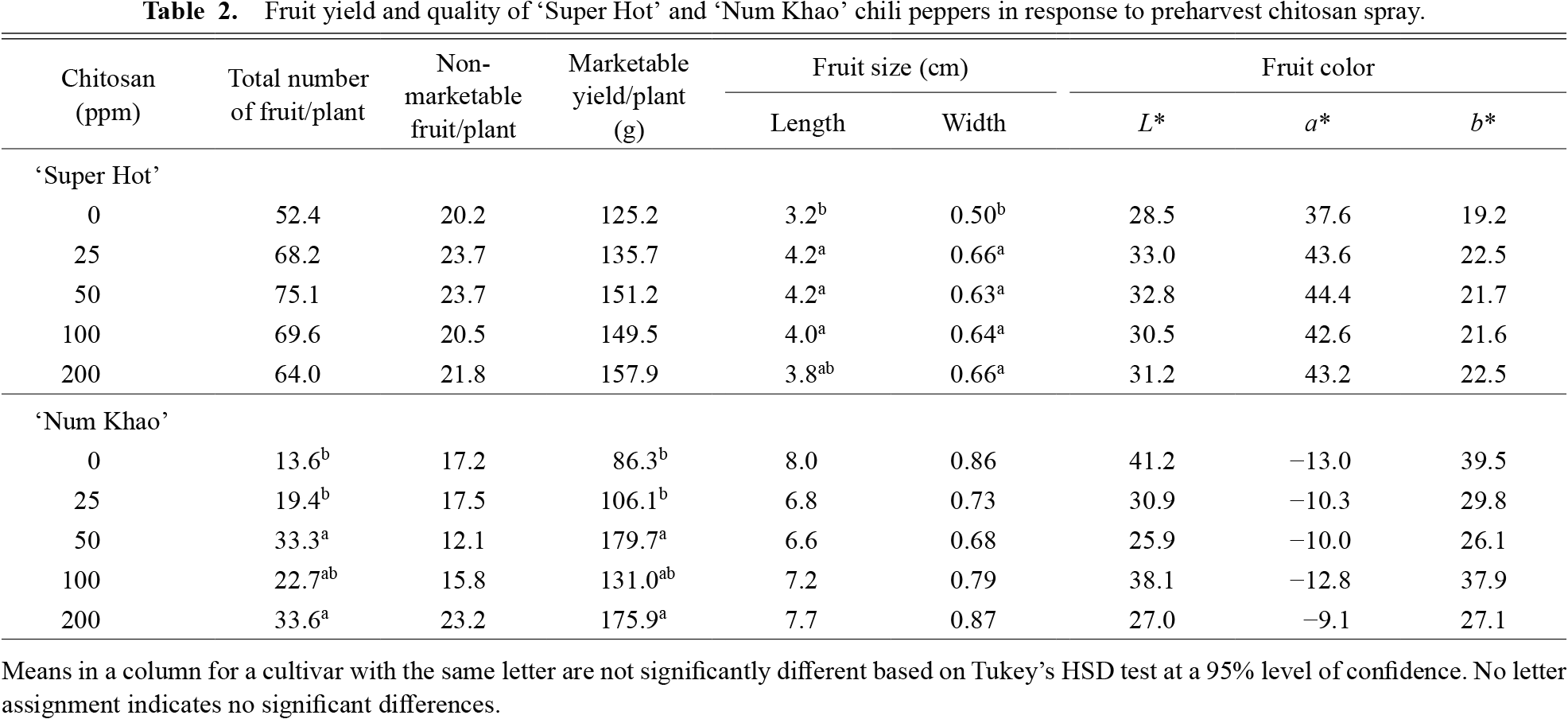
Fruit yield and quality of ‘Super Hot’ and ‘Num Khao’ chili peppers in response to preharvest chitosan spray.
Fruit yield largely depends on the capacity of the plant to produce and translocate photosynthates for fruit production. Chitosan treatment seemed to increase the photosynthetic capacity of both chili cultivars through increased vegetative growth and canopy size. Increased photosynthetic capacity has been implicated in chitosan-enhanced growth (Esyanti et al., 2019). Dzung et al. (2017) showed increases in fruit yield of chili pepper in response to chitosan treatment as a result of the production of more fruit rather than increase in fruit size and weight. A chitosan-induced increase in fruit yield was also observed in squash (Sabreen et al., 2015), cucumber (Shehata et al., 2012), okra (Mondal et al., 2016), tomato and eggplant (Sultana et al., 2017). In other studies, the increase in yield due to chitosan application has been attributed to improved nutrient uptake (Bakiyalakshmi et al., 2016; O’Herlihy et al., 2003; Spiegel et al., 1988). This could be investigated in future studies.
Fruit qualityFruit color did not show significant variations with chitosan treatment in either cultivar (Table 2). Color coordinates L*, a* and b* values ranged from 28.5–33.0, 37.6–44.4 and 19.2–22.5 in ‘Super Hot’ and 25.9–41.2, −9.1 to −13.0 and 26.1–39.5 in ‘Num Khao’, respectively. The much higher a* values of ‘Super Hot’ indicate the characteristic red color of the fruit in contrast to the light green color of ‘Num Khao’ fruit as the usual harvest maturity indicators. These objective color measurements corresponded to the sensory color, which did not differ with treatment and ranged from 4.0–4.5 in ‘Super Hot’ and 3.5–4.1 in ‘Num Khao’ (Table 3). Overall acceptability was improved by chitosan in both cultivars and a 50 ppm concentration was sufficient to elicit the effect. This was due to improved size and quality in ‘Super Hot’ and improved appearance, size, and glossiness in ‘Num Khao’. Sensory defects did not vary with treatment in either cultivar.
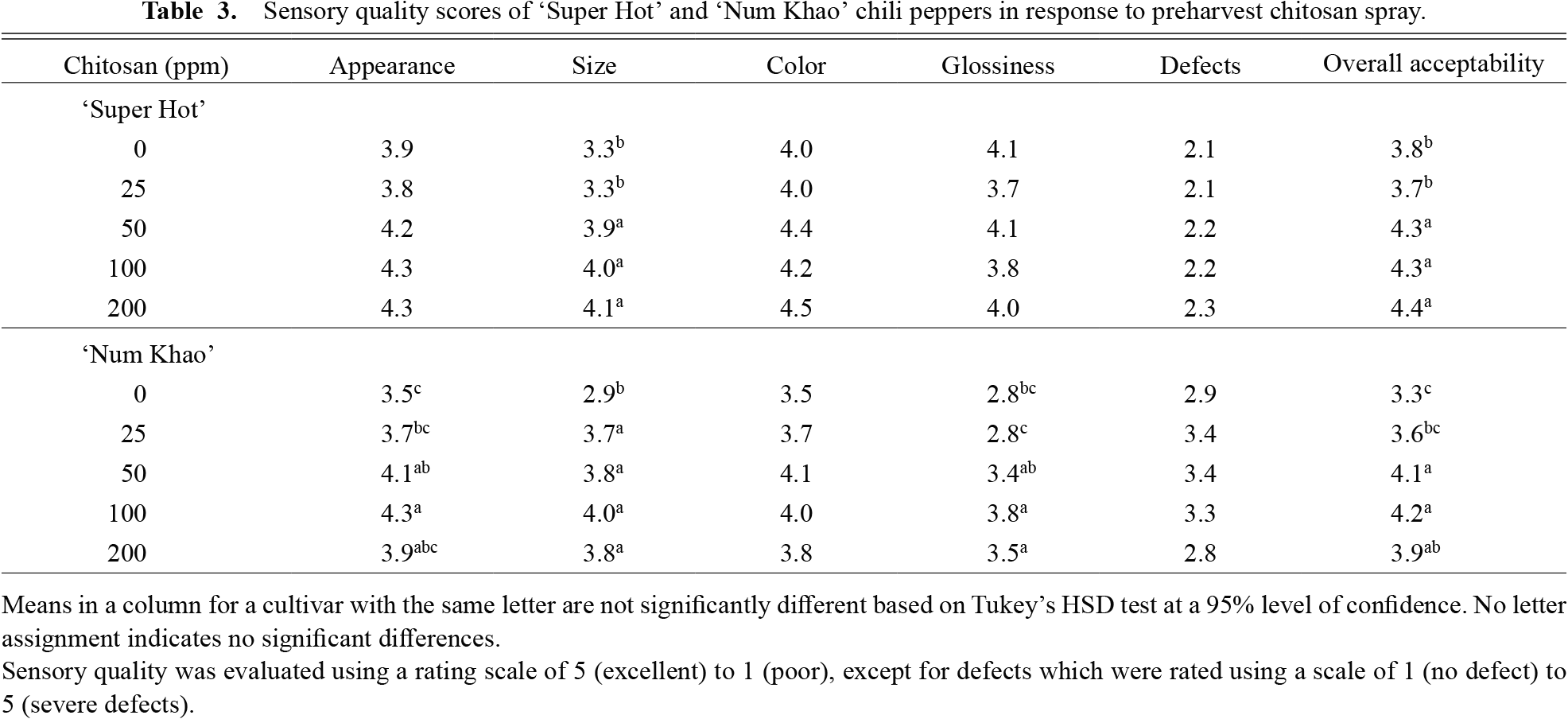
Sensory quality scores of ‘Super Hot’ and ‘Num Khao’ chili peppers in response to preharvest chitosan spray.
Previous studies showed various effects of chitosan on fruit quality even from the same crop. Chitosan spray increased fruit size of chili pepper, squash, cucumber, strawberry, tomato, and eggplant (Dzung et al., 2017; Rahman et al., 2018; Sabreen et al., 2015; Shehata et al., 2012; Sultana et al., 2017), soluble solids content of squash, cucumber, eggplant, sweet pepper, strawberry and common bean (Abdel-Mawgoud et al., 2010; Abu-Muriefah, 2013; Sabreen et al., 2015; Shehata et al., 2012; Sultana et al., 2017), and carotenoid content and color of strawberry (Rahman et al., 2018). However, it reduced the soluble solids and acidity contents of tomato (Sultana et al., 2017) and had no effect on the soluble solids content of tomato and strawberry (El-Miniawy et al., 2013; El-Tantawy, 2009) or the acidity of eggplant (Sultana et al., 2017). Overall, the results of the present study indicate that chitosan spray had no detrimental effect on fruit quality; rather, it improved the sensory quality of both cultivars.
ConclusionChitosan spray improved growth, yield, and quality of chili pepper, but some responses were cultivar-specific. Growth promotion was expressed as increased plant height in the ‘Super Hot’ cultivar, increased canopy size of both the ‘Super Hot’ and ‘Num Khao’ cultivars, and reduced leaf curl incidence in the ‘Num Khao’ cultivar. This contributed to an increase in the fruit size of ‘Super Hot’ and an increased number of fruits, a significant increase in the fruit yield of ‘Num Khao’, while the fruit yield of chitosan-treated plants significantly increased. The improved sensory quality of the ‘Super Hot’ cultivar was due to improved size and quality, while that of the ‘Num Khao’ cultivar was due to improved appearance, size and glossiness. Chitosan at 50 ppm was sufficient to cause these effects. Further studies are needed to establish the effectiveness and practicality of chitosan spray for commercial chili production.
The author would like to thank Miss Naphawan Yimyam and Miss Nittaya Janepakwan for data collection and technical support and the Department of Plant Production Technology, School of Agricultural Technology, King Mongkut’s Institute of Technology Ladkrabang for providing laboratory facilities.