Abstract
The aim of this study was to select new pollinizers for Prunus spp. with high pollen germination rates at low temperatures and assess their effect on the fruit set of Japanese plum cultivars. In this study, we examined in vitro pollen germination in 17 plum cultivars and two Myrobalan plum lines (420-2-2 and 421-3-1) at eight temperatures (7.5°C, 10.0°C, 12.5°C, 15.0°C, 17.5°C, 20.5°C, 22.5°C, and 25.0°C). The extent of pollen germination was affected by the incubation temperature. The germination rates of most cultivars were highest between 20.0°C to 25.0°C and ≤ 20% between 7.5°C to 10.0°C. However, the two Myrobalan plum lines (420-2-2 and 421-3-1) showed higher germination rates than the other cultivars at 10.0°C with ≥ 25% germination. The high germination rate in Myrobalan 420-2-2 was further confirmed in experiments conducted by our group in 2020. Open field studies on the Japanese plum ‘Kiyo’ revealed that the fruit setting rate was 17.6% using Myrobalan 420-2-2 and only 9.9% in the control using ‘Hollywood’. The fruit setting rate of the Japanese plum ‘Taiyo’ was approximately 20% when pollinated with both the cultivars. However, both fruit setting rate and fruit quality did not differ significantly between ‘Kiyo’ and ‘Taiyo’ with either pollination treatment. The formation rates of perfect seeds in ‘Taiyo’ were 90% and 65% by pollination using Myrobalan 420-2-2 and ‘Hollywood’, respectively. However, pollination treatment using pollen from both cultivars did not show any variations in the early development of the ovary and ovule. S-genotyping in Myrobalan 420-2-2 was determined as S7S10; therefore, we assumed that Myrobalan could be cross-compatible with many other plum cultivars. In conclusion, we selected Myrobalan 420-2-2 as a new plum pollinizer as it can effectively pollinate Japanese plum and germinate at low temperatures with no adverse effect on fruit set and quality.
Introduction
The Japanese plum belonging to the Prunus genus is self-incompatible, and requires cross-pollination for fruit setting, as members of this genus are unable to bear fruits parthenocarpically (Guerra and Rodrigo, 2015; Hartmann and Neumuller, 2009). Prunus spp. ‘Hollywood’, with high pollen content, is one of the most common pollinizers for Japanese plum in Japan. However, extremely low pollen germination rates may cause fruit setting failure (Vasilakakis and Porlingis, 1985). Therefore, pollen germination when pollinating is very important to achieve stable fruit setting and production of high-quality fruit. Among the plum varieties cultivated in Japan in recent years, the Japanese plum ‘Kiyo’ is popular for its taste; however, it has the problem of a low fruit set rate. The low fruit set rate in the ‘Kiyo’ variety is caused by triploidy and delayed embryo sac development (Nakajo et al., 2014). Additionally, the effects of different pollens and delayed growth of the ovary or ovule on poor fruit set have not yet been studied in the Japanese plum ‘Taiyo’, the parent cultivar of ‘Kiyo’.
Although it varies among cultivars and growing regions, flowering in Japanese plum generally occurs from mid to late March in Japan. Additionally, the best time for pollination is only 1–3 days after the opening of the flowers (Bajwa et al., 1991; Hartmann and Neumuller, 2009). Low temperatures at flowering have negative effects on pollen germination and fruit setting in Japanese plums (Yaegaki et al., 2007); fruit yield is low when the average temperature during the flowering period is ≤ 11°C (Muraoka et al., 1987). The optimum temperature for pollen germination varies between cultivars in several woody species, such as avocados (Sedgley and Annells, 1981), almonds and peaches (Weinbaum et al., 1984), walnuts (Luza et al., 1987), pistachios (Polito et al., 1988), apricots (Egea et al., 1992), mangoes (Sukhvibul et al., 2000), pears (Kuroki et al., 2017), and apples (Kobayashi et al., 2021). In Japanese plum, pollen germination at 20°C differs significantly among cultivars; the germination rates were 0–31.2% after 12 h of incubation (Yaegaki et al., 2007). However, previous studies reported that the germination rates in two Myrobalan plum lines, 420-2-2 and 421-3-1 (Prunus cerasifera), were slightly higher than in other cultivars (Yaegaki et al., 2007). Myrobalan plums native to the Balkan Peninsula and Caucasus (Faust and Surányi, 1999) are expected to be cross-compatible with many Japanese plum cultivars. However, the germination capacity of plum pollen under the low temperature conditions of < 20°C has not been tested. To ensure optimal yield and fruit quality, it is necessary to identify pollen with a high germination capacity at low temperatures and evaluate fruit set.
Therefore, the aim of this study was to select new pollinizers for Japanese plum with pollen having high germination rates at low temperatures, and assess their effect on fruit set of Japanese plum cultivars. At first, we performed a comparative assessment of the germination of pollen in plum plants and selected cultivars with pollen that exhibited high germination rates under low temperature conditions. Then, we investigated the effect of pollination treatments, using the pollen of selected cultivars, on the fruit set and fruit quality in ‘Taiyo’ and ‘Kiyo’, the main Japanese plum cultivars. Simultaneously, to assess the effect of the pollination treatments on the early development of the ovary and ovule, we measured the height and width of the ovary and ovule in pistils of ‘Taiyo’ using a microscope. Finally, we analyzed S-genotyping of the plum cultivar selected as a new pollinizer to consider its potential for cross-compatibility in several other plum cultivars.
Materials and Methods
Amount of pollen and pollen germination at different temperatures on Prunus spp. (Experiment 1)
We used pollen from 17 Prunus spp. and two Myrobalan plum lines (P. cerasifera) in 2017 (Shown in Table 1) and five Myrobalan plum lines and ‘Hollywood’ in 2020 (Shown in Table 2), grown in the orchards of the Institute of Fruit Tree and Tea Science, (NARO) Genebank, Ibaraki, Japan (36.2°N, 140.2°E). We collected pollen from one or two standing trees (> 5 years old) from each cultivar in March 2017. Anthers were collected from flowers in the balloon stage. They were then dried for 24 h at 24°C, and the pollen was extracted. For each cultivar, the amount of pollen per 100 flowers was recorded.
The dried pollen was sieved through a 0.25 mm mesh and immediately germinated in polystyrene Petri dishes (35 × 10 mm) at eight temperatures (7.5°C, 10.0°C, 12.5°C, 15.0°C, 17.5°C, 20.0°C, 22.5°C, and 25.0°C) in 2017 and two temperatures (10.0°C and 20.0°C) in 2020. The pollen was scattered in an in vitro system, which had been injected with a solidified germination medium consisting of 10.0% (w/v) sucrose, 1.0 mM boric acid, and 1.0% (w/v) agar. The pollen germination rate was determined 5 h after culturing using a microscope (Optiphot, Nikon, Tokyo, Japan). Pollen was considered to have germinated when the length of the pollen tube exceeded the diameter of the pollen grain. For each treatment, the pollen germination rate was determined using nine replications by counting 20 or more pollens at a time for each replication. Statistical analyses among germination rates of plum cultivars were performed for the same temperature treatment using Tukey-Kramer’s HSD tests. Statistical significance was set at P < 0.05 using the rates data of the nine replications for each plum cultivar.
Effect of the pollination treatments consisting of pollen from Myrobalan plums applied in an open field on fruit set and fruit quality (Experiment 2)
The experiment in 2018 was conducted using the Japanese plum ‘Kiyo’ [trellis training (> 5 years old)] planted in a field at the Gunma Agricultural Research Center, Gunma, Japan (36.3°N, 139.2°E), and the experiment in 2020 was conducted using the Japanese plum ‘Taiyo’ [trellis training (> 5 years old)] planted in a field at the Shimane Prefectural Agricultural Technology Center, Shimane, Japan (35.3°N, 132.7°E). Using the pollen collected in 2017 (Experiment 1), artificial pollination was performed using pollen (stored at −20°C) of Myrobalan 420-2-2 and ‘Hollywood’ (control). The temperature data near the experimental field at the time of pollination were extracted from the weather data obtained from the Japan Meteorological Agency. After unopened flower buds were removed, hand pollination was performed in late March (March 23rd, 2018 and March 20th, 2020) for > 250 flowers using nine lateral branches. Pollinated flowers were unbagged and used to evaluate the effects of pollination on fruit set in each lateral branch by t-tests after approximately eight weeks of pollination. Fruit quality was evaluated on the day of fruit harvest (Fruits of ‘Kiyo’ and ‘Taiyo’ were harvested on July 19th, 2018 and August 20th, 2020, respectively) by t-tests (n = 20). In 2020, the formation rate of perfect seeds was evaluated in ‘Taiyo’ based on the ratio of perfect or imperfect seeds (browned seed or wrinkled seed) by chi-square test (n = 20). Statistical significance was set at P < 0.05.
Effect of the pollination treatment using pollen of the selected cultivar on the early development of the ovary and ovule in the ‘Taiyo’ (Experiment 3)
To evaluate the effects of the pollination treatment on the early development of the ovary and ovule, pistils from about 100 Japanese plum ‘Taiyo’ flowers in Experiment 2 were collected on day 0, day 4, and day 10 each post pollination treatment (total n = 300/treatment). The pistils were fixed with FAA fixative solution [70% (v/v) ethanol, formaldehyde, and acetic acid (8:1:1, v/v/v)] and washed with running water for 24 h. They were then dehydrated in a graded series of ethanol [30% (v/v), 50% (v/v), 70% (v/v), 80% (v/v), and 96% (v/v)] for 2 h and in 100% (v/v) ethanol for 1 h and subsequently embedded in Technovit 7100 resin (Kulzer and Co., Wehrheim, Germany). For biological microscopy, the samples were cut to a thickness of 2 μm using a microtome (Yamato Kohki Industrial, NS-31, Japan) and stained with 0.1% safranin solution and 0.1% fast green solution. From the samples, 20 pistils with ovules were selected to measure the height (maximum diameter in the vertical direction) and length (maximum diameter in the horizontal axis direction) of the ovaries and ovules using a microscope (Optiphot, Nikon, Japan) and calculated as mean length.
Analysis of S-genotyping of Myrobalan (Experiment 4)
DNA was extracted from young leaves of five Myrobalan plum lines using a modified cetyltrimethylammonium bromide (CTAB) protocol (Yamamoto et al., 2001). The leaves were homogenized in liquid nitrogen and the resulting tissue powder was suspended in 10 mL of CTAB extraction buffer, 1 mL of lysis buffer, and 0.2 mL of 2-mercaptoethanol, and then incubated at 60°C for 1 h. The suspension was then purified twice in a chloroform: isoamyl alcohol (24:1) solution and precipitated with ice-cold 2-propanol. The recovered DNA was dissolved in TE buffer. PCR amplification was conducted using allele-specific primer pairs for P. cerasifera S-alleles from S1 to S15 (Sutherland et al., 2009). PCR conditions were as previously described by Sutherland et al. (2009). The reaction products were separated by electrophoresis at 100 V for 20 min in 2.0% TAE agarose gels. Gels were stained with ethidium bromide (0.5 μg·mL−1) and bands were visualized under ultraviolet light.
Results
Amount of pollen and pollen germination at different temperatures on Prunus spp. (Experiment 1)
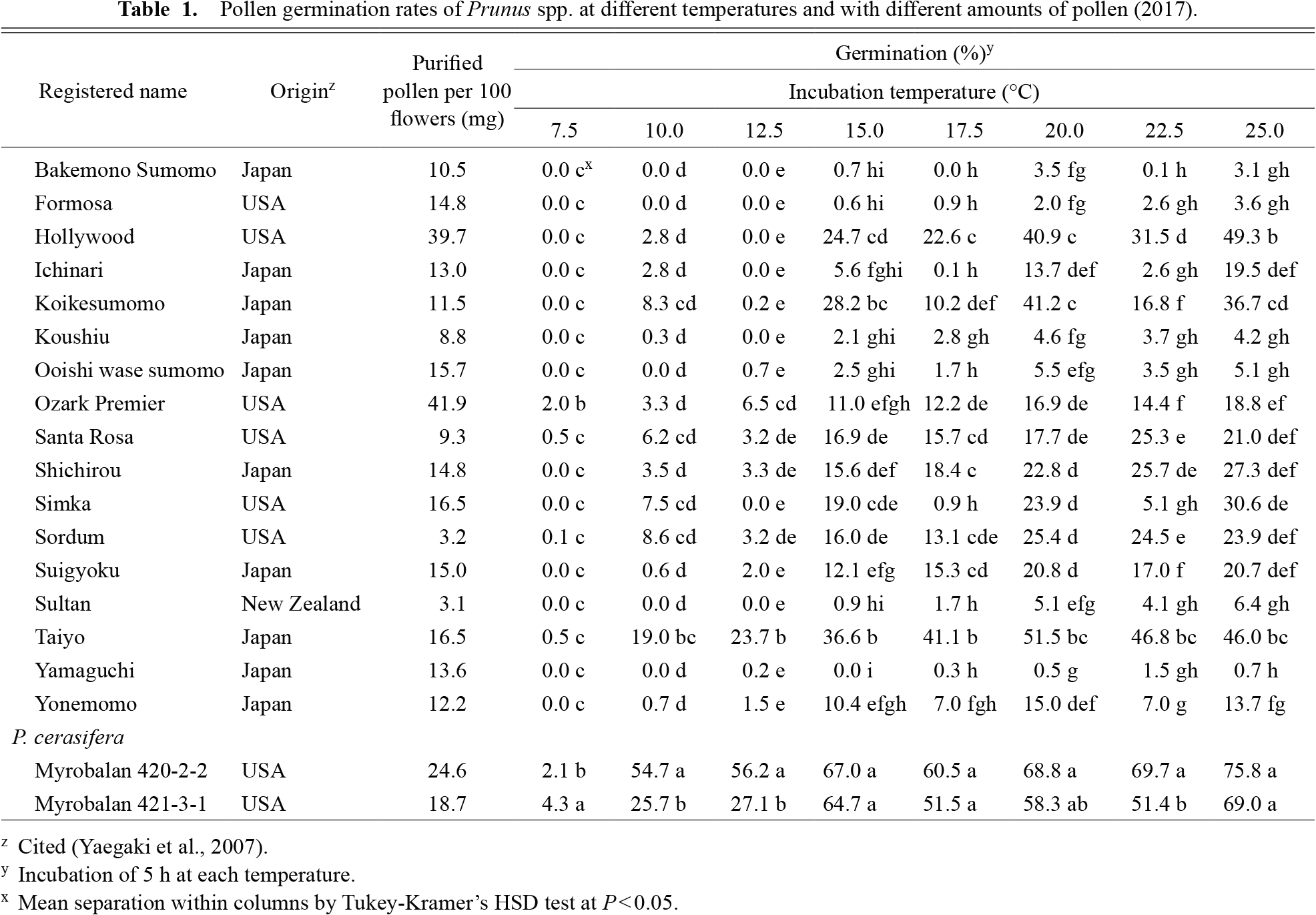
The amount of pollen per 100 flowers in all cultivars used in this study (2017) ranged from 3.2 mg to 41.9 mg (Table 1), while that of the two Myrobalan plum lines (420-2-2 and 421-3-1) was 24.6 mg and 18.7 mg, respectively.
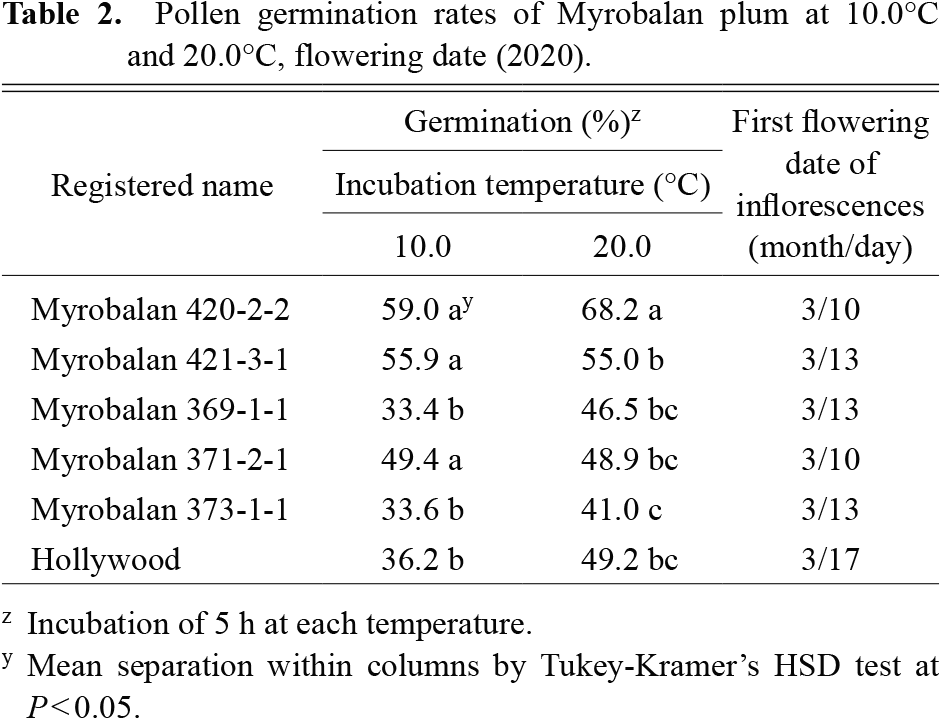
Among all cultivars used in 2017, the germination rates of six cultivars (‘Bakemono Sumomo’, ‘Formosa’, ‘Koushiu’, ‘Ooishi wase sumomo’, ‘Sultan’, and ‘Yamaguchi’) were < 10% at all induction temperatures (Table 1). In most other cultivars, the germination rates were highest between 20.0°C–25.0°C, and in < 20% between 7.5°C–10.0°C. However, Myrobalan 420-2-2 showed a significantly higher germination rate than the other cultivars at 10.0°C and 12.5°C. Furthermore, the germination rate of Myrobalan 420-2-2 at 10.0°C in 2017 was > 50%. pollen germination rates of the three Myrobalan plum lines (420-2-2, 421-3-1, and 371-2-1) were higher than that of ‘Hollywood’, which was used as a control at 10.0°C in 2020 (Table 2).
Among all cultivars used in 2017, the germination rates of six cultivars (‘Bakemono Sumomo’, ‘Formosa’, ‘Koushiu’, ‘Ooishi wase sumomo’, ‘Sultan’, and ‘Yamaguchi’) were < 10% at all the induction temperatures (Table 1). In most other cultivars, the germination rates were highest between 20.0°C–25.0°C and in < 20% between 7.5°C–10.0°C. However, Myrobalan 420-2-2 showed a significantly higher germination rate than the other cultivars at 10.0°C and 12.5°C. Furthermore, the germination rate of Myrobalan 420-2-2 at 10.0°C in 2017 was > 50%. The pollen germination rates of the three Myrobalan plum lines (420-2-2, 421-3-1, and 371-2-1) were higher than the ‘Hollywood’, which was used as a control at 10.0°C in 2020 (Table 2).
Effect of the pollination treatments consisting of pollen from Myrobalan plums applied in an open field on fruit set and fruit quality (Experiment 2)
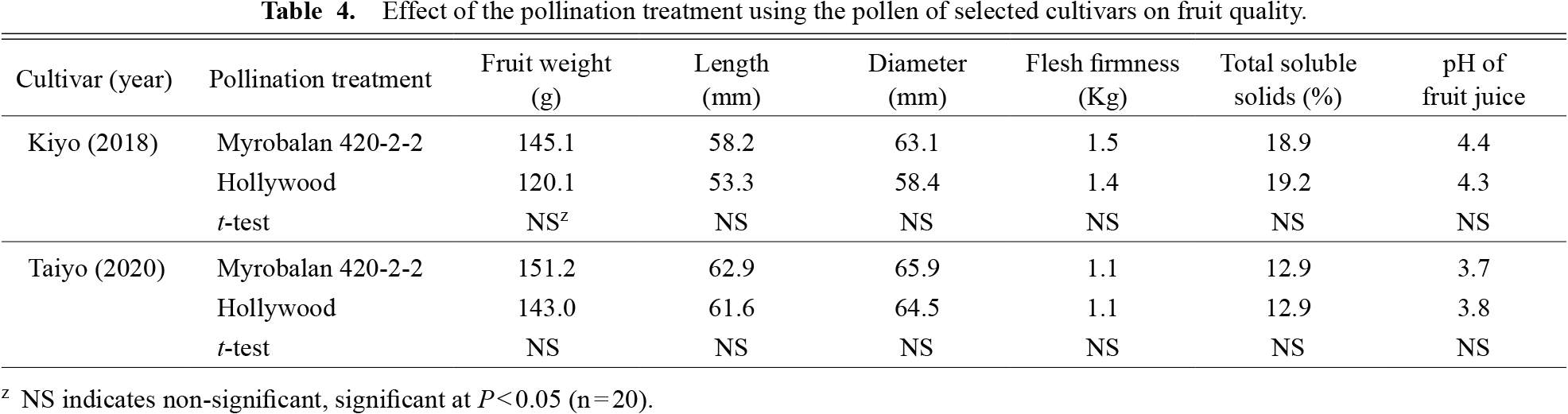
In 2018, at the start of pollination phase, the temperature at 10:00 am was 14.5°C near Gunma Agricultural Research Center (Isesaki city: 36.2°N, 139.1°E). The fruit setting rates of the ‘Kiyo’ were 17.6% and 9.9% by pollination using Myrobalan 420-2-2 and ‘Hollywood’, respectively (Table 3). In 2020, at the start of the pollination phase, the temperature at 10:00 am was 11.2°C near the Shimane Prefectural Agricultural Technology Center (Izumo city: 35.2°N, 132.4°E). The fruit setting rate of the ‘Taiyo’ was approximately 20% for both cultivars (Table 3).

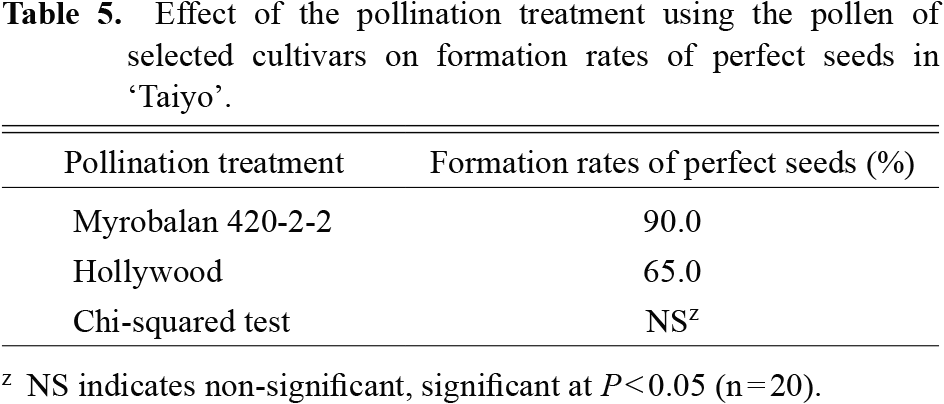
Fruits of ‘Kiyo’ and ‘Taiyo’ were harvested on 19th July 2018 and 20th August 2020, respectively. The fruit quality results (fruit weight, length, diameter, flesh firmness, total soluble solids, and pH of the fruit juice) of the ‘Kiyo’ and ‘Taiyo’ did not differ significantly with different pollination treatments (Table 4). The formation rates of perfect seeds in ‘Taiyo’ by pollination using Myrobalan 420-2-2 and ‘Hollywood’ were 90% and 65%, respectively (Table 5). However, neither of the formation rates were significant according to a chi-squared test.
Effect of the pollination treatment using the pollen of the selected cultivar on the early development of the ovary and ovule in ‘Taiyo’ plants (Experiment 3)
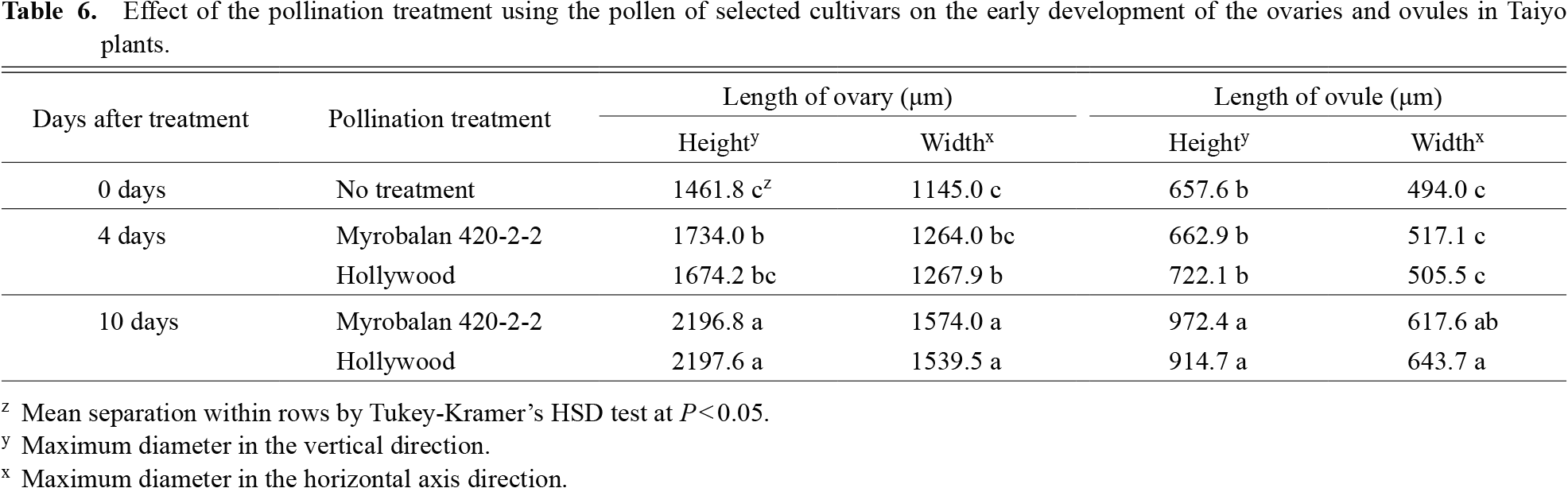
After four days of pollination, the ovaries under the Myrobalan 420-2-2 treatment were longer than those in the untreated group (Table 6). Both the height and width of the ovaries were greater after 10 days of the treatment and did not vary between the Myrobalan 420-2-2 and ‘Hollywood’ treatments.
There was no variation in the height or width of the ovule in all the groups (both the treatment and control) after four days of pollination. However, after 10 days of pollination, both the height and the width of the ovule, although longer in both the treatment groups, did not vary.
Analysis of S-genotyping of Myrobalan (Experiment 4)
PCR with a set of primers resulted in PCR products of different lengths from five cultivars without any incorrect amplification. S-genotyping in each line was determined as follows: Myrobalan 420-2-2 (S7S10), 421-3-1 (S1S10) (Fig. 1), 369-1-1 (S1S2), 371-2-1 (S2S12), and 373-1-1 (S8S12) (Fig. 2).
Discussion
In general, Japanese plum flowering occurs very early in the spring season and low temperatures at the time of flowering reduce the speed of pollen tube growth (Sanzol and Herrero, 2001). The pollen germination rate is very important for fertilization and subsequent fruit development (Mellenthin et al., 1972; Therios et al., 1985). Therefore, the present study was performed to select candidate cultivars as new pollinizers with a stabilizing influence on the fruit setting of Japanese plum at low temperatures.
Regarding the extent of pollen germination at different temperatures in Prunus spp., most varieties did not germinate at 12.5°C as indicated by the experimental results of 2017 (Table 1). However, ‘Taiyo’ and Myrobalan 420-2-2 and 421-3-1 showed higher germination rates than the other cultivars at the same temperature condition; the germination rates of the three cultivars were > 20%. Additionally, the germination rate in Myrobalan 420-2-2 was > 50% at 10.0°C. The high germination rate in Myrobalan 420-2-2 was also confirmed in the 2018 (Supplementary Table S1) and 2020 (Table 2) experiments and the rates in Myrobalans 420-2-2, 421-3-1, and 371-2-1 were higher than in ‘Hollywood’, which was used as a pollinizer in a large area of Japan. Previous studies have reported that the viability, germination, and tube growth of pollen vary significantly according to the species and cultivars (Du et al., 2006; Hedhly et al., 2004). It was also reported that pollen germination in plum plants grown at 20°C differs significantly among cultivars and the germination rate is higher in Myrobalan plums than in Japanese plums (Yaegaki et al., 2007). A new finding that resulted from this study was that the pollen of Myrobalan plums had an extremely high germination rate at low temperatures. Previous studies established that in vitro germination tests of several fruit trees provide an index of the fertilizing capacity of the pollen at each temperature condition, as in vitro germinability and fertilizing capacity in the field are correlated (Kobayashi et al., 2021; Kuroki et al., 2017). On the other hand, it was also reported that yearly variations in pollen germination are due to many environmental factors (Kuroki et al., 2017; Szabo and Nyeki, 2000; Yaegaki et al., 2007). In fact, six cultivars showed germination rates of < 10% in 2017. However, a previous study reported the germination rates of these cultivars to be 15.8%–22.9% after 12 h of incubation (Yaegaki et al., 2007). The pollen germination rate in Myrobalan 420-2-2 after 5 h of incubation at 10°C was stable and high in all investigated years; thus, we concluded that this line possesses high germinating capacity at low temperatures. In addition, it is important for a pollinizer to efficiently collect a large amount of pollen. The amount of pollen per 100 flowers was higher in Myrobalan 420-2-2 than in the other cultivars used in this study (Table 1). In addition, the flowering start date of plums in Tsukuba in 2020 was approximately one week earlier than that of ‘Hollywood’, which was used as a pollinizer in a large area of Japan (Table 2). Thus, we suggest that this Myrobalan plum line can be used to harvest a stable amount of pollen at a relatively early time.
Next, we assessed fruit set in an open field to determine cross-compatibility using a pollination treatment with Myrobalan 420-2-2. The fruit setting rates of Japanese plums ‘Kiyo’ and ‘Taiyo’ were approximately 10–20% under the pollination treatment using pollen of Myrobalan 420-2-2 and using ‘Hollywood’ as the control (Table 3). Previous studies report that the proportion of flowers that develop into fruit is much lower in the Japanese plum (approximately 10%) (Beppu et al., 2005; Guerra et al., 2010) than in other Prunus almonds (approximately 30%) (Kester and Griggs, 1959) and peach (approximately 30%) (Harrold, 1935). However, Guerra and Rodrigo (2015) reported that the higher number of flowers produced usually results in adequate fruit production, as a fruit set of 5% can be sufficient to obtain a good yield, depending on the cultivar. Therefore, we suggest that the effect on fruit set by pollination using the pollen of Myrobalan 420-2-2 is sufficient for cultivating. However, the temperature at the start of the pollination phase in both the experiments (2017 and 2020) was higher than 11°C, and a subsequent decrease in temperature was not confirmed. In this study, we could not perform pollination tests in significantly low temperature conditions; therefore, this point requires further investigation.
On the other hand, neither the fruit size nor fruit quality of the cultivars ‘Kiyo’ and ‘Taiyo’ varied with the source of pollen used (Myrobalan 420-2-2 or ‘Hollywood’; Table 4). Also, the formation rates of perfect seeds in ‘Taiyo’ were 90% and 65% by pollination using Myrobalan 420-2-2 and ‘Hollywood’, respectively, although these formation rates were not significant according to the chi-squared test. The phenomenon whereby the pollen source affects fruit characteristics is known as xenia (Focke, 1881) and has been reported to affect the quality and quantity of fruits and nuts produced by some plant species (Denney, 1992). In pistachio, pollen has been reported to affect the size of the embryo and seeds (Cran and Iwakiri, 1980). Additionally, in previous studies on Japanese plum, the observation of ovule development in the days following pollination revealed a high incidence of premature ovule degeneration in the final fruit set in the low-producing cultivars (Guerra et al., 2011). Therefore, we measured the size of the ovary and ovule using a microscope to assess the effect of the pollination treatment (Myrobalan 420-2-2 and ‘Hollywood’) on the early development of these organs in ‘Taiyo’. The size of the ovary and ovule were not significantly different after they were pollinated by both cultivars even after 10 days of treatment. These results indicate that there was no effect of pollination using pollen of Myrobalan 420-2-2 on the early development of the ovary and ovule.
Finally, we analyzed S-genotyping of Myrobalan 420-2-2, selected as a new pollinizer, to consider the possibilities of cross-compatibility in several other Japanese plum cultivars. In Myrobalan plums, specific 15 S-alleles have been identified using a combination of RT-PCR and direct cloning of genomic PCR products (Sutherland et al., 2009); in this study, the S-genotype in Myrobalan 420-2-2 was determined as S7S10 (Fig. 1). On the other hand, S-genotype in Japanese plum ‘Kiyo’ and ‘Taiyo’ has already been determined in previous studies as SbScSf and SbSc, respectively (Beppu et al., 2002; Jun et al., 2007). Furthermore, it has been confirmed that many cultivars of Japanese plum have the Sb or Sc gene (Amemiya et al., 2015). We also performed PCR using primers of the same sequence according to the method described by Beppu et al. (2002). As a result in Myrobalan 420-2-2, bands of about 400 bp and about 1200 bp were confirmed, but at least the band of about 1550 bp of the Sb gene possessed by both ‘Kiyo’ and ‘Taiyo’ was not detected (data not shown). From the results of the pollination test in this study, we determined that Myrobalan 420-2-2 showed cross-compatibility with both the Japanese plum cultivars ‘Kiyo’ and ‘Taiyo’. In addition, as the deduced amino acid sequence of S-genotyping in Myrobalan 420-2-2 (Sutherland et al., 2009) and Sa-Sn in Japanese plums (Beppu et al., 2002, 2003; Yamane et al., 1999) is different (Supplementary Fig. S1), we assumed that Myrobalan 420-2-2 also showed cross-compatibility with many other plum cultivars. In conclusion, our results show that Myrobalan 420-2-2 has the potential to be a new plum pollinizer as it can effectively pollinate the Japanese plum and germinate at low temperatures with no adverse effect on fruit set and quality.
Literature Cited
- Amemiya, H., M. Miyake, A. Sato, T. Tezuka, A. Tomita, M. Inomata and T. Sakurai. 2015. Research relating to S-haplotypes and pollination compatibility of Japanese plum cultivars. Bull. Yamanashi Fruit Tree Exp. Stn. 14: 1–9.
- Bajwa, G. S., A. S. Bindra, J. S. Bal and P. P. S. Minhas. 1991. Problems of pollination and fertilisation in plum. Acta Hortic. 283: 157–162.
- Beppu, K., H. Yamane, H. Yaegaki, M. Yamaguchi, I. Kataoka and R. Tao. 2002. Diversity of S-RNase genes and S-haplotypes in Japanese plum (Prunus salicina Lindl.). J. Hortic. Sci. Biotechnol. 77: 658–664.
- Beppu, K., Y. Takemoto, H. Yamane, H. Yaegaki, M. Yamaguchi, I. Kataoka and R. Tao. 2003. Determination of S-haplotypes of Japanese plum (Prunus salicina) cultivars by PCR and cross-pollination test. J. Hortic. Sci. Biotechnol. 78: 315–318.
- Beppu, K., N. Komatsu, H. Yamane, H. Yaegaki, M. Yamaguchi, R. Tao and I. Kataoka. 2005. Se-haplotype confers self-compatibility in Japanese plum (Prunus salicina Lindl.). J. Hortic. Sci. Biotechnol. 80: 760–764.
- Cran, J. C. and B. T. Iwakiri. 1980. Xenia and metaxenia in pistachio. HortScience 15: 184–185.
- Denney, O. J. 1992. Xenia includes metaxenia. HortScience 27: 722–728.
- Du, Y. H., S. L. Zhang, T. Jiang and J. Wu. 2006. Characteristics of pollen germination and pollen tube growth of Prunus mume in vitro. Acta Bot. 26: 1846–1852.
- Egea, J., L. Burgos, N. Zoroa and L. Egea. 1992. Influence of temperature on the in vitro germination of pollen of apricot (Prunus armeniaca, L.). J. Hort. Sci. 67: 247–250.
- Faust, M. and D. Surányi. 1999. Origin and dissemination of plums. Hortic. Rev. (Am. Soc. Hortic. Sci.) 23: 179–231.
- Focke, W. O. 1881. Die Pflanzen-Mischlinge: ein Beitrag zur Biologie der Gewächse (In Germany). pp. 510–518. In: Gebruder Bornträger. Berlin.
- Guerra, M. E., A. Wunsch, M. Lopez-Corrales and J. Rodrigo. 2010. Flower emasculation as the cause for lack of fruit set in Japanese plum crosses. J. Amer. Soc. Hort. Sci. 135: 556–562.
- Guerra, M. E., A. Wunsch, M. Lopez-Corrales and J. Rodrigo. 2011. Lack of fruit set caused by ovule degeneration in Japanese plum. J. Amer. Soc. Hort. Sci. 136: 375–381.
- Guerra, M. E. and J. Rodrigo. 2015. Japanese plum pollination. A review. Sci. Hortic. 197: 674–686.
- Hartmann, W. and M. Neumuller. 2009. Plum breeding. p. 161–231. In: P. M. Priyadarshan and S. Mohan Jain (eds.). Breeding Plantation Tree crops: Temperate Species. Springer, New York.
- Harrold, T. J. 1935. Comparative study of the developing and aborting fruits of Prunus persica. Bot. Gaz. 96: 585–620.
- Hedhly, Y., J. Hormaza and I. M. Herrero. 2004. Effect of temperature on pollen tube kinetics and dynamics in sweet cherry, Prunus avium (Rosaceae). Am. J. Bot. 91: 558–564.
- Jun, J., J. H. Kwon and K. H. Chung. 2007. Identification of self-incompatibility genotypes in Japanese plum (Prunus salicina Lindl.) by polymerase chain reaction and cross-pollination tests. Hortic. Environ. Biotechnol. 48: 228–234.
- Kester, D. E. and W. H. Griggs. 1959. Fruit setting in the almond: the pattern of flower and fruit drop. Proc. Am. Soc. Hortic. Sci. 74: 214–219.
- Kobayashi, T., A. Sawada, S. Kasai, S. Goto, K. Matsumoto and S. Kudo. 2021. Apple pollen selection using higher germination properties at low temperature. Hort. Res. (Japan) 30: 287–294 (In Japanese with English abstract).
- Kuroki, K., Y. Takemura, M. Jiang, H. Marumori, N. Teratani, K. Matsumoto, T. Matsumoto and F. Tamura. 2017. Pear pollen selection using higher germination properties at low temperatures and the effect on the fruit set and quality of Japanese pear cultivars. Sci. Hortic. 216: 200–204.
- Luza, J. G., V. S. Polito and S. E. Weimbaum. 1987. Staminate bloom date and temperature responses of pollen germination and the tube growth in two walnut (Juglans) species. Am. J. Bot. 74: 1898–1903.
- Mellenthin, W. M., C. Y. Wang and S. Y. Wang. 1972. Influence of temperature on pollen tube growth and initial fruit development in d’Anjou pear. HortScience 7: 557–559.
- Muraoka, K., T. Miyoshi and T. Matsunami. Effect of air temperature on flowering and fruiting of plum. 1987. Gunma Agri. Res. (Japan) 3: 23–28 (In Japanese with English abstract).
- Nakajo, H., S. Obayashi, M. Yahata, Y. Nagashima, H. Naruse, Y. Masuda, H. Mukai, H. Harada and T. Takagi. 2014. Histological study of ovule development in triploid Japanese plum “Kiyo”. Hort. Res. (Japan) 13: 107–111 (In Japanese with English abstract).
- Polito, V. S., J. G. Luza and S. A. Weinbaum. 1988. Differential low temperature germination responses by pollen of Pistacia vera clones with different bloom dates. Sci. Hortic. 35: 269–274.
- Sanzol, J. and M. Herrero. 2001. The effective pollination period in fruit trees. Sci. Hortic. 90: 1–17.
- Sedgley, M. and C. M. Annells. 1981. Flowering and fruit-set response to temperature in the avocado cultivar ‘Hass’. Sci. Hortic. 14: 27–33.
- Sukhvibul, N., A. W. Whiley, V. Vithanage, M. K. Smith, V. J. Doogan and S. E. Hetherington. 2000. Effect of temperature on pollen germination and pollen tube growth of four cultivars of mango (Mangifera indica L.). J. Hortic. Sci. Biotechnol. 75: 214–222.
- Sutherland, B. G., R. Cerovic, T. P. Robbins and K. R. Tobutt. 2009. The Myrobalan (Prunus cerasifera L.): a useful diploid model for studying the molecular genetics of self-incompatibility in plums. Euphytica 166: 385–398.
- Szabo, Z. and J. Nyeki. 2000. Floral biology of plum. Int. J. Hortic. Sci. 6: 11–27.
- Therios, I. N., V. M. Tsirakoglou and K. N. Dimassi-Therous. 1985. Physiological aspects of pistachio (Pistacia vera L.) pollen germination. Rivista Ortoflorofruit Italy 3: 161–170.
- Vasilakakis, M. and I. C. Porlingis. 1985. Effect of temperature on pollen germination, pollen tube growth, effective pollination period, and fruit set of pear. HortScience 20: 733–735.
- Yaegaki, H., T. Haji, Y. Suesada and M. Yamaguchi. 2007. Cultivar differences, and rates of stained and germinated pollen in apricot and plums. Bull. Natl. Inst. Fruit Tree Sci. 6: 23–29 (In Japanese with English abstract).
- Yamamoto, T., T. Kimura, Y. Sawamura, K. Kotobuki, Y. Ban, T. Hayashi and N. Matsuta. 2001. SSRs isolated from apple can identify polymorphism and genetic diversity in pear. Theor. Appl. Genet. 102: 865–870.
- Yamane, H., R. Tao and A. Sugiura. 1999. Identification and cDNA cloning for S-RNases in self-incompatible Japanese plum (Prunus salicina Lindl. cv. Sordum). Plant Biotechnol. 16: 389–396.
- Weinbaum, S. A., D. E. Parfitt and V. S. Polito. 1984. Differential cold sensitivity of pollen grain germination in two Prunus species. Euphytica 33: 419–426.