2023 年 92 巻 1 号 p. 66-76
2023 年 92 巻 1 号 p. 66-76
Floral induction by grafting without vernalization treatment (NV grafting method) has potential to shorten breeding times and to diversify the seed production of cabbage, an important leafy vegetable with a long and absolute low temperature exposure requirement for its floral induction. However, it is unknown whether the NV grafting method can be actually used for cabbage breeding and seed production. This is because the NV grafting method’s effect on the field performance of obtained progenies has not been investigated, as opposed to the conventional floral induction method by vernalization treatment. Therefore, in this study we compared the effects of two different floral induction methods on the agricultural traits of the obtained progenies. Two clonal lines of ‘Watanabe-seiko No.1’ cabbage were used for the experiment. In the two-year field experiment, we observed a consistent effect of clonal lines on vegetative growth; however, almost no effects of the floral induction methods on either vegetative or reproductive growth were observed. This was further supported by similar expression levels of FLOWERING LOCUS C homologs in the progenies at the young seedling stage. Pollen production and seed formation of the progenies were confirmed regardless of the floral induction method. In conclusion, cabbage seeds obtained by the NV grafting method are likely to show the same traits as those obtained by the conventional vernalization method. This indicates the direct applicability of this method to cabbage breeding and seed production.
Cabbage (Brassica oleracea var. capitata) is an important leafy vegetable cultivated around the world. Like other cruciferous crops, cabbage is induced to flower after exposure to low temperatures (Miller, 1929), a phenomenon known as vernalization. Due to a juvenile phase and a strong vernalization requirement for flowering (Ito and Saito, 1961; Miller, 1929), cabbage requires a longer time for flowering than other cruciferous crops, taking about six months or more for one generation, even when grown under optimal conditions in a climate chamber. In an extreme cabbage accession, flowering may not occur even after overwintering (Kinoshita et al., 2021). Several studies have identified genes that regulate the vernalization requirement of B. oleracea, and the major ones are homologs of FLOWERING LOCUS C (FLC), a master regulator of the vernalization floral pathway in Arabidopsis thaliana (Abuyusuf et al., 2019; Irwin et al., 2016; Lin et al., 2018; Michaels and Amasino, 1999; Okazaki et al., 2007). This strong requirement for vernalization is advantageous in agricultural production of cabbage because it can suppress the occurrence of bolting, stem elongation followed by flowering, which damages harvesting sites (Jung and Müller, 2009). On the other hand, this difficulty in floral induction is problematic for breeding programs, in which rapid floral induction to shorten the generation time is required. Also, such flowering characteristics limit the available regions and conditions for cabbage seed production (EL-Eslamboly and Hamed, 2021; Nyarko et al., 2007; Wang et al., 2000). If several weeks of vernalization treatment could be avoided and direct induction of cabbage flowering were realized, it would be possible to improve breeding efficiency and diversify cabbage seed production.
With this background, we have developed a method to induce cabbage flowering without vernalization treatment by grafting cabbage onto florally induced radish (Raphanus sativus L.) rootstocks (Non-Vernalization-grafting floral induction method, NV grafting method; Fig. 1). This method was developed based on a report by Kagawa (1957), who found that grafting onto radish allowed the cabbage to flower without vernalization treatment, while grafting onto florally induced cabbage did not induce the grafted cabbage to flower. This technique utilizes the action of florigen, a universal, transmissible floral inducer produced in the leaves of florally induced plants, through the graft junction (Chailakhyan, 1936; Zeevaart, 1958). FLOWERING LOCUS T (FT) protein and its homologs have been identified as a major component of florigen in several plant species (Corbesier et al., 2007; Lifschitz et al., 2006; Lin et al., 2007; Notaguchi et al., 2008), and were shown to potently induce flowering at the transmitted sites.

Current procedure and key steps in the Non-Vernalization-grafting floral induction method (NV grafting method) for cabbage. (A) Schematic illustration of conventional vernalization based on the floral induction method and NV grafting method. LD means long-day condition (16 h light/8 h dark). Fluorescent lamps are usually used for growing the plants. Conditions are described when the cabbage cultivar ‘Matsunami’ (Ishii Seed Growers Co., Ltd., Shizuoka, Japan) was used as the scion, and early-bolting type radish (‘Rat’s tail-CH’) was used as the rootstock. The optimal conditions may vary depending on the cabbage and radish cultivars. The picture shown at the top right is a typical ‘Watanabe-seiko No.1’ cabbage induced to flower by the vernalization method (bar = 10 cm). The picture shown at the bottom right is a typical ‘Matsunami’ cabbage scion induced to flower by the NV grafting method (bar = 10 cm). White arrowhead indicates the graft junction. (B) Key points for successful floral induction using the NV grafting method. (C) Floral induction of young cabbage seedlings by the NV grafting method (‘Matsunami’). Left: Cabbage seedlings grown at 22°C, with a 16 h day length condition for 3–4 weeks after sowing. Middle left: A cabbage seedling cut to a wedge shape for grafting. Middle right: Cleft grafting of a cabbage seedling onto the flower stem of a radish rootstock. White arrowhead indicates the graft junction. Right: Grafted cabbage scions started to flower 50 days after grafting. The picture was taken 53 days after grafting.
The report of Kagawa (1957) was groundbreaking, showing that floral induction of cabbage without vernalization treatment could be achieved by grafting. However, his findings were overlooked for more than 50 years, probably due to the low reproducibility of this phenomenon. We investigated the grafting-induced flowering of cabbage in detail under a controlled environment and found that a high success rate for floral induction could be achieved by using specific accessions of radish as a rootstock (Motoki et al., 2019, 2022). Expression analysis of flowering-related genes was conducted to deduce the mechanism of floral induction by grafting, and it was revealed that FLC homologs were highly expressed and FT homologs were lowly expressed in cabbages which were induced to flower by grafting (Motoki et al., 2019). FLC acts as a repressor of FT in A. thaliana (Helliwell et al., 2006; Michaels et al., 2005); therefore, it was assumed that the grafted cabbage scions were induced to flower by exogeneous FT protein from the rootstock (Motoki et al., 2019). Supporting this observation, the accumulation levels of FT protein in the grafted scions were found to be positively correlated with the floral induction of the grafted cabbage, and a higher expression level of FT and a larger leaf area of the rootstocks were necessary for the high accumulation of FT and stable floral induction of the grafted cabbage (Motoki et al., 2022).
We improved the grafting procedure so that a higher amount of FT protein could be transported from the rootstock to the grafted cabbage by doing the following: (i) selection of radish accessions with good genetic potential to highly express FT homologs in their leaves; (ii) environmental control such as seed vernalization treatment and long day treatment to increase the expression of FT homologs of radish rootstocks; (iii) keeping the leaf area of the rootstock as large as possible (Fig. 1A, B). Removing leaves of the grafted cabbage to ensure that the cabbage could preserve sink activity was also important, as done in previous studies (Hamner and Bonner, 1938; Kagawa, 1957). By using this NV grafting method, we could induce flowering and obtain viable seeds in several cabbage cultivars (Motoki et al., 2019). We confirmed that the NV grafting method could induce flowering of young cabbage seedlings (3–4 weeks after sowing) −1.5 to 2 months after grafting (Fig. 1C, Motoki et al., 2022). In this way, our NV grafting method is close to becoming a viable seed production technology.
However, it is not clear if NV grafting can actually be used for breeding programs or seed production because we have not yet investigated the possibility of alteration in the growth traits of the obtained progenies. It has been reported in several crops that conditions during seed production affect the seed traits and growth characteristics of the progenies (Hagiya, 1949, 1950; Shinohara, 1959). In this study, we conducted seed production trials by the conventional vernalization-based floral induction method (vernalization method) and the NV grafting method in the same growth environment, and investigated the field performance of the obtained progenies over a two-year field cultivation period.
Two clonal lines isolated from ‘Watanabe-seiko No.1’ cabbage (Genebank project, NARO, Japan, accession No. 25974) were used for the seed production experiment (line #1 and #2). Clonal lines were used to avoid the effect of genetic heterogeneity within this cultivar. These lines were independently isolated from a single seed and clonally propagated in vitro by cuttings for more than a year. The plants were cultured on modified Murashige and Skoog medium (Murashige and Skoog, 1962) with 10% strength ammonium nitrate. Culture conditions were 25 ± 3°C and a 16 h photoperiod provided by fluorescent lamps. Open-pollinated ‘Watanabe-seiko No.1’ seeds (Original population), and a commercial cabbage cultivar ‘Kinkei No.201’ (Sakata Seed Corp., Yokohama, Japan) were used as a control for the field experiments. ‘Watanabe-seiko No.1’ is a summer sowing-type cultivar and ‘Kinkei No.201’ is an autumn sowing-type cultivar. ‘Rat’s tail-CH’ radish (Motoki et al., 2019, originally bought from Chiltern Seeds, Wallingford, United Kingdom) was used as a rootstock for the grafting.
Seed production of cabbage by the vernalization methodTwo clonally propagated cabbage lines were transplanted into 9 cm diameter plastic pots filled with Nippi gardening soil No. 1 (Nihon Hiryo Co., Ltd., Gunma, Japan) and acclimated in a plant growth room at 22 ± 2°C, 16 h day length with a light intensity of 80–120 μmol·m−2·s−1 PPFD provided by fluorescent lamps (NEC Lighting, Ltd., Tokyo, Japan). Seedlings with stem diameters exceeding 1 cm were vernalized for 12–13 weeks in the growth cabinet at 6 ± 1°C, using a 16 h day length with a light intensity of 90 μmol m−2·s−1 PPFD provided by LED light (660 nm:450 nm = 8:2). After vernalization treatment, the seedlings were returned to the growth room. Self-pollination by CO2 treatment (Nakanishi and Hinata, 1975) was performed as follows: opened flowers were pollinated with self-pollen, and the inflorescence was immediately covered by a one-layered clear polyethylene bag. Then, CO2 gas accounting for almost 20% of the volume of the polyethylene bag was injected into the bag, and the opening was tied. The polyethylene bags were removed 12–24 h after pollination. Pollination was continued until the plants ceased flowering. Mature pods were harvested after drying and before splitting. All plants were irrigated and fertilized from below using a half-strength nutrient solution (NO3-N 8 mEq·L−1, PO4-P 2 mEq·L−1, K 4 mEq·L−1, Ca 4 mEq·L−1, Mg 2 mEq·L−1, Enshi-shoho, formulated by the National Horticultural Research Station, Japan).
Seed production of cabbage by the NV grafting methodSeeds of ‘Rat’s tail-CH’ radish were sown on wet filter paper and germinated in the dark at 22°C for a day. Germinated seeds were then vernalized at 2°C in the dark for 14 days, and then sown in 7.5 cm diameter plastic pots filled with granular rockwool (Nippon Rockwool Corp., Tokyo, Japan) in the growth room described above. Vernalization at 2°C can arrest seed growth, thereby preventing their elongation, while it is effective for radish floral induction. Grafting was conducted according to a previously published protocol (Motoki et al., 2019), except that the lateral shoots of the rootstocks were partially untouched, with their flower buds being removed. Two clonally propagated cabbage lines acclimated in the same growth room were used as scions for grafting. After the first flower opened, all leaves of cabbage scions longer than 2 cm were regularly removed. Self-pollination was performed in the same way as the vernalization method described above until the plants ceased flowering. After the plants ceased flowering, scion leaf removal was stopped. Mature pods were harvested after drying and before splitting. All plants were irrigated and fertilized from below using a half-strength nutrient solution (Enshi-shoho).
Growth conditions and investigation of vegetative and reproductive growth of cabbage in the field experiments‘Kinkei No.201’ and an original population of ‘Watanabe-seiko No.1’ as control lines, and self-pollinated seeds of two clonal lines of ‘Watanabe-seiko No.1’ produced by the two floral induction methods were used for the field experiments. In 2019, the sterilized seeds were directly sown in a 105-hole cell tray filled with a soil mixture composed of Nippi gardening soil No. 1 (Nihon Hiryo Co., Ltd.) and a peat moss-based growing media, PRO-MIX PGX (Premier Tech Ltd., Quebec, Canada) at a ratio of 2:1 (v/v) on August 26th. After grown in a growth chamber (20°C, all day light condition using fluorescent lamps) for five days, seedlings were grown in a plastic greenhouse until transplanting. They were transplanted in an open field of the Kizu Farm, Kyoto University, on September 24th, with an inter bed distance of 1.4 m, two rows in a bed, an inter row distance of 0.3 m, and inter plant distance of 0.4 m (plant density 357.1 plants·a−1). In 2021, the sterilized seeds were firstly sown on wet filter paper on September 19th and incubated in the dark at 22°C for a day. Because the seed coat did not naturally split in most seeds of line #1 irrespective of the floral induction method, seed coats were removed by tweezers from all lines and then sown in a 128-hole cell tray filled with the same medium used in 2019. Seedlings were grown in a plastic greenhouse until they were transplanted in the open field of the Kizu Farm on October 13th, with an inter bed distance of 1.4 m, two rows in a bed, inter row distance of 0.4 m, and inter plant distance of 0.4 m (plant density 357.1 plants·a−1). In 2019, 40 seedlings were transplanted for each treatment, except for the seeds of line #1 produced by the vernalization method for which we could not obtain enough established seedlings. In 2021, 24 seedlings were transplanted for each treatment. All cultivation, fertilization, and pest management activities were conducted according to commercial practice. After March of the following year, after transplanting, cabbage heads were divided vertically with a knife so that the main stem could bolt.
The emergence rate of the seedlings was measured five days after sowing in 2019, and 10 days after sowing in 2021. The seedlings were regarded as emerged if the greened cotyledons were visible from above. The length and width of the largest leaf and maximum plant width were measured on December 17th in 2019, and on December 18th in 2021. Half of the transplanted plants were harvested, and their head traits (diameter, height, and weight) were investigated on January 7th in 2020, and on February 11th in 2022. Leaves covering over the half of the inside sphere were included as a head. Remaining half plants were used for the investigation of reproductive growth. The flowering date was defined as the day when the petals of the first flower expanded. Pollen production was confirmed by touching the flowers by hand and seeing if pollen grains were released. Seed formation was investigated for the open-pollinated flowers by inspecting the presence of the developing seeds in the young pods about three weeks after the initial flower opening. Plants which were heavily infected by disease were removed and excluded from the reproductive growth investigation.
Expression analysis of FLOWERING LOCUS C homologs in young seedlings of the cabbage progeniesIn B. oleracea, three FLC homologs [BoFLC1, BoFLC3, and BoFLC4 (BoFLC2)] have been reported to be involved in the vernalization requirement for flowering (Abuyusuf et al., 2019; Lin et al., 2018; Okazaki et al., 2007). To investigate the possibility that the NV grafting method may alter the physiological state for flowering in the obtained progenies, we measured the expression of these three FLC homologs as representative genes for the vernalization-induced flowering response in the young cabbage seedlings.
Self-pollinated seeds of two clonal lines of ‘Watanabe-seiko No.1’ produced by the two floral induction methods were used for the experiment. Sterilized seeds were sown on wet filter paper and incubated in the dark at 22°C for two days. Then, the seedlings were transplanted with Jiffy-7® 42 mm peat pellets (Jiffy Products of America, Inc., Batavia, IL, USA) and grown for two more days in the plant growth room at 22 ± 2°C, 16 h day length with a light intensity of 120 μmol·m−2·s−1 PPFD provided by fluorescent lamps (NEC Lighting). Cotyledons were sampled from four individuals in each line/method at 0–0.5 h before the end of the light period on the fourth day after sowing.
Total RNA was extracted from the cotyledons using Sepasol RNA I Super G (Nacalai Tesque, Inc., Kyoto, Japan), purified using an EconospinTM for RNA (Epoch Life Sciences, Missouri, TX, USA), and reverse transcribed using ReverTra Ace® qPCR RT Master Mix with gDNA Remover (Toyobo Co., Ltd., Osaka, Japan) according to the manufacturer’s instructions. Subsequently, 1 μL of 20-fold diluted RT product was used as a template for quantitative reverse transcription‐PCR (RT-qPCR). RT-qPCR was performed using a THUNDERBIRD® SYBR® qPCR Mix kit (Toyobo Co., Ltd.) according to the manufacturer’s instructions. The reaction was performed using a LightCycler® 480 system (Roche Diagnostics K.K., Tokyo, Japan). RT-qPCR cycling was performed as follows: 95°C for 5 min, followed by 40 cycles at 95°C for 10 s, 60°C for 30 s, and 72°C for 30 s. Single-target product amplification was evaluated using a melting curve. All RT-qPCR primers used in this study are listed in Supplementary Table S1.
Statistical analysisStatistical analyses were performed using R v. 4.1.0. (R Core Team, 2021). Data were subjected to two-way analysis of variance (ANOVA).
Seed of two clonal lines of ‘Watanabe-seiko No.1’ were produced for several plants in the growth room using the vernalization method and NV grafting method. These clonal lines were used to avoid the effect of any genetic heterogeneity traits on the progenies because we observed morphological variability among the individuals of ‘Watanabe-seiko No.1’ cabbage. In the vernalization method, cabbage plant flowers started to open from around three weeks after the end of the vernalization treatment. In the NV grafting method, cabbage plant flowers started opening from 6–8 weeks after grafting. Self-pollination of these plants by CO2 treatment produced mature seeds with all lines and methods. The results of the seed production are summarized in Table 1. The floral induction method had a significant effect on the seed yield, although there was large variability depending on the plants in both the vernalization and NV grafting methods. The amount of seeds produced by the NV grafting method was comparable in line #1 and lower in line #2 than the vernalization method; 0.03–6.46 g of seeds were obtained per plant using the vernalization method and 0.10–1.08 g of seeds were obtained per plant using the NV grafting method. The floral induction method had a significant effect on the seed size, and the vernalization method produced larger seeds than the NV grafting method. No effect of lines was observed for these seed traits.
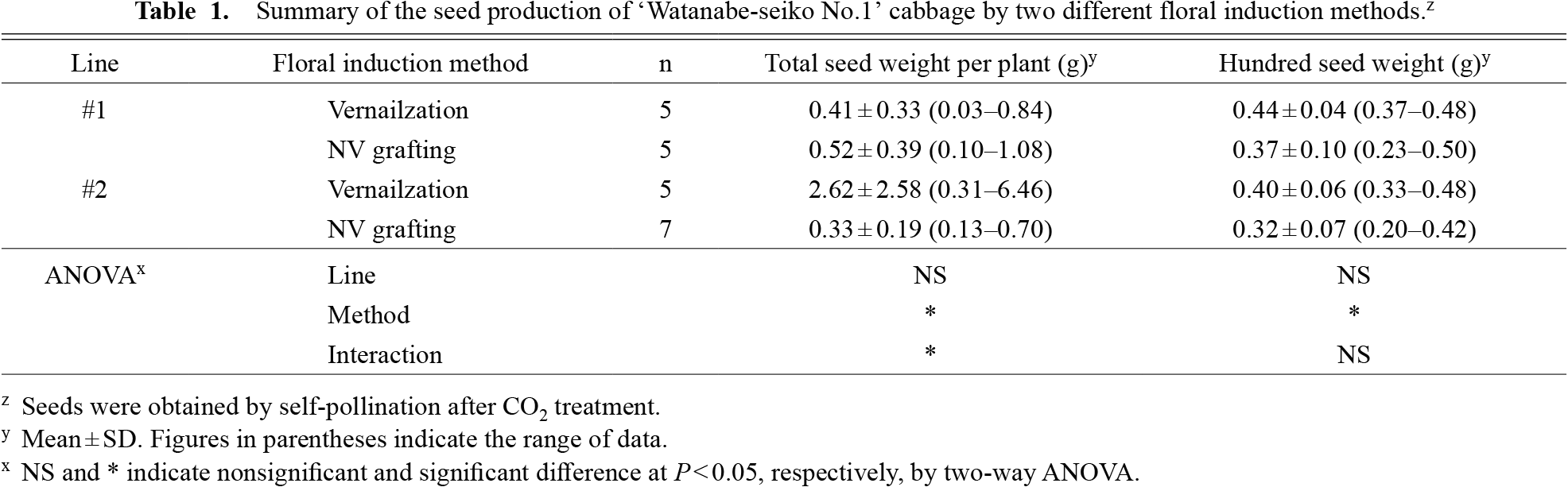
Summary of the seed production of ‘Watanabe-seiko No.1’ cabbage by two different floral induction methods.z
Field experiments of the progenies produced by the two floral induction methods were conducted from summer to the following spring in 2019 and 2021. The sizes of the seeds used for the field experiments were smaller for seeds produced by the NV grafting method (Table 2). We observed a lower emergence rate in line #1 in both 2019 and 2021, regardless of the floral induction method (Table 2). The vegetative growth of each line measured in December is shown in Table 3. A significant effect of the floral induction method was only observed for the plant width in 2019. For the length of the largest leaf, a significant effect of lines was observed in both 2019 and 2021.
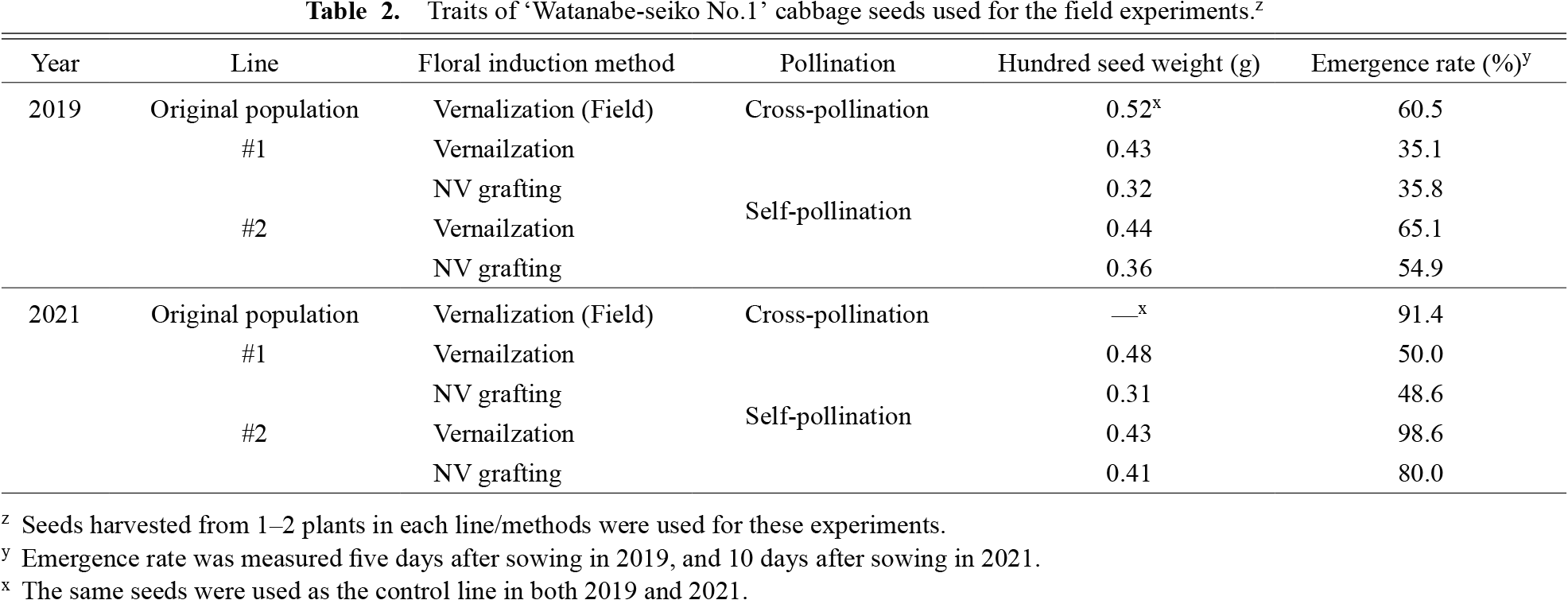
Traits of ‘Watanabe-seiko No.1’ cabbage seeds used for the field experiments.z

Vegetative growth of ‘Watanabe-seiko No.1’ cabbage progenies obtained by two different floral induction methods.
The head trait measurements are shown in Table 4. In 2021, heads were small in all lines, probably due to the late transplanting. The commercial hybrid cultivar ‘Kinkei No.201’ which was grown as a control produced heads averaging about 1 kg in weight in 2019. Meanwhile, the ‘Watanabe-seiko No.1’ cabbage used in this study tended to produce smaller heads (less than 1 kg on average for each line), including the original population. We observed no significant effect of the floral induction methods on any of the head traits investigated in 2019 and 2021. On the other hand, the effect of lines on the head height was significant in both 2019 and 2021, which was likely caused by the difference in the leaf length between the two lines (Table 3). In summary, we observed a consistent effect on the vegetative growth of ‘Watanabe-seiko No.1’ cabbage by the clonal lines, but not by the floral induction method.
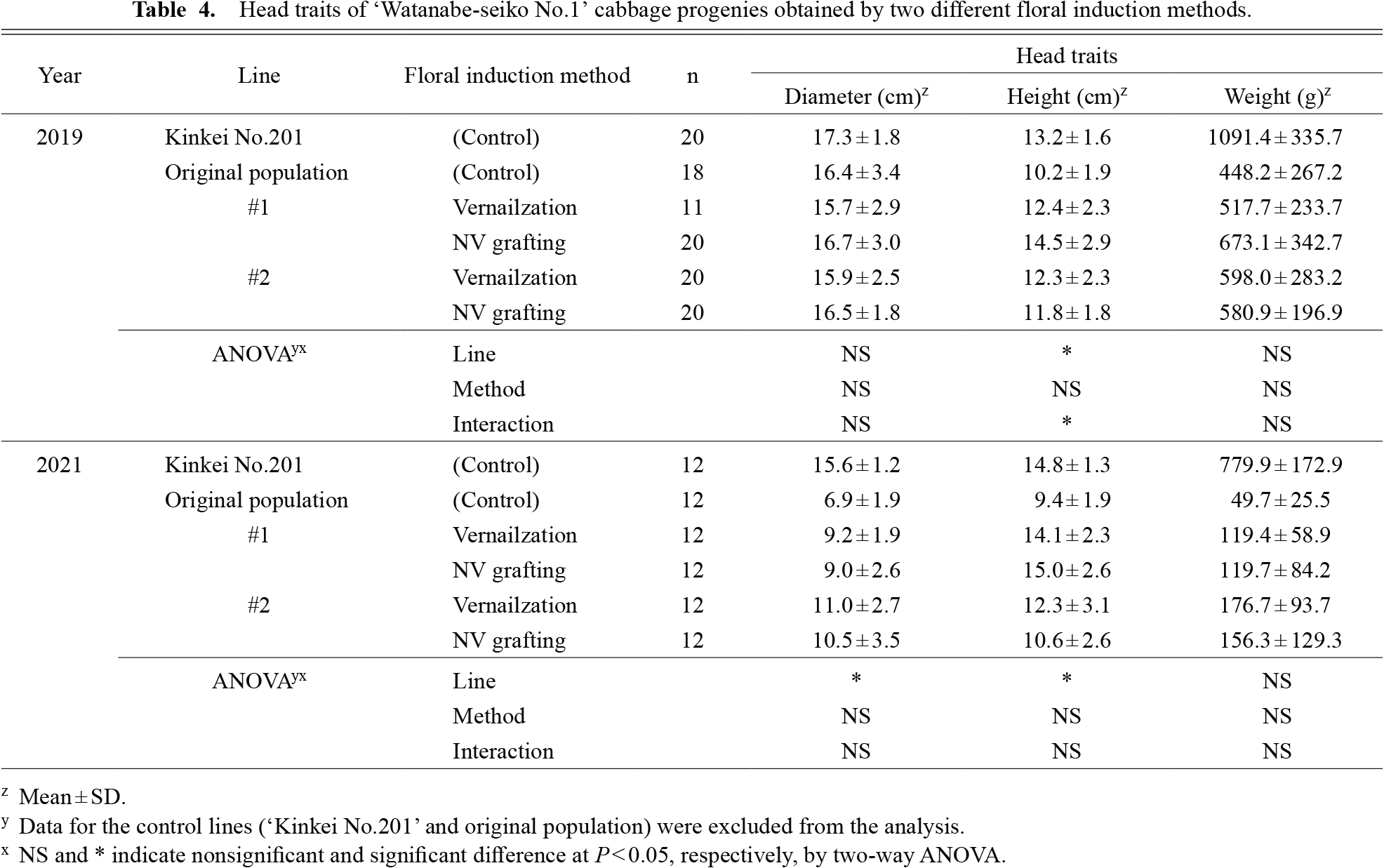
Head traits of ‘Watanabe-seiko No.1’ cabbage progenies obtained by two different floral induction methods.
After overwintering, bolting and flower opening of all lines started in April in both the 2019 and 2021 field experiments. The results of the flowering time measurement are shown in Table 5. All plants flowered by the end of April, except for one individual in line #1 produced by the vernalization method. The original population of ‘Watanabe-seiko No.1’ flowered about 10 days earlier than the two clonal lines in 2019, but was almost the same as the two clonal lines in 2021. We did not observe any significant effect of either clonal lines or floral induction methods for flowering time in 2019. A significant effect of clonal lines was observed in 2021, but the difference in average days to flowering was only one day. All the flowered plants produced pollen and formed seeds normally when they were open pollinated in the 2021 experiment, regardless of the floral induction method (Table 5).
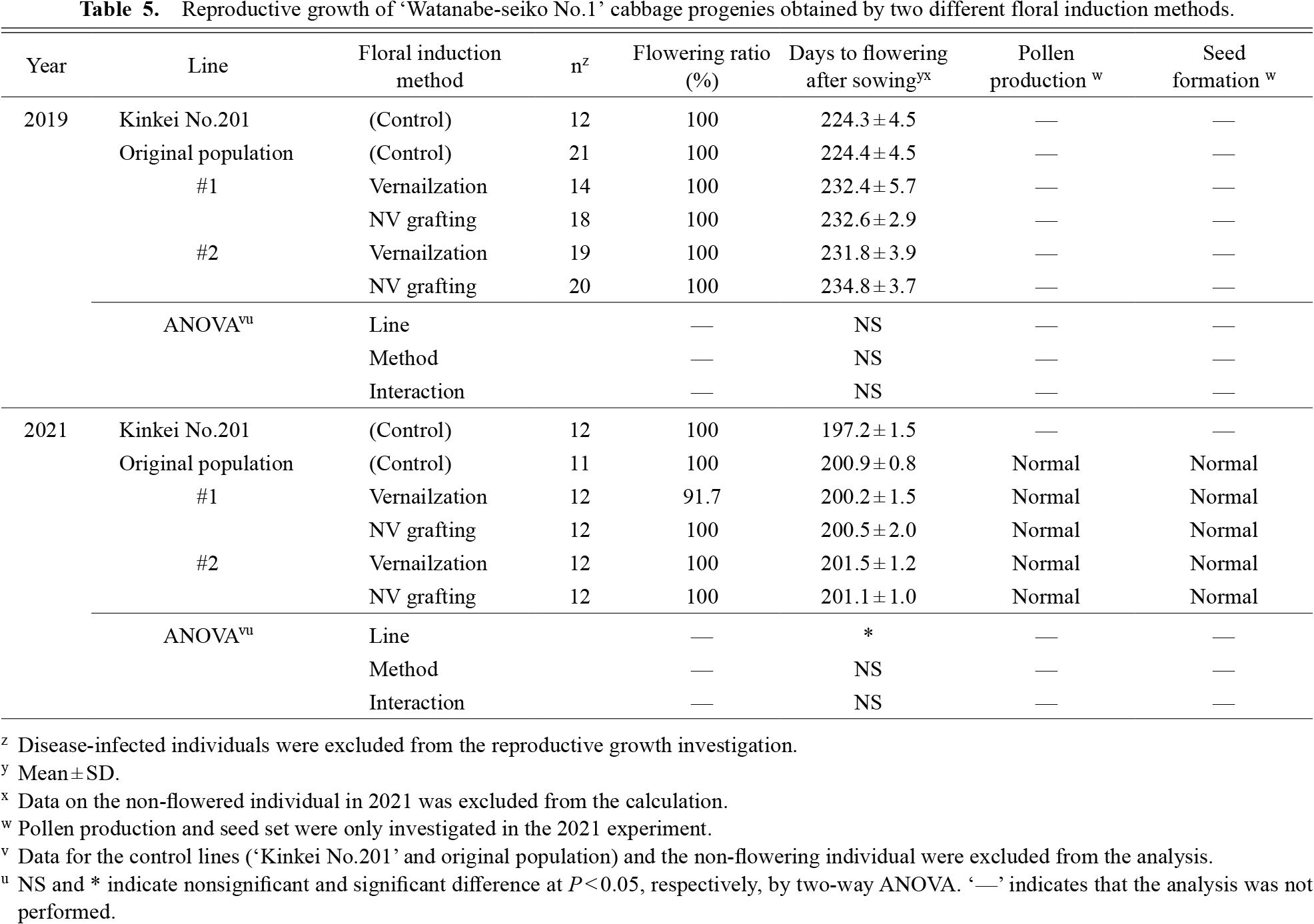
Reproductive growth of ‘Watanabe-seiko No.1’ cabbage progenies obtained by two different floral induction methods.
In parallel with field trials, to investigate the possible effect of NV grafting methods on the physiological state of the progenies for reproductive growth, we conducted expression analysis of three FLC homologs in young seedlings of the progenies. In a two-way ANOVA, the expression levels of the three FLC homologs did not differ with the floral induction method, although a significant difference was observed between clonal lines for BoFLC1 (Fig. 2). This suggested that the NV grafting method may not have an effect on the physiological state of the progenies in terms of reproductive growth, even at the start of the developmental stage. In conclusion, we did not observe any differences in the reproductive growth of the cabbage progenies produced by the two floral induction methods.

Expression level of (A) BoFLC1, (B) BoFLC3, and (C) BoFLC4 transcripts in the cabbage progenies obtained by two different floral induction methods. Cotyledons were sampled at four days after sowing and used for RNA extraction. The expression level of each gene was determined by RT-qPCR (n = 4). BoActin was used as an internal control. Two-way ANOVA results are displayed above each graph. NS and * indicate nonsignificant and significant difference at P < 0.05, respectively.
In plant breeding, floral induction is an important step that determines the time required to conduct crossing and advance generations to establish inbred lines (Watson et al., 2018). Also, it limits the suitable conditions for seed production. Especially in crops such as cabbage, for which artificial floral induction requires time and is a laborious process, floral induction can be a major limitation for breeding and seed production (EL-Eslamboly and Hamed, 2021; Nyarko et al., 2007; Wang et al., 2000). Our NV grafting method enables direct induction of flowering by skipping the prolonged exposure to low temperatures that cabbage usually requires for its floral induction. In this study, to examine the applicability of the NV grafting method to cabbage breeding and seed production, we investigated the field performance of the obtained progenies.
Seed yield and quality of cabbages obtained by the NV grafting methodSeed yields of the cabbage lines tended to be lower with the NV grafting method than with the vernalization method (Table 1). This was likely due to the difference in the number of opened flowers between the two flowering inducing methods. From a rough observation, cabbage plants tended to produce more inflorescences with the vernalization method than the NV grafting method in this study. On the other hand, we also observed that seed yield varied among the grafted individuals and some of the plants produced comparable amount of seeds to that of the vernalization method (Table 1). It was shown in a previous grafting experiment that the number of opened flowers was affected by the condition of the radish rootstocks, such as leaf area and the degree of floral induction (Motoki et al., 2022). Therefore, seed yield in the NV grafting method may be increased by using radish rootstocks with larger and more florally induced leaves. The actual cause of the variation in the seed yield in the NV grafting method should be further investigated; however, data on factors related to seed yield, such as number of flowers, fruiting rate, and number of seeds per pod, were not measured in this study. Further investigation into these factors will lead to stable seed production using the NV grafting method.
Seed size also tended to be smaller with the NV grafting method than with the vernalization method (Table 1). This could be related to the difference in the source capacity of the plants between the two floral induction methods. In the vernalization method, cabbage plants grown for 2–3 months that had many leaves were used for floral induction. In contrast, with the NV grafting method, cabbage plants were grafted onto small radish rootstocks grown for less than one month that had only 3–5 leaves. This may have resulted in the difference in the ratio of source leaves to seed pods, lead to different seed sizes.
Field performance of the progenies obtained by the NV grafting methodIn the field experiment, while there was difference in seed size among the tested seeds depending on the floral induction method (Table 2), there was little difference in their vegetative growth (Tables 3, 4). It has been reported that in cabbage, seed size affected initial growth, but had little effect on later growth and yield (Lingegowda and Andrews, 1973). Similarly in this study, the effect of different seed sizes seemed to be compensated for during the growth period before the vegetative growth measurement in December. One concern regarding the growth measurement was the low emergence rates in both clonal lines (Table 2), which may have caused variation in growth during seedling raising. While there were large differences in the emergence rates between clonal lines in both 2019 and 2021, the differences in the emergence rates between the floral induction methods were relatively small (Table 2). Therefore, we assume that the low emergence rates may not have had much effect on the vegetative growth with the different floral induction methods.
The average head weight in 2019 was about 1 kg for the commercial hybrid cultivar ‘Kinkei No.201’. This is the standard market size in Japan. On the other hand, ‘Watanabe-seiko No.1’ cultivated under the same conditions, including the original population, had a much lower head weight than ‘Kinkei No.201’ (Table 4), although the plant size was comparable between these cultivars (Table 3). The small head size in ‘Watanabe-seiko No.1’ may be one of its original characteristics because it is a classic open-pollinated cabbage cultivar raised in Japan more than 60 years ago. In this study, although no differences in the head traits were observed between the two floral induction methods, studies using cultivars with more stable heading are needed to further confirm that the NV grafting method does not affect the head traits of the progenies.
‘Watanabe-seiko No.1’, including the original population, had a more slender leaf resulting in a smaller head, especially in 2021 (Tables 3, 4). This seemed to be due to the late sowing date and its sowing-type. However, in ‘Kinkei No.201’, its vegetative and head traits were not so different between 2019 and 2021. Thus, we consider that the cultivation conditions did not affect our results.
We also observed no difference in the reproductive growth of cabbage seeds produced by the two different floral induction methods (Table 5). This was supported by a similar expression level of the FLC homologs, key genes in the flowering regulation of cabbage (Fig. 2). This suggested that the temperature history of the mother plant may not have an effect on the flowering response of the resulting cabbage seeds. In summary, it is unlikely that the NV grafting method alters the traits of the progenies as compared to the vernalization method.
The NV grafting method for cabbage breedingWe believe that the NV grafting method can be applied to various cabbage breeding scenarios. For example, it can be used to shorten the time for backcrossing in the introduction of cytoplasmic male sterility and disease resistance to existing cultivars, or for establishing inbred lines. Another possible application is simultaneous floral induction for crosses between cultivars that flower with different timing under natural conditions. In tropical regions, attempts have also been reported to introduce foreign cabbage varieties that are difficult to flower under natural conditions into local breeding programs by using the NV grafting method (EL-Eslamboly and Hamed, 2021).
The results of the two-year field evaluation conducted in this study indicated that it was unlikely that the NV grafting method would alter the traits of any progenies, that is, both vegetative and reproductive growth (Tables 3, 4, and 5). Therefore, it is likely that seeds obtained by the NV grafting method could be used in breeding programs in the same way as conventional vernalization methods.
There are some issues with the NV grafting method for breeding programs. First, it tends to produce fewer seeds than the conventional vernalization method (Table 1). Although, there was variability among individuals, it was possible to obtain more than 100 seeds from one plant. This is enough for hybridization or backcrossing. On the other hand, if a large number of seeds is needed for a large-scale trait evaluation in the field, it may be necessary to collect seeds by conducting multiple grafting. Another problem may be the labor-intensive nature of the grafting operation and cultivation management, such as leaf removal from the scions after grafting. These problems with the NV grafting method need to be solved through genetic improvements in the rootstock and development of more labor-saving management methods.
The NV grafting method for cabbage seed productionIn commercial seed production, the NV grafting method has the potential to be used in the development of novel seed production, such as off-season seed production when cabbage does not naturally flower, seed production of extremely late-bolting cabbage cultivars which are difficult to induce to flower under natural conditions, or hybrid seed production between lines with different natural flowering periods. In this study, it was considered unlikely that NV grafting would affect traits in the obtained progenies, so it is possible to use this method directly for seed production. However, the NV grafting method currently produces much smaller numbers of seeds per plant compared to the reported yield of seed production in the field (Al-Khatib et al., 1995), and is more labor-intensive. Thus, its applicability to commercial seed production is currently limited. For example, the NV grafting method could be used for seed production of parental lines for hybrid seed production. On the other hand, for non-commercial use, it could be used for small-scale seed production to maintain genetic resources or for local seed self-sufficiency by farmers. To realize broader application of the NV grafting method for seed production, the seed productivity of this method will be needed to be improved.
We would like to say a special thanks to Prof. Susumu Yazawa for all his valuable information on the grafting induced flowering of cabbage, based on his long-term investigations into flowering of cruciferous crops. We are grateful to Mr. Toshio Sakakibara, Mr. Katsutoshi Nonaka, Mr. Koji Nishikawa, Mr. Noboru Nara, Mr. Masaru Matsuda, and Mrs. Saori Kawaguchi for technical assistance with cabbage cultivation. Seeds of ‘Watanabe-seiko No.1’ (JP No. 25974) were kindly provided from the Genebank Project, NARO, Japan.