2023 年 92 巻 1 号 p. 1-12
2023 年 92 巻 1 号 p. 1-12
Self-incompatibility in Citrus species is an important trait related to fruit set and seed formation. In particular, self-incompatible citrus varieties combined with sufficient parthenocarpy produce seedless fruits. The characteristics of self-incompatibility have been studied for many years, and essential traits, such as pollen tube elongation behavior and self-incompatibility genotypes, have been characterized. Recently, it has been shown that the genetic mechanism of self-incompatibility in citrus varieties is S-RNase-based gametophytic self-incompatibility. To date, 18 S-RNases (17 self-incompatible alleles and 1 self-compatible allele) have been identified. The DNA markers for S-RNases can enable the early identification of self-incompatibility/compatibility status. The expression of self-compatibility in Citrus species is ascribed to the presence of the self-compatibility Sm allele, which is a defective S-RNase, and to the suppression of S-RNase expression. Polyploidization induces self-compatibility in Citrus species: Citrus tamurana ‘Hyuganatsu’ is substantially self-incompatible; however, its bud mutation, ‘Nishiuchi Konatsu’, is self-compatible. ‘Nishiuchi Konatsu’ is diploid; however, it forms unreduced pollen, which causes the breakdown of self-incompatible reaction when self-pollinated because of a competitive interaction within the same individual. In addition, after fertilization by unreduced pollen, seed formation is also inhibited by triploid block caused by interploid hybridization between diploid pollen and haploid egg cells. Therefore, ‘Nishiuchi Konatsu’ shows self-compatibility regardless of the self-incompatibility haplotype and produces fruits with few seeds. The seedlessness trait could be beneficial for citrus breeding in the future; however, the genetic mechanisms involved in the expression of this trait remain unclear. This review focuses on the recent advances in the genetics of self-incompatibility in citrus plants, implicating the mechanisms involved in self-incompatibility and their applications for achieving the desired trait of seedlessness in citrus fruits.
Self-incompatibility (SI) in plants is a mechanism to avoid inbreeding deterioration through self-fertilization and promote outcrossing to ensure genetic diversity (de Nettancourt, 1977). Genetic studies of the phenotype of SI (S phenotype), i.e., whether interaction with a mating object is compatible or incompatible, have determined two distinct types of SI: sporophytic SI (SSI) and gametophytic SI (GSI). In SSI, the S phenotype of pollen is determined by the S genotype of the diploid parent plant that produces the pollen, whereas in GSI, the S phenotype of pollen is determined by the S genotype of the pollen itself (Hiscock and McInnis, 2003). The GSI system is the most prevalent SI system (Franklin-Tong and Franklin, 2003), for which two different mechanisms have been identified. First is S-RNase-based GSI, wherein the S-RNase gene acts as a female S determinant and the S-locus F-box/S-haplotype-specific F-box (SLF/SFB) gene acts as a pollen determinant; this molecular mechanism has been identified in the Solanaceae, Rosaceae, and Plantaginaceae families (Franklin-Tong and Franklin, 2003; Hiscock and McInnis, 2003). The second is a Papaveraceae-type GSI discovered in poppy, Papaver rhoeas, wherein incompatibility is caused by the interaction between the S factor (PrsS) in the stigma and the pollen S factor (PrpS) (Dresselhaus and Franklin-Tong, 2013; Foote et al., 1994; Wheeler et al., 2009). An early genetic analysis in Citrus species showed that they exhibit GSI (Soost, 1965, 1969). In the present review, recent advances in the genetics of SI in citrus plants and the possible mechanisms involved in SI are discussed. Moreover, the applications of this new knowledge for the production of seedless citrus fruits are considered.
The presence/absence of seeds in fruits is essential for determining the commercial value of citrus fruits in the market. Seedless fruit production and breeding of seedless cultivars have a high demand in the citrus industry. Self-incompatible Citrus species and varieties can stably produce seedless fruits if they are also parthenocarpic. In contrast, if they do not have sufficient parthenocarpic ability, SI can cause problems in setting fruit. Trees with SI need a compatible pollen donor for pollination and fertilization, which ultimately leads to seed formation in the fruits. Therefore, the status of SI and its mechanism needs to be clarified for better fruit production in Citrus species and cultivars. Hence, extensive research has been conducted to determine the SI status of citrus accessions.
Some citrus varieties, such as pummelos, mandarins, and their relatives, are self-incompatible (Table 1; Yamamoto et al., 2006, 2012). Among Japanese citrus accessions, ‘Hyuganatsu’ [C. tamurana hort. ex Tanaka (Honsho et al., 2012; Miwa, 1951; Yamashita, 1978, 1981)], ‘Hassaku’ [C. hassaku hort. ex Tanaka (Yamashita, 1980)], ‘Keraji’ [C. keraji hort. ex Tanaka (Yamamoto and Tominaga, 2002)], pummelos [C. maxima (Burm.) Merr.], including ‘Tosa Buntan’, ‘Tanikawa Buntan’, ‘Banpeiyu’ (Ngo et al., 2001), ‘Egami Buntan’ (Iwamasa and Oba, 1980), and some bred cultivars, such as ‘Ariake’ mandarin (Yamamoto et al., 2006, 2012) and ‘Hayasaki’ pummelo (Nesumi et al., 2003; Okudai et al., 1991), are self-incompatible. Clementine (C. clementina hort. ex Tanaka) is a global representative of self-incompatible mandarins (Chao, 2005; Distefano et al., 2009a; Ton and Krezdorn, 1966). Some tangelo varieties, ‘Minneola’ and ‘Orlando’, both of which are generated by the outcross between ‘Duncan’ grapefruit (Citrus × paradisi Macfad.) and ‘Dancy’ tangerine (C. reticulata Blanco), are self-incompatible (Krezdorn and Robinson, 1958), whereas ‘Seminole’, another variety with the same parents, is self-compatible (Ngo et al., 2019). Six sibling cultivars developed by hybridization between self-incompatible Clementine and ‘Orlando’ (‘Nova’, ‘Fairchild’, ‘Lee’, ‘Fortune’, ‘Osceola’, and ‘Robinson’) are self-incompatible (Hearn et al., 1969; Montalt et al., 2021; Reece and Register, 1961; Takahara et al., 1982; Yamamoto et al., 2006). A hybrid of ‘Minneola’ and Clementine, ‘Page’, is also self-incompatible (Hearn et al., 1969; Yamamoto et al., 2006). ‘W. Murcott’ mandarin known as ‘Afourer’ or ‘Nadorcott’, a natural hybrid between ‘Murcott’ and an unknown pollen parent, is also self-incompatible (Gambetta et al., 2013). In addition, ‘Moncada’ [C. clementina × ‘Kara’ (C. unshiu Marc. × C. nobilis Lour.)], ‘Imperial’ (C. reticulata), and ‘Ellendale’ [C. reticulata × C. sinensis (L.) Osbeck] tangor have been reported to be self-incompatible (Montalt et al., 2021).

Major representative self-incompatible citrus cultivars.
There are a few reports on cross-incompatibility in citrus varieties. ‘Minneola’ and ‘Orlando’, which are siblings from the same parents, are cross-incompatible. Hearn et al. (1969) and Reece and Register (1961) reported the cross-incompatibility among tangerine hybrid cultivars, including ‘Page’ and the siblings from a cross between Clementine and ‘Orlando’. Kitajima et al. (2001) found that ‘Muroto Konatsu’, a bud mutation of self-incompatible ‘Hyuganatsu’, is self-incompatible and cross-incompatible with other cultivars. Unilateral cross-incompatibility was observed in Tachibana [C. tachibana (Makino) Tanaka] (Ueno, 1978). A cross-incompatible reaction occurred when Tachibana was pollinated with Hassaku or Natsumikan (C. natsudaidai Hayata); however, it did not occur for the reciprocal crosses. A self-incompatible ‘Ariake’ (‘Seike’ navel orange × Clementine) is cross-incompatible with Clementine, one of its parental cultivars (Yamamoto et al., 2006).
Some self-incompatible citrus varieties, such as ‘Egami Buntan’ (Iwamasa and Oba, 1980), ‘Keraji’ (Yamamoto and Tominaga, 2002), ‘Wuzishatangju’ mandarin (C. reticulata) (Ye et al., 2009), ‘Xiangshui’ lemon (Zhang et al., 2012), ‘Ariake’ (Yamamoto, 2014), ‘Afourer’ mandarin (Gambetta et al., 2013), and some Clementine cultivars (Garcia-Papi and Garcia-Martinez, 1984; Montalt et al., 2021), have been reported to produce seedless fruits. They are presumed to have sufficient parthenocarpic ability. The original self-incompatible cultivar ‘Hyuganatsu’ does not have sufficient parthenocarpic ability to produce seedless fruit in solid planting cultivation. However, ‘Koyama New Summer’ (Hamabe et al., 2020a, b) and ‘Ishikawa Hyuga’, discovered independently as bud sports of ‘Hyuganatsu’ in recent years, show stronger parthenocarpy, and it is expected that they will enable C. tamurana to produce seedless fruit steadily.
Upon self-pollination, pollen can germinate at the stigma; however, pollen tube growth is inhibited in the stigma and style rather than on the stigmatic surface (Kahn and Demason, 1985; Ngo et al., 2001, 2019; Ton and Krezdorn, 1966; Yamashita, 1980). Some studies have reported the inhibition of self-pollen tubes found in the ovary (Ye et al., 2009). The inhibition of pollen tube growth has been observed in different locations in ‘Hyuganatsu’—in the stigma (Yamashita, 1978), in the center of the style (Uchida et al., 2012), and at the border between the stigma and style (Honsho et al., 2012).
Some practical methods to break SI have been reported. Bud pollination (Distefano et al., 2009b; Montalt et al., 2022; Wakana et al., 2004), repeated pollination of immature buds (Yamashita, 1980, 1981), and high-temperature treatment (Aloisi et al., 2020) can artificially break the SI in Citrus species. Wakana et al. (2004) reported that the most appropriate time for producing self-fertilized seeds by the bud pollination of self-incompatible Citrus species is when flowers are half of their total length. Distefano et al. (2009b) noted that when flowers of ‘Fortune’ and ‘Nova’ were self-pollinated 1 day before anthesis, the pollen tubes grew towards the ovary. Thus, the incompatibility reaction appears to occur particularly late, just before anthesis. Moreover, tetraploidized self-incompatible citrus plants emerged to become self-compatible (Montalt et al., 2022; Yamashita et al., 1990). More on this subject is mentioned in a later section.
The genetic basis of SI in Citrus species has been identified to be of the gametophytic type from observations of SI type segregation in several hybrid combinations (Soost, 1965, 1969). Researchers have previously attempted to determine the genotypes for SI in citrus varieties. The crosses with self-incompatible cultivars sometimes resulted in segregation distortion in the progenies for glutamate oxaloacetate transaminase (GOT) isozymes, some of which are supposed to be linked with SI genes (S genes). Ngo et al. (2011) used Got-3 allozyme markers to determine the S genotypes in citrus cultivars. The self-incompatible genotypes of ‘Banpeiyu’, ‘Tosa Buntan’, ‘Hassaku’, ‘Yuge-hyokan’ (C. yuge-hyokan hort. ex Yu. Tanaka), ‘Shishiyuzu’ (C. pseudogulgul hort. ex Shirai), and ‘Hyuganatsu’ were suggested to be S1S2, S1S3, S4S5, S6S7, S1S6, and S1S8, respectively, albeit with some uncertainty.
Later, the S genotypes of ‘Banpeiyu’, ‘Hassaku’, ‘Clementine’, and ‘Hirado Buntan’ were defined as S1S2, S4S5, S3S11, and S9S10, respectively, and their self-pollinated progenies with the homozygous S allele were produced by bud self-pollination (Kim et al., 2011, 2020, 2021; Zhou et al., 2018). Pollen grains from individuals homozygous for the S-allele were used to pollinate a citrus accession to assess whether its S allele was solely contained in the pollen by observing the degree of pollen tube elongation in pistils. In addition to the abovementioned definitions for S alleles, a self-compatible individual is assumed to carry one or two self-compatible Sf alleles. The genotypes of some citrus cultivars have been determined based on the presence of Sf alleles and the parentage information of cultivars, e.g., satsuma mandarin (SfS4), ‘Sweet Spring’ (SfS5), sweet oranges (SfS3), ‘Ellendale’ (S3S11), ‘Kiyomi’ tangor (SfSf), and ‘Kawano Natsudaidai’ (SfS2) (Kim et al., 2011, 2020, 2021; Zhou et al., 2018). Kim et al. (2020) also found that the S genotype of all sweet orange cultivars is S3Sf, except for blood orange cultivars. The blood orange cultivars are a subgroup of sweet oranges developed from a mutation in the S3 allele, resulting in S3sm, which is demonstrated by the cross-compatibility with S3 pollen in the style. This suggests that a common ancestor of blood oranges acquired a mutation in the S3 allele after diverging from other sweet orange cultivars.
Many attempts have been made to elucidate the molecular mechanisms of SI in Citrus species (reviewed by Zhang et al. (2018)). For example, homologs of genes that have demonstrated their involvement in SI reactions in other plant species, e.g., RNase (see below), F-box (Chai et al., 2011b), and S-phase kinase-associated protein1-like gene (Chai et al., 2010a; Li et al., 2015), have been isolated from Citrus species. In addition, differentially expressed genes between self-compatible ‘Shatangju’ and its self-incompatible mutant ‘Wuzishatangju’ mandarins (C. reticulata) have been surveyed using suppression subtractive hybridization (Miao et al., 2011b, 2013). The transcript profiles of non-pollinated and self-pollinated pistils of self-incompatible Clementine ‘Comune’ and its self-compatible mutant ‘Monreal’ (Distefano et al., 2009a) and those of the self-pollinated styles with stigmas of ‘Comune’ and ‘Monreal’ (Caruso et al., 2012) were compared using cDNA-amplified fragment length polymorphism (AFLP) and microarray analysis, respectively. RNAseq was used for comparing no-, self-, and cross-pollination in the stigmas and styles of self-incompatible ‘Xiangshui’ lemon (Zhang et al., 2015). In ‘Hyuganatsu’, protein expression between different stages at 1-, 3-, and 5-day before anthesis was compared using 2D polyacrylamide gel electrophoresis (PAGE) and matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry (Uchida et al., 2012).
It is reasonable to hypothesize that citrus SI is based on the S-RNase system because several phenotypic features of citrus SI, such as pollen tube behavior in the SI reaction, are similar to those of known S-RNase-based GSI plants. The S-RNase homologs from citrus plants were cloned after overcoming many challenges (Chai et al., 2011a; Honsho et al., 2019b; Liang et al., 2017; Miao et al., 2011a). Although some genes did not show tissue-specific expression (Chai et al., 2011a; Miao et al., 2011a), indicating that they are inappropriate as S-RNase genes, Liang et al. (2017) identified a tissue-specific S-RNase homolog, named CgRNS3, from ‘Shatian’ pummelo based on information obtained from the genome sequence of sweet oranges (Xu et al., 2013). Honsho et al. (2019b) also cloned three S-RNase homologs from ‘Hyuganatsu’, CtRNS1–CtRNS3, showing specific expression in the female organ.
Recently, Liang et al. (2020) reported that some RNases act as crucial female S-determinants in Chinese Citrus species. They cloned RNases with pistil-specific gene expression and showed their segregation along with the S-haplotype in a GSI manner. Furthermore, the recombinant protein coded by the cloned RNase gene showed a S-haplotype-specific inhibitory effect on pollen tube growth in vitro. Thus, citrus SI is governed by S-RNase-based GSI. Fourteen self-incompatible S-RNase genes, S1–S14-RNase, and one self-compatible S-RNase gene, Sm-RNase, coding a truncated protein owing to a frameshift with a gap insertion into a functional sequence, were reported by Liang et al. (2020). In addition, the S-RNase genes of some Japanese citrus varieties, including self-incompatible ‘Hyuganatsu’, ‘Nomabeni Hassaku’, ‘Tosa Buntan’, and ‘Banpeiyu’ varieties, and probably self-compatible Sweet Spring variety, were identified, and the three novel S-RNase genes, S15–S17-RNase, were reported (Honsho et al., 2021). To date, 18 S-RNase genes, S1–S17-RNase and Sm-RNase, have been identified (Table 2). As stated earlier, the SI genotypes of Citrus species are typically defined based on two approaches, the Got-3 allozyme pattern followed by confirmation with a pollination test using pollen tube growth parameters and the molecular type of S-RNase. Different numbering systems can assign multiple different genotypes to a cultivar, potentially leading to confusion. For example, the genotype of ‘Tosa Buntan’ was determined as S1S3 by Kim et al. (2011), whereas Honsho et al. (2021) designated the genotype as S5-RNase and S16-RNase and its S-haplotype was expressed as S5S16 (Table 3). To avoid this confusion, a unified notation system of S-haplotypes is desirable.
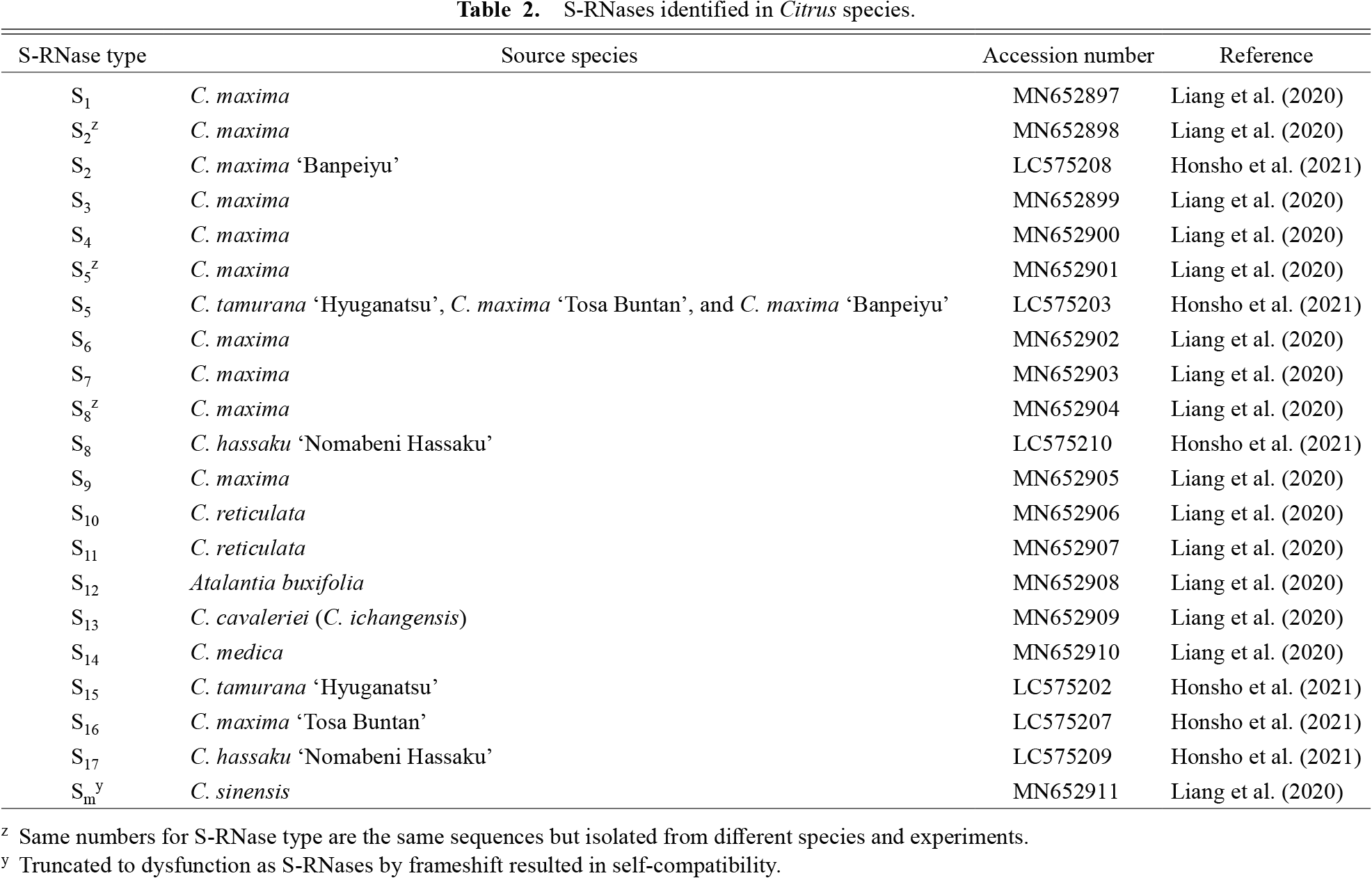
S-RNases identified in Citrus species.

Correspondence between S genotype, determined from incompatibility reactions, and S-RNase genotype.
Citrus S-RNase proteins are members of the ribonuclease T2 family, T2 RNases, which are similar to other S-RNase-based GSI plants. The structure of the S-RNase gene in Citrus species comprises one intron at the 1st hypervariable region like in other S-RNase-based GSI plants, except for the Prunus species belonging to the Rosaceae family (Fig. 1; Honsho et al., 2019a, 2021; Liang et al., 2020). The coding region has five conserved regions and five hypervariable regions (Honsho et al., 2019a, 2021; Liang et al., 2020).
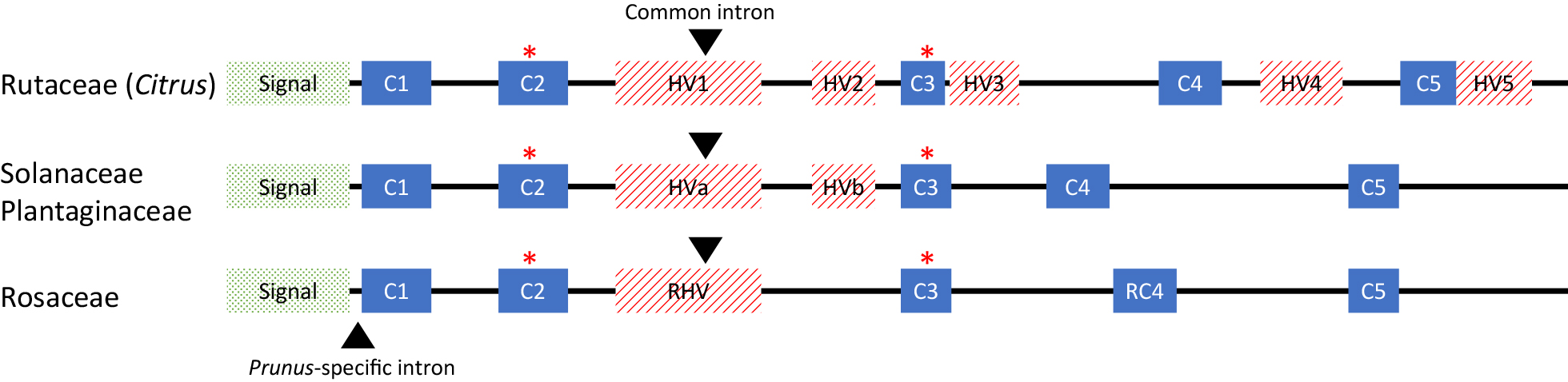
S-RNase protein features of the Rutaceae (which includes Citrus), Solanaceae, Plantaginaceae, and Rosaceae families. The positions of introns in their S-RNase genes are indicated by black arrowheads. Two histidine residues essential for RNase activity are indicated by asterisks. Signal: signal peptide; C1–C5: conserved regions; HV1–5, HVa, HVb, and RHV: hypervariable regions.
Honsho et al. (2021) obtained sequences of T2 RNases from the Citrus genome available in public databases and annotated transcriptomes from RNAseq data to construct a maximum likelihood tree using a combined dataset of T2 RNases from citrus and other S-RNase-based GSI plants. In the maximum likelihood tree, the T2 RNases were divided into three clusters, Class I–III, as defined by Igic and Kohn (2001). All known S-RNases were included in the Class III cluster, and the citrus S-RNases formed a monophyletic group within Class III. In addition, abundant citrus T2 RNases were observed in three subclusters within the Class III cluster with a low polymorphism insufficient for S-RNases (Fig. 2). Moreover, the segregation of those T2 RNases in the progeny did not follow the S allele inheritance, indicating that their location on the chromosomes was different from that for S genes. Thus, they are considered distinct from S-RNases (Honsho et al., 2021). The previously reported homologous genes of S-RNase, CtRNS1 (Honsho et al., 2019b) and CgRNS3 (Liang et al., 2017), were placed in low polymorphic subclusters 1 and 2, respectively, both of which are non-S-RNase subclusters (Fig. 2). As the CtRNS2 sequence is a partial sequence of CtRNS1 and is also incomplete (Honsho et al., 2019b), it may be a non-S-RNase. T2 RNase proteins, including S-RNases present in the Class III cluster, showed a basic isoelectric point (pI), although their functional association with SI was unclear, whereas T2 RNase proteins in Class I and II clusters were acidic (Ramanauskas and Igic, 2017). Citrus S-RNase and other T2 RNases in the Class III cluster were also predicted to possess a basic pI (Honsho et al., 2019b, 2021).
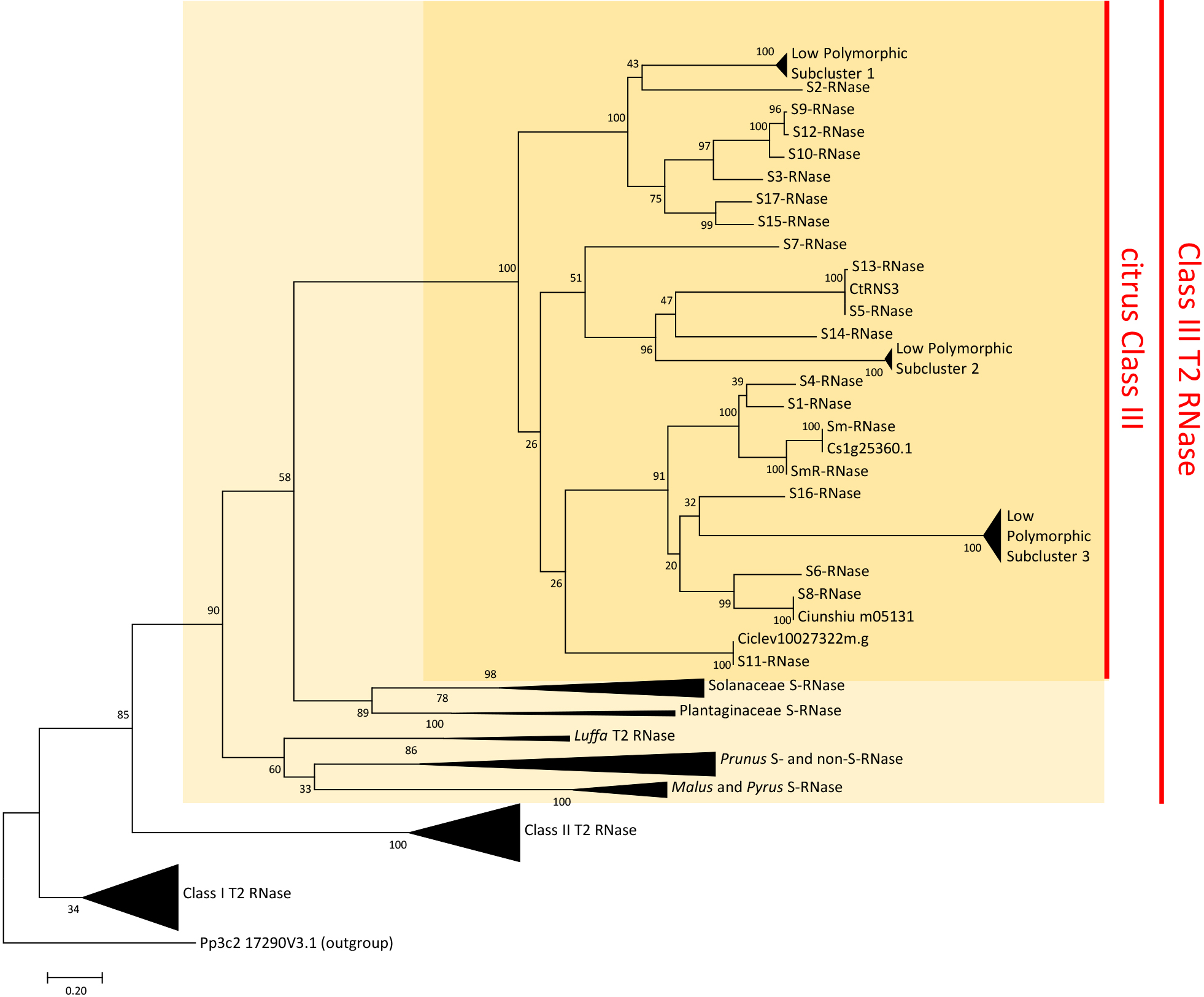
Maximum likelihood tree of T2 RNase in plants. Citrus S-RNases form a monophyletic group in Class III T2 RNase. In the citrus Class III cluster, there are three subclusters formed by non-S-RNases, which are referred as the low polymorphic subcluster in the tree. The numbers above the branches indicate the bootstrap value of 1,000 replications. A full list of datasets used for the tree is available in Supplementary Table 1. The tree was constructed according to Honsho et al. (2021).
The segregation analysis of progenies of reciprocal crosses between ‘Fortune’ and ‘Ellendale’, both of which are self-incompatible and semi-cross-incompatible (i.e., one of the two S alleles is shared in the two cultivars), using genome-wide single nucleotide polymorphism (SNP) markers, indicated distorted segregation at the edge region of chromosome 7 of the Clementine genome (Wu et al., 2014), on which the T2 ribonuclease gene and several F-box genes were located (Ollitrault et al., 2021). This result supported the S-RNase-based GSI system in citrus found by Liang et al. (2020) and Honsho et al. (2021).
In the S-RNase-based GSI system, the female S determinant is S-RNase, whereas the male S determinant is SLF/SFB. In S-RNase-based GSI plants except for Prunus (Rosaceae), multiple SLFs/SFBs linked to S-RNases cooperate to recognize collaborative “non-self” S-RNases for degradation (Claessen et al., 2019; De Franceschi et al., 2012; Kubo et al., 2010). In contrast, in the genus Prunus, only one SFB gene linked to S-RNase is involved in SI and is considered to recognize the “self” S-RNase, thereby degrading a general inhibitor that blocks the activity of the S-RNase (Matsumoto and Tao, 2016; Tao and Iezzoni, 2010). Liang et al. (2020) identified 12 SLF/SLFL (SLF-like) genes linked to S-RNase in the S1 and S2 haplotypes of Chinese pummelo. The genomic structure of the region around S-RNase obtained from the public genome database also revealed the presence of multiple SLF/SFB genes. This structure seems to fit the non-self-recognition model. The presence of competitive interactions in Citrus species also supports this model, because S-RNase-based-GSI plants that display the self-recognition model (i.e., Prunus species) do not show competitive interaction.
Several individuals of self-incompatible Citrus species have been reported to develop self-compatibility independent of the introduction of the self-compatible Sf gene (Sm-RNase). ‘Monreal’, a mutant of the self-incompatible Clementine, is a self-compatible cultivar (Distefano et al., 2009a); ‘Ihara Hyuga’, ‘Nishiuchi Konatsu’, ‘Shiratori Hyuga’, and an unregistered line (Hyuganatsu self-compatible: HYSC), are self-compatible mutants of self-incompatible ‘Hyuganatsu’ (Araki, 2006; Honsho et al., 2009; Okada et al., 1992). The Chinese cultivars ‘Guiyou No. 1’ and ‘Zigui Shatian’ are self-compatible mutants of self-incompatible ‘Shatian’ pummelo (Chai et al., 2010b; Hu et al., 2021). A contrasting example of the development of self-compatibility from a self-incompatible cultivar has been reported in ‘Wuzishatangju’, which is self-incompatible and developed from the self-compatible ‘Shatangju’ (Ye et al., 2009).
Self-compatible mutants developed from SI lines are useful for elucidating the mechanisms of SI, because genetic transformation for the analysis using reverse genetics is difficult in citrus plants, even if genetically-modified citrus plants are produced, evaluating their traits is very time-consuming. In the self-compatible pummelo ‘Guiyou No. 1’, which is a spontaneous mutant of self-incompatible ‘Shaitan’ pummelo, self-compatibility has been attributed to the downregulation of S2-RNase (Hu et al., 2021). Similarly, a bud mutation of ‘Hyuganatsu’, HYSC, is self-compatible likely because of the low expression level of S15-RNase (Honsho et al., 2021). In both cases, no mutation was found in the S-RNase gene sequences; thus, an alteration of transcriptional regulation likely caused the lower expression of S-RNases. In ‘Guiyou No. 1’, CgHB40 was identified as the candidate gene for a transcription factor that regulates the transcription of S2-RNase in styles and it may be related to the downregulation of ‘Guiyou No. 1’, leading to the loss of SI (Hu et al., 2021).
A comparison of transcripts from stylar canal cells of self-compatible ‘Monreal’ and self-incompatible ‘Comune’ Clementine cultivars revealed that three aspartic-acid-rich (ASP-rich) proteins present in tandem in the genome were over-represented in ‘Comune’ (Caruso et al., 2012). It has been confirmed that this protein is highly expressed in a self-compatible reaction during the pollen–pistil interaction. Ectopic expression of this protein in transgenic tobacco resulted in altered Ca2+ distribution and intracellular pH, organization of actin filaments, and secretion of new cell wall material, thereby affecting the growth rate. It is assumed that the expression of ASP-rich proteins in Clementines affects the level of cytosolic calcium, and in turn, the organization of actin filaments leading to the modulation of pollen tube growth (Parrotta et al., 2022).
Polyploidization is a direct cause of conversion from self-incompatibility to self-compatibility of S-RNase-based GSI plants, except for the Prunus species, and this mechanism can be explained by competitive interaction (Adachi et al., 2009; Golz et al., 2000; Lewis and Modlibowska, 1942). The allelic composition of a pollen grain produced by a tetraploidized SI plant is considered to be either homoallelic or heteroallelic, and only heteroallelic pollen grains with different S alleles elongate the pollen tube, regardless of the S-RNase composition in the pistil. Because citrus SI is considered to be an S-RNase-based GSI with a non-self-recognition system, polyploidization is considered to be significant for SI breakdown. Based on the same mechanism, when a diploid (2x) pollen from a tetraploidized (4x) plant pollinates the original diploid plant, the self-incompatible reaction is avoided and fertilization occurs (Montalt et al., 2022; Yamashita et al., 1990). Tetraploid ‘Hyuganatsu’ and ‘Clemenules’ Clementine are self-compatible (Montalt et al., 2022; Yamashita et al., 1990). It was reported that diploid pollen grains from tetraploidized ‘Hyuganatsu’, when used to pollinate the original diploid self-incompatible ‘Hyuganatsu’, can grow pollen tubes by avoiding SI reaction in the pistil, leading to successful fertilization with x egg cells in the ovary.
As an additional effect of interploid fertilization, where most of the fertilized zygotes are triploid because of the hybridization of a haploid egg and a diploid pollen, embryos are not likely to survive and may be aborted during fruit development, resulting in an aborted seed. This phenomenon is generally known as “triploid block,” which likely occurs because of an imbalance in chromosome numbers between the embryo and endosperm (Köhler et al., 2010; Ramanna and Jacobsen, 2003; Scott et al., 1998). For seedless fruit production, the induction of seed abortion by pollen grains is as beneficial as SI that does not trigger seed formation. Trials for low-seed production with the pollination of tetraploid cultivars have been conducted using self-incompatible citrus cultivars (Takahara et al., 1982). Considering the benefits, an autotetraploid of ‘Hyuganatsu’ was generated and released as ‘Kuchinotsu 41 gou’ as the pollinizer cultivar for diploid ‘Hyuganatsu’ (Imai et al., 2014).
The self-compatible ‘Nishiuchi Konatsu’ was found as a bud sport of self-incompatible ‘Hyuganatsu’ and displays the abortion of most seeds during fruit development (Honsho et al., 2009, 2015). This self-compatibility of ‘Nishiuchi Konatsu’ has been attributed to unreduced pollen (2n pollen) production (Honsho et al., 2012). ‘Nishiuchi Konatsu’ produces both reduced and unreduced pollen grains; however, it is likely that only pollen tubes of unreduced pollen can grow in the pistil upon self-pollination, considering that reduced haploid pollen is rejected by the self-incompatible reaction. On the contrary, an unreduced pollen (2x) with heteroallelic S gene can avoid the self-incompatible reaction through competitive interaction, considering the non-self-recognition system of GSI in citrus. These unreduced pollen grains were considered to be a result of the first division restitution (FDR) during meiosis (Honsho et al., 2016, 2022). FDR can thus maintain the parental heterozygosity in unreduced gametes rather than the second division restitution (Bretagnolle and Thompson, 1995; Cuenca et al., 2011, 2015; Park et al., 2007). Thus, the majority of unreduced pollen grains produced by ‘Nishiuchi Konatsu’ should have multiple S alleles (Fig. 3). Pollen tube elongation by reduced (haploid) pollen is inhibited by the self-incompatible reaction, whereas unreduced (diploid) pollen breaks the SI through competitive interaction, leading to fertilization. Furthermore, unless the egg cells are unreduced, fertilized triploid embryos are aborted, inducing the failure of seed formation and resulting in a seedless (or low-seeded) fruit crop. This is an interesting case of competitive interactions found during the self-pollination of a diploid plant, and is likely the only example in the genus Citrus. This trait is very useful for agricultural production because unreduced gametes can be used for triploid citrus breeding (Aleza et al., 2010; Yasuda et al., 2021) and the competence of unreduced gamete production results in diploid self-compatible cultivars producing fruits with fewer seeds.

Self-compatible system of ‘Nishiuchi Konatsu’. ‘Nishiuchi Konatsu’ (contains S5- and S15-RNase) produces both reduced haploid (x) pollen (n pollen) and unreduced diploid (2x) pollen (2n pollen). When self-pollinated, the pollen tube elongation of the haploid pollen and unreduced pollen homoallelic for the S locus is arrested by S-RNase in the pistil. In contrast, in unreduced pollen with a heteroallelic S locus, all S-RNases are recognized by SLFs followed by degradation by competitive interaction, avoiding the self-incompatibility reaction and allowing continuous pollen tube elongation. In addition, triploid (3x) zygotes resulting from fertilization between haploid egg cells and diploid pollen are aborted during seed development to form aborted seeds, resulting in a fruit with less seeds. Part of this figure is reproduced from Honsho et al. (2012) with permission from the Japanese Society for Horticultural Science.
In stone fruit production, the presence of seeds has little effect on the commercial value of fruits as there is a single and relatively large seed in the fruit. In contrast, the presence or absence of seeds has a significant effect on the commercial value of citrus fruits because they contain many relatively small seeds that greatly affect palatability and can negatively impact consumer acceptance. Therefore, under the presence of parthenocarpy, SI in citrus plants is a positive trait that prevents fertilization and seed production, although causing poor fruiting. Elucidation of the SI system in citrus is thus beneficial. Since the genetic basis of citrus SI was revealed as S-RNase-based GSI, DNA marker development for self-(in)compatibility haplotyping is possible. This can be a useful tool for citrus breeding. In addition, the system of the breakdown of SI with 2n pollen by competitive interaction and the seed abortion by triploid block in diploid plants through unreduced pollen production found in ‘Nishiuchi Konatsu’ could be utilized for breeding new citrus varieties that are self-compatible and produce low-seeded fruits at the diploid level, regardless of the SI haplotype.
The author would like to thank Editage (www.editage.com) for English language editing.