2023 年 92 巻 1 号 p. 13-21
2023 年 92 巻 1 号 p. 13-21
The development of intergeneric hybrids for horticultural crops has been attempted to introduce new quality and resistance traits and to enlarge the gene pool. Interspecific and intergeneric hybridization are often hindered by incompatibility reactions occurring at various stages of hybridization, from early pollination to initial growth, and the reproductive stages of the progeny. In this study, we investigated intergeneric and interspecific cross-compatibility among six species in the tribe Maleae (Rosaceae), namely, Pyrus communis (European pear), P. pyrifolia (Japanese pear), Malus × domestica (apple), Eriobotrya japonica (loquat), Cydonia oblonga (quince), and Pseudocydonia sinensis (Chinese quince). In vivo pollen tube growth tests showed the presence of a postmating, prezygotic barrier in many cross-combinations, in which cross-compatibility was regulated by both genetic distance and crossing direction. Strong hybridization barriers were observed in pollen tube growth and fruit setting when intergeneric hybridization was performed with E. japonica, a species phylogenetically distant from the others studied. Different compatibility reactions in reciprocal crosses were observed in some intergeneric hybridizations; C. oblonga as a pollen donor was incompatible with P. sinensis, whereas the reciprocal cross was compatible, resulting in the development of hybrid seedlings. Furthermore, the pollen tube growth rate differed among Pyrus species when pollinated on the apple pistils, suggesting divergence of cross-compatibility response in a specific linage. Factors affecting intergeneric hybridization are discussed with reference to the genetic distance between species and morphological characteristics such as pistil length. Our comprehensive assessment of intergeneric cross-compatibility will help provide a way to overcome crossing barriers and develop new hybrid crops in the tribe Maleae.
Hybridization involving different species is an important reproductive mechanism that has facilitated speciation and domestication in plants (Purugganan, 2019). However, wide hybridization events are often hindered by reproductive barriers, which are generally classified into premating and postmating types (Moyle et al., 2014). Postmating reproductive barriers can be further divided into prezygotic and postzygotic types, in which incompatible reactions take place before (prezygotic) and after (postzygotic) fertilization, respectively. In the prezygotic type, the incompatible reaction usually involves failure of pollen germination and pollen tube growth, which may occur in various tissues, such as the stigma, style, or ovary (Moyle et al., 2014). In contrast, in the postzygotic type, the incompatible reaction may act on various tissues and stages from early embryo development to the initial growth and reproductive stages of the progeny (Bikard et al., 2009; Bomblies et al., 2007; Yamamoto et al., 2010).
Different crop species show a wide range of useful agronomic traits, such as resistance to biotic and abiotic stresses, growth behaviors, and fruit qualities that can be used for agriculturally important developments through interspecific or intergeneric hybridizations (Chetelat, 2016; Gradziel, 2022). Understanding the mechanisms of reproductive isolation between related species is indispensable to overcoming cross-incompatibility barriers and enabling the introduction of useful agronomic traits. The tribe Maleae in the family Rosaceae includes a large number of economically important fruit tree species, such as Malus × domestica (apple), Pyrus communis (European pear), and P. pylifolia (Japanese pear). Taxonomically, the Maleae, which has a base chromosome number of 17, originated with a whole-genome duplication event in an ancestral relative of Gillenia (Evans et al., 2000; Ireland et al., 2021). The current Maleae tribe consists of at least 500 species in over 30 genera (Xiang et al., 2017), but the development of intergeneric hybrids has only been reported for a few cross-combinations (Postman, 2011). Additionally, although cross-compatibility across some Maleae genus and species has been partially clarified on the basis of fruit set and in vivo pollen tube growth (Mahoney and Brand, 2021; Yang and Fu, 2020), classification of, and behavior in, cross-incompatibility remain to be investigated across a wide range of combinations.
In this study, we characterized intergeneric cross-incompatibility across six major fruit tree species in the tribe Maleae. The presence of postmating, prezygotic barriers was observed in various cross-combinations. Based on the crossing barriers found in this study, we discuss the possibility of developing new Maleae tribe hybrids.
Eighteen cultivars and accessions representing five genera and six species in the tribe Maleae were used in this study. These included Cydonia oblonga ‘Smyrna’ (S genotype is unknown), Eriobotrya japonica ‘Mogi’ (EjS6, S10) (Zhang et al., 2017), P. communis ‘Alexandrine Douillard’ (PcS103, S104), ‘Aurora’ (PcS101, S105), ‘La France’ (PcS101, S119), ‘Le Lectier’ (PcS104, S118), ‘Max Red Bartlett’ (PcS101, S102), ‘Silverbell’ (PcS110, S119) (Goldway et al., 2009), P. pyrifolia ‘Gold Nijisseiki’ (PpS2, S4), ‘Hosui’ (PpS3, S5), ‘Kikusui’ (PpS2, S4), ‘Kosui’ (PpS4, S5), ‘Osa Gold’ (PpS2, S4m) (Yamane and Tao, 2009), Malus × domestica ‘Fuji’ (MdS1, S9), ‘Golden Delicious’ (MdS2, S3), ‘Jonathan’ (MdS7, S9), ‘Ohrin’ (MdS2, S7) (Yamane and Tao, 2009), and Pseudocydonia sinensis unknown cultivar (S genotype is unknown). The S haplotype numbering is specific to the respective species. All these cultivars are diploid (2n = 2x = 34). Seed parent cultivars including C. oblonga ‘Smyrna’, E. japonica ‘Mogi’, P. communis ‘Alexandrine Douillard’, P. pyrifolia ‘Gold Nijisseiki’, M × domestica ‘Fuji’, and P. sinensis, were grown at the experimental farm of Kyoto Prefectural University (Kyoto, Japan) and Kyoto University (Kyoto, Japan).
Investigation of in vivo pollen tube growthPollen tube growth within pollinated pistils was observed to assess cross-incompatibility occurring at the postpollination, prezygotic stage. Pollination tests were conducted in two successive years, 2019 and 2020. Anthers were harvested from unopened flowers at the balloon stage of development, incubated for 24 h at room temperature for dehiscence, and stored at 4°C with silica gel until use. In vitro pollen germination was evaluated after 2 h of incubation at 20°C on a medium containing 15% sucrose, 0.01% boric acid, and 1.5% agar. Before the pollination experiment, pollen was incubated for 1 h at 20°C for acclimation. One- or 2-year-old branches with unopened flowers were collected just before anthesis. The basal part of the branches was immersed in tap water and maintained at 20°C under a 16 h photoperiod. Due to an insufficient number of flowering branches, an exception was made in 2019 for the pollination using P. sinensis as a seed parent, in which flowers were collected at the balloon stage and placed in 1.5% agar medium. The flowers were emasculated at the balloon stage of development and pollinated 24 h later.
The pollinated pistils were collected five days after pollination and immediately fixed in a solution containing chloroform:ethanol:acetic acid (1:3:1) at 4°C. After 24 h, the fixative was replaced with 99% ethanol, and the pollinated pistils were stored at 4°C until use. The fixed pistils were incubated in 40% sodium hydroxide at 60°C for 1 h followed by 20°C for 4 h. The pistils were then washed three times with deionized water and stained with 0.1% aniline blue in 0.1 N K3PO4 (Martin, 1959). Fluorescent microscopic observation of pollen tube growth was performed with squashed samples using a fluorescence microscope (model BX60; Olympus, Tokyo, Japan) equipped with ultraviolet epifluorescence. After observing pollen germination on the stigma, pollen tube growth in the pistil was investigated. The length of the pollen tube that grew the longest distance was recorded for each pistil, and the pollen tube growth rate was expressed as a percentage of the longest pollen tube to the pistil length. Five to 10 pistils were investigated for each cross-combination, and a mean percentage (± S.E.) was calculated. Pollen tube growth of 100% indicates a compatible cross, at least for the in vivo pollen tube growth test.
Field pollinationPollination tests were conducted in two successive years, 2019 and 2020. Flowers at the balloon stage of development were emasculated and then pollinated using a thin paintbrush or cotton swab. Pollinated flowers were covered with an insect-proof net to prevent insect pollination. Fruit set was assessed at harvest time when fruits reached maturity. Seeds obtained from crosses using P. communis, P. pyrifolia, and M. × domestica as the seed parent was subjected to in vitro culture to obtain hybrid seedlings (Morimoto et al., 2020). The in vitro cultures were maintained at 25°C under a 16 h photoperiod. Seeds obtained from a cross between C. oblonga and P. sinensis were subjected to stratification at 4°C for five months, and germinated seeds were transplanted into pots containing decomposed granite soil:compost (1:1).
Confirming the hybrid status of seedlingsTo confirm that the developed seedlings resulted from hybridization between different species, polymerase chain reaction (PCR) tests based on the S-RNase allele genotype and simple sequence repeat (SSR) markers were conducted. Genomic DNA was isolated from young leaves using a DNeasy Plant Mini Kit (Qiagen, Tokyo, Japan). S allele typing was performed with the ASPF3 and ASPR3 primer pair (Kim et al., 2009). PCR was conducted in a 20 μL reaction volume containing 2 μL 10 × ExTaq polymerase buffer (TaKaRa Bio, Shiga, Japan), 0.2 mM dNTPs, 100 nM each of the forward and reverse primers, 0.5 U ExTaq DNA polymerase, and 20 ng of genomic DNA. The PCR cycle was as follows: initial denaturation at 94°C for 1 min; then 35 cycles at 94°C for 1 min, 55°C for 1.5 min, and 72°C for 2.5 min; followed by a final elongation at 72°C for 5 min. The PCR products were electrophoresed in a 2.0% agarose gel, stained with ethidium bromide, and visualized under ultraviolet light. An SSR marker CH04g09 (Liebhard et al., 2002) was used to analyze the hybrid seedlings as described by Morimoto and Banno (2015).
For all cultivars, more than 70% of pollen grains germinated in vitro. Pollen germination was also observed on the stigma in all crossing combinations (data not shown), indicating that pollen used in this experiment were viable and that there was no hybridization barrier at the time of pollen germination on the stigma. In contrast, in vivo pollen tube growth differed among crossing combinations (Tables 1–6).
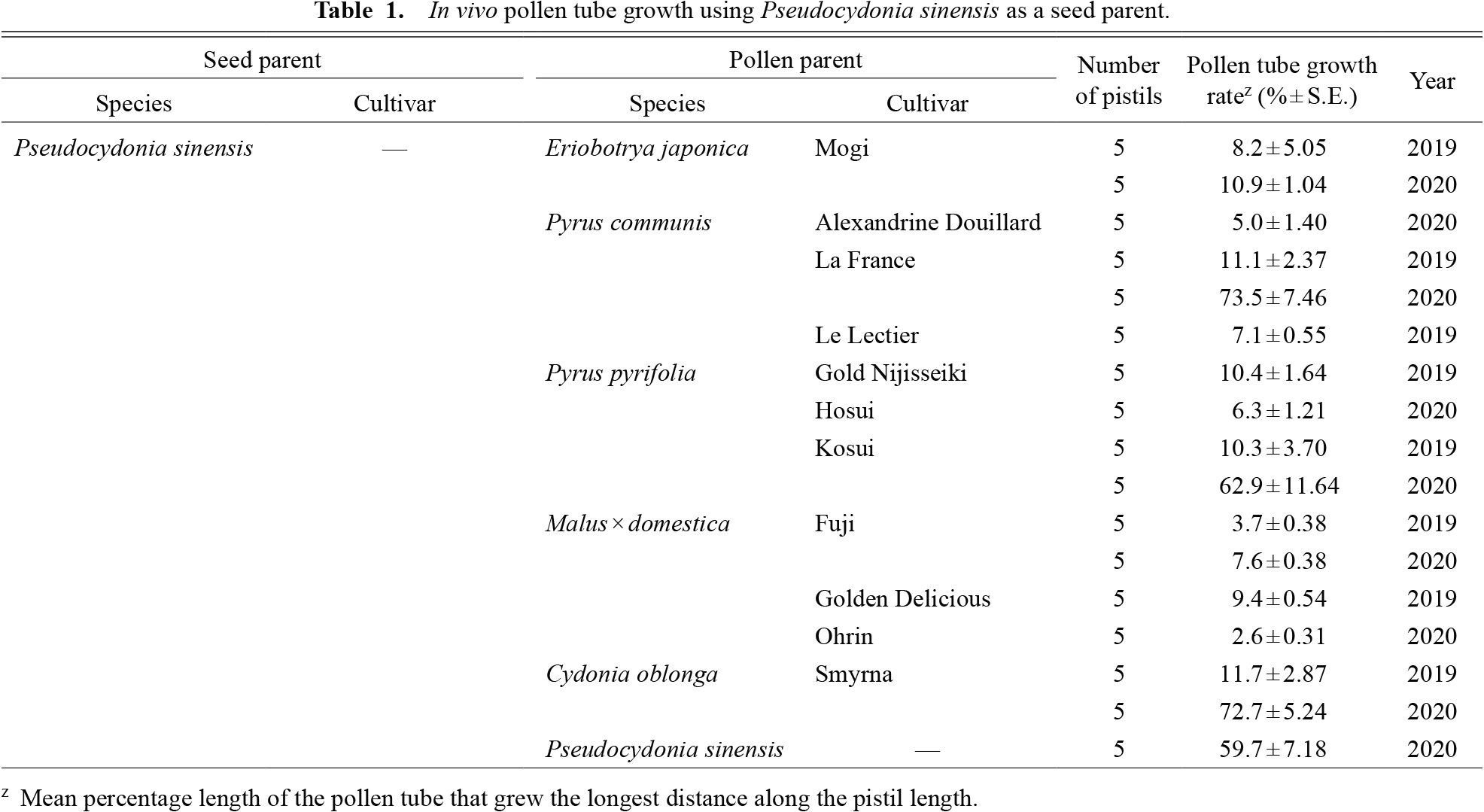
In vivo pollen tube growth using Pseudocydonia sinensis as a seed parent.
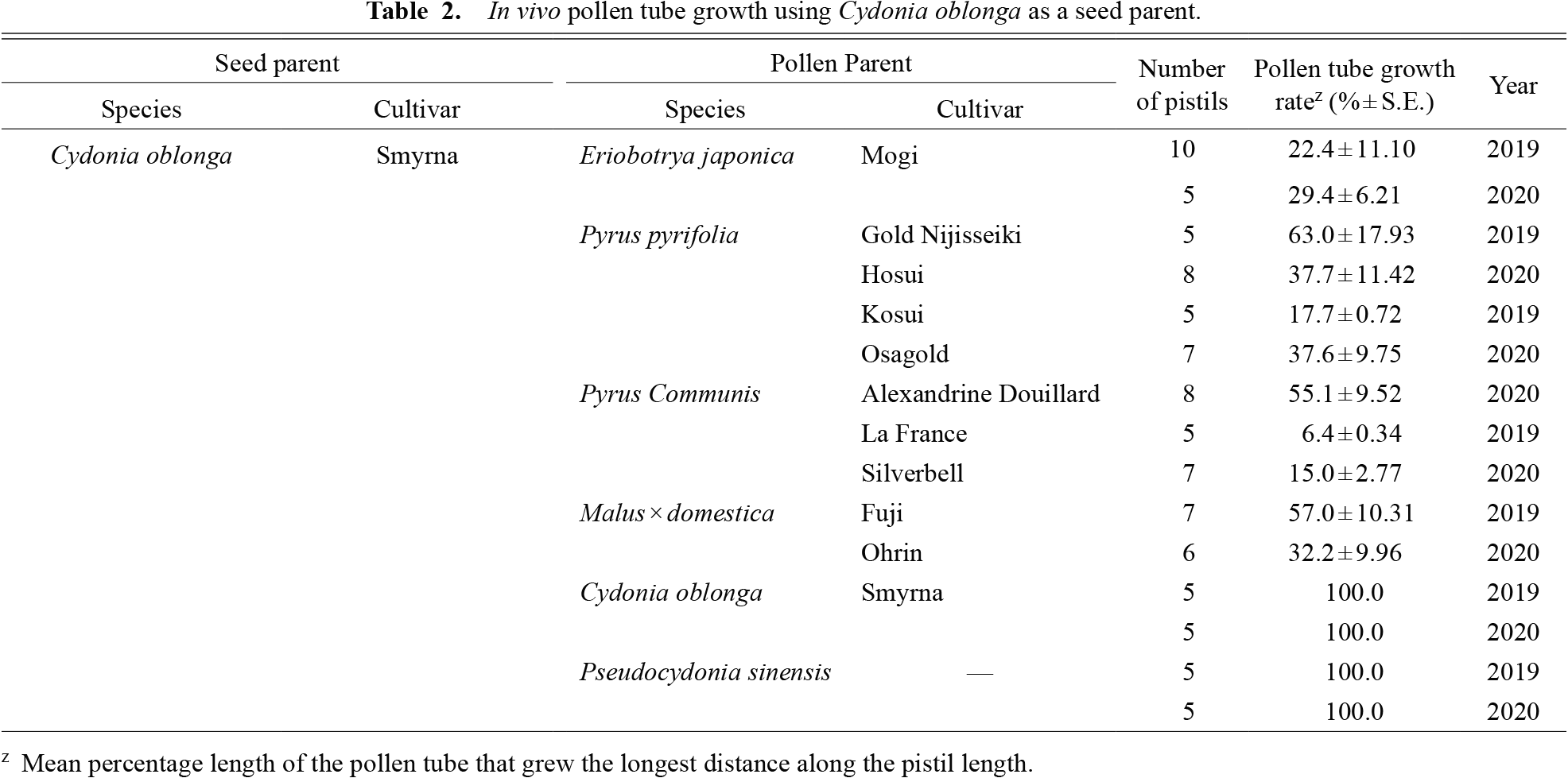
In vivo pollen tube growth using Cydonia oblonga as a seed parent.
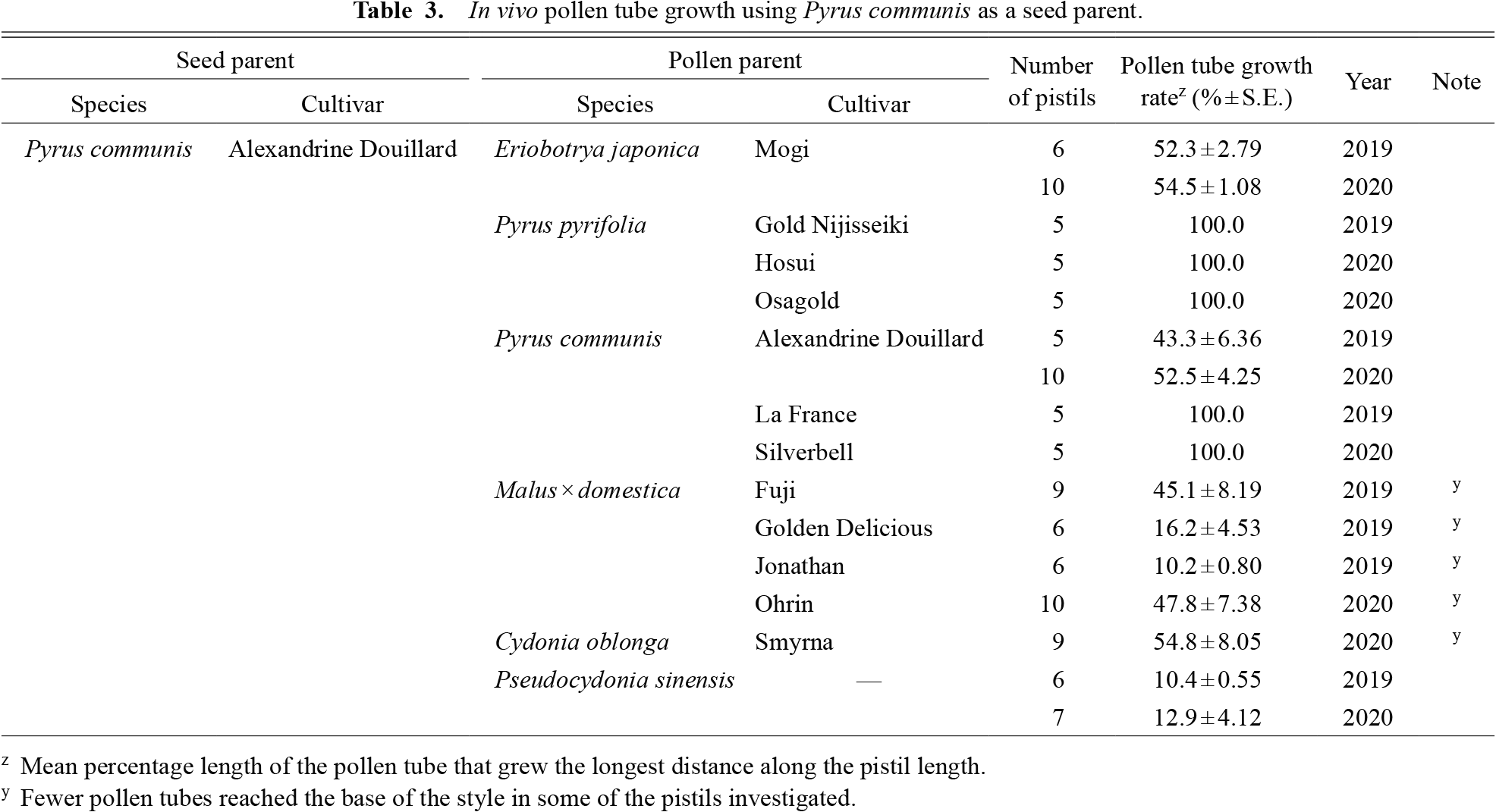
In vivo pollen tube growth using Pyrus communis as a seed parent.

In vivo pollen tube growth using Pyrus pyrifolia as a seed parent.
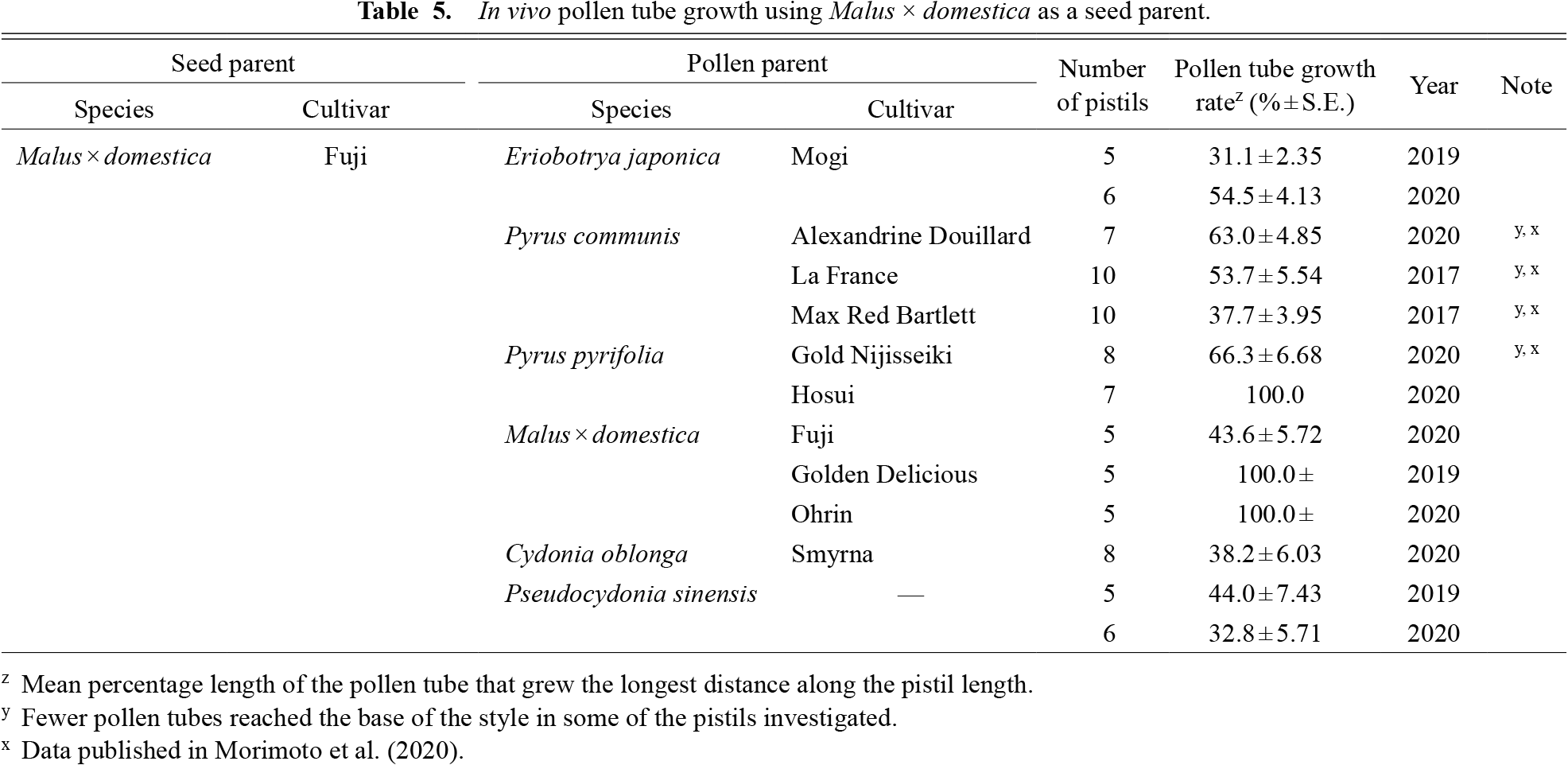
In vivo pollen tube growth using Malus × domestica as a seed parent.

In vivo pollen tube growth using Eriobtrya Japonica as a seed parent.
Successful hybridization in the tribe Maleae was generally dependent on phylogenetic relationships. This tendency was clearly found in E. japonica, a species phylogenetically distant from the other species used in this study (Xiang et al., 2017). When used as a pollen parent, E. japonica had insufficient pollen tube growth for fertilization in the pistils of P. sinensis (Table 1), C. oblonga (Table 2), P. communis (Table 3), P. pyrifolia (Table 4), and M. × domestica (Table 5), with the longest pollen tube growing only to about the halfway point within the pistils. This cross-incompatibility response may be partially determined by the relationship between the pistil length of the seed parent and the growth potential for pollen tube length on the part of the pollen parent. Species with longer pistils are thought to have longer pollen tubes, even after interspecific crossing (Morimoto et al., 2019; Perez and Moore, 1985), and the converse may also be true. In 2020, we investigated the relationship between the pistil length of the six species and the maximum pollen tube length of Eriobotrya in these pistils. The pistil length of E. japonica was significantly shorter than in other species (Fig. 1), suggesting that E. japonica does not need a long pollen tube for successful fertilization in intraspecific crossing. On the other hand, E. japonica pollen may be cross incompatible in species with longer pistils. This hypothesis is supported by our in vivo observation that the pollen tubes of E. japonica were arrested at a similar distance from the stigma irrespective of the female species, except for P. sinensis (1.41 ± 0.13 mm in P. sinensis, 4.69 ± 0.99 mm in C. oblonga, 5.85 ± 0.44 mm in M. × domestica, 5.38 ± 0.11 mm in P. communis and 6.09 ± 0.21 mm in P. pyrifolia pistils); this length is equivalent to or longer than the pistil length of E. japonica (3.94 ± 0.08 mm, Fig. 1). Consistent with this observation, pistils of E. japonica accepted pollen of Pyrus species, with pollen tubes reaching the base of the style (Table 6). On the other hand, pollen tubes of P. sinensis, C. oblonga, and M. × domestica did not reach the base of the style of E. japonica. Also, pollen tubes of E. japonica were observed to be arrested at the upper part in the pistil of P. sinensis when compared within that of other species. These observations suggest the presence of factors other than pistil length that regulate the intergeneric hybridization barrier. Our results indicated that pistil length and the potential for pollen tube growth are prezygotic hybridization barriers in a part of Maleae tribe cross combination.

Pistil length of six species from the tribe Maleae and the maximum pollen tube length of E. japonica in these pistils. Co: Cydonia oblonga ‘Smyrna’, Ps: Pseudocydonia sinensis, Md: Malus × domestica ‘Fuji’, Pp: Pyrus pyrifolia ‘Gold Nijisseiki’, Pc: Pyrus communis ‘Alexandrine Douillard’, Ej: Eriobotrya japonica ‘Mogi’. Data are means ± S.E. (n = 5–10). Different letters indicate significant difference (P < 0.05) based on Tukey’s test, in which uppercase and lowercase letters indicate the statistical significance for pistil length and pollen tube length, respectively.
Because there have been no reports on the development of intergeneric hybrids of E. japonica and Pyrus species (Fukuda et al., 2007), we conducted field pollination to test for intergeneric cross-compatibility (Table 7). Consistent with in vivo pollen tube growth, intergeneric hybridization using E. japonica as a pollen parent resulted in no fruit set in any crossing combinations, except for self-pollination. However, even as a seed parent, E. japonica did not bear any fruits by intergeneric hybridization with Pyrus pollen, the same as other species. Previous studies also reported no fruit set in a cross between E. japonica and P. pyrifolia (Fukuda et al., 2007). We suggest that the hybridization barrier observed in E. japonica as a seed parent may be regulated at a later stage of pollen tube guidance, such as when pollen enters the ovary and embryo sac (Higashiyama and Yang, 2017) or at the postzygotic stage during early seed development (Bikard et al., 2009). Further studies are needed to assess whether Pyrus pollen tubes reach the ovule in E. japonica and whether fertilization takes place.
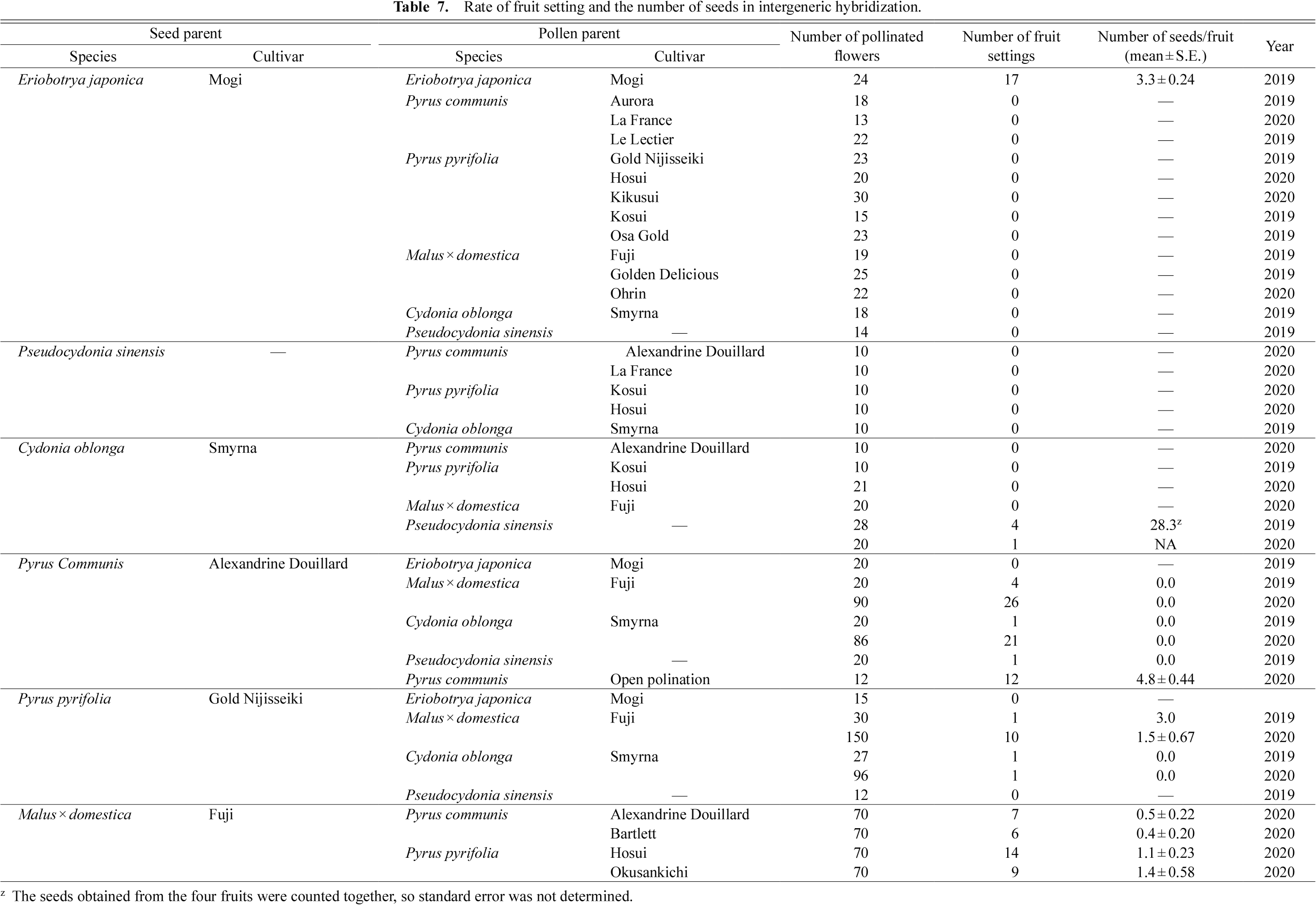
Rate of fruit setting and the number of seeds in intergeneric hybridization.
C. oblonga (quince) and P. sinensis (Chinese quince) belong to different genera but are close relatives in the phylogeny of the tribe Maleae (Xiang et al., 2017). As a female parent, P. sinensis inhibited the pollen tube growth of all species (Table 1), whereas the pistils of C. oblonga accepted pollen of P. sinensis, but not that from other species (Table 2). It should be noted that the pollen tube growth rate using P. sinensis as a seed parent differed largely among years. This may have been due to the pollination method, as we used flowers on branches in 2020, but detached flowers were used in 2019. There was no relationship between reciprocal cross-compatibility and pistil length, since C. oblonga had longer pistils than P. sinensis (Fig. 1). In field pollination, C. oblonga pollinated with P. sinensis pollen bore fruits, whereas the reciprocal cross resulted in no fruit set (Table 7), consistent with the in vivo pollen tube growth test. Although the fruit set percentage was low (5%–14%), the resulting fruits had seeds (on average 28 seeds in a fruit).
Of 113 seeds obtained from crossing C. oblonga as a seed parent with P. sinensis as a pollen parent, 44 seedlings developed and were maintained in the greenhouse for two years. The hybrid status of the seedlings was confirmed by PCR genotyping using an SSR marker CH04g09. Bands specific to each parental species were detected in all the seedlings, confirming the hybrid status of C. oblonga × P. sinensis (Fig. 2). Thus, our pollination experiment indicates the presence of unilateral cross-incompatibility between C. oblonga and P. sinensis, and that an unidentified factor apart from pistil length is involved in this response. Intergeneric hybrids with Cydonia have previously been recorded for Pyrus (known as Pyronia) and Malus (Cydomalus), but not for P. sinensis (Postman, 2011). At the time of writing this manuscript (2022), hybrid seedlings were three-years old and were transplanted in the experimental farm of Kyoto Prefectural University. Although we have not yet seen flowering or fruiting of the hybrid seedlings because they are in their juvenile phase, postzygotic incompatibility such as reported in other plants (Bikard et al., 2009; Bomblies et al., 2007; Yamamoto et al., 2010) was not observed, at least in the initial growth stage of the seedlings. The newly developed C. oblonga × P. sinensis hybrid seedlings are a promising material for future breeding programs.

Genotyping of seedlings derived from a cross between C. oblonga and P. sinensis using SSR marker CH04g09. Co: Cydonia oblonga ‘Smyrna’, Ps: Pseudocydonia sinensis.
Malus × domestica (cultivated apple) and Pyrus spp. (pears) are the most economically important species in the tribe Maleae. For Pyrus, two species, P. pyrifolia (Japanese pear) and P. communis (European pear), were used as seed parents in this study. Interspecific crosses of the two Pyrus species showed cross-compatibility in both directions (Tables 3 and 4), consistent with the development of pear interspecific hybrids in previous research (Bell and Itai, 2011; Yamamoto et al., 2002). In contrast, differences in compatibility were observed in the intergeneric crosses with M. × domestica. In pistils of M. × domestica, P. pyrifolia pollen tubes reached the base of the style, whereas most pollen tubes of P. communis were arrested within the apple style, with the pollen tube growth rate varying from 37.7% in ‘Max Red Bartlett’ to 63.0% in ‘Alexandrine Douillard’ (Table 5). In Pyrus pistils, M. × domestica pollen tubes were observed at the style base in P. pyrifolia, but were arrested within the styles of P. communis.
Pollination tests in the field were conducted to confirm the results obtained from the pollen tube growth tests in the lab. In intergeneric crossing, P. communis bore only seedless fruits, which are derived from parthenocarpy, and hybrid seed formation was not observed (Table 7). Seeded fruit was obtained when M. × domestica was pollinated with P. pyrifolia and P. communis and when P. pyrifolia was pollinated with M. × domestica (Table 7). The hybrid status of the seedlings was confirmed by S-RNase allele genotyping PCR. All the seedlings investigated possessed one of the parental S-RNase alleles (Fig. 3). Taken together, our pollination experiments indicate intergeneric cross-compatibility for the following three combinations: 1) M. × domestica pollinated by P. pyrifolia, 2) M. × domestica pollinated by P. communis, and 3) P. pyrifolia pollinated by M. × domestica. Although most pollen tubes were arrested in the cross between M. × domestica pollinated with P. communis, some pollen tubes did reach the base of the style (Table 5). We previously reported that pollens having the P. communis-derived allele at chromosome 5 are selectively inhibited within M. × domestica pistils, but some pollen tubes can grow down the full length of the pistil and fertilization takes place (Morimoto et al., 2020). Moreover, the development of intergeneric hybrid seedlings between M. × domestica and P. communis has also been reported (Fischer et al., 2014). These observations combined with our results suggest that the crossing combination of M. × domestica pollinated by P. communis is partially compatible and that intergeneric hybrids can develop despite inferior pollen tube growth. For breeding purposes, the identification and selection of cultivar combinations that show superior pollen tube growth and thus result in higher fruit setting, would be a possible solution to more efficiently develop new hybrids between M. × domestica and P. communis.
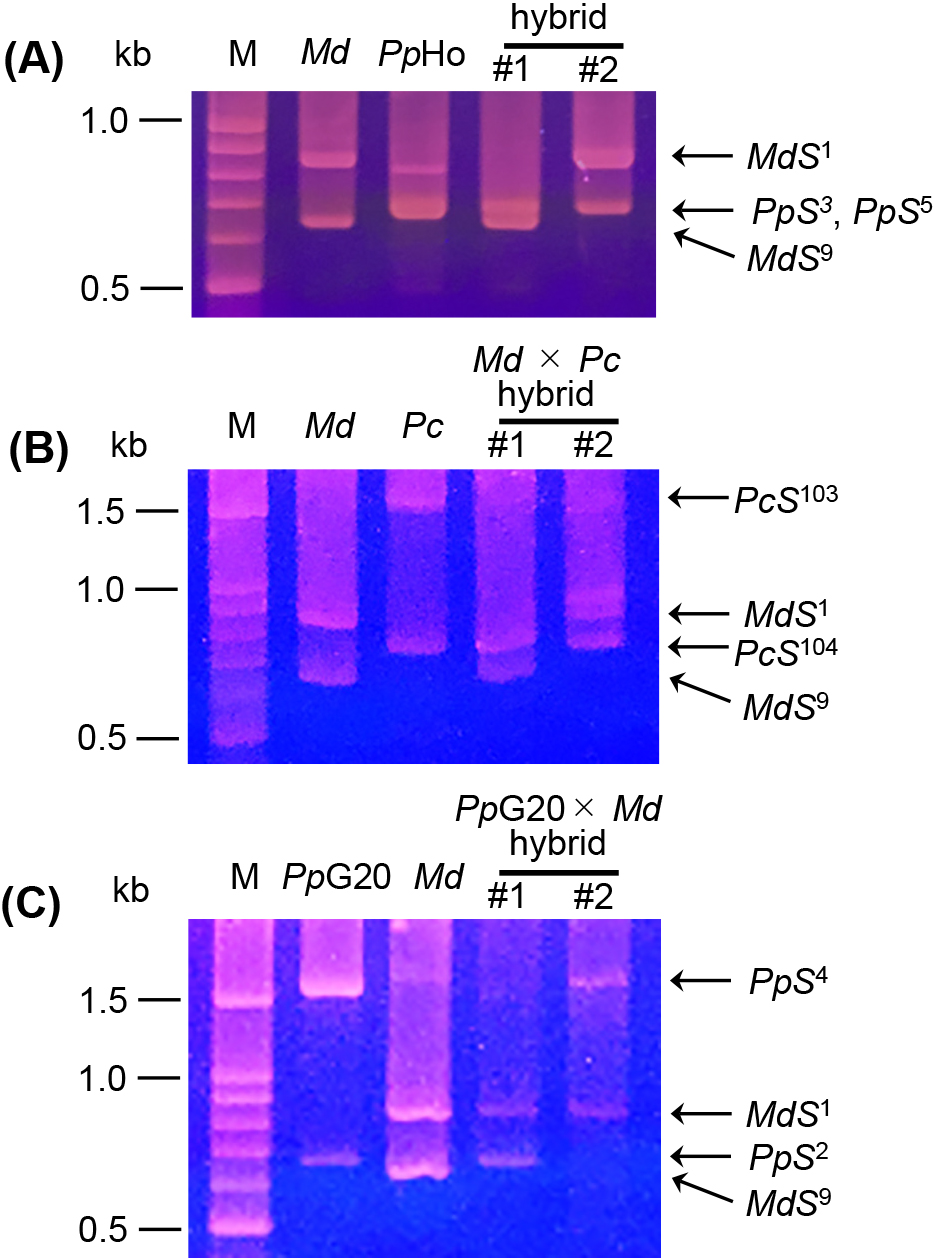
S-RNase allele genotyping of the apple-pear hybrids. (A) ‘Fuji’ × ’Hosui’, (B) ‘Fuji’ × ‘Alexandrine Douillard’, (C) ‘Gold Nijisseiki’ × ‘Fuji’. M: ladder marker, Md: Malus × domestica ‘Fuji’, PpHo: Pyrus pyrifolia ‘Hosui’, Pc: Pyrus communis ‘Alexandrine Douillard’, PpG20: Pyrus pyrifolia ‘Gold Nijisseiki’.
In conclusion, our pollination experiments showed diverse patterns of the intergeneric cross-incompatibility in the tribe Maleae, in which phylogenetic distance, cross-combinations, and crossing direction are major determinants of species identity. We propose that diverse patterns of cross-incompatibility in the tribe Maleae can be a useful model system to uncover the underlying mechanisms that have contributed to species speciation in nested genetic backgrounds. For breeding purposes, appropriate crossing direction and combinations should be considered when breeding new hybrids.
We would like to thank the laboratory of pomology at Kyoto University for providing plant materials.